Abstract
The presence of Fe(III), but not that of Fe(II), resulted in ca. 20-fold-lower levels of mRNA for fumarate reductase, inhibiting fumarate reduction and favoring utilization of fumarate as an electron donor in chemostat cultures of Geobacter sulfurreducens, despite the fact that growth yield with fumarate was 3-fold higher than with Fe(III).
The study of Fe(III) reduction has been greatly facilitated by the finding that microorganisms in the family Geobacteraceae are the predominant Fe(III)-reducing microorganisms in a diversity of subsurface environments (1, 3, 6, 11) and the fact that Geobacteraceae similar to those that predominate in these environments can be recovered in pure culture. Geobacter sulfurreducens, which commonly serves as the model organism for the Geobacteraceae in subsurface environments, can use both Fe(III) and fumarate as an electron acceptor (2). Understanding the relative utilization of Fe(III) and fumarate as electron acceptors could aid in developing strategies to best promote the growth of Geobacter species for bioremediation applications, such as the reductive precipitation of uranium from contaminated groundwater (1). In previously studied microorganisms, the regulation of electron acceptor utilization favors the reduction of the electron acceptor that can yield the most energy to support cell growth (5, 7, 8, 12). However, it is not clear whether the same trend applies to microorganisms in subsurface environments.
Inhibition of fumarate respiration in the presence of Fe(III).
In order to evaluate potential controls on fumarate and Fe(III) respiration, G. sulfurreducens was grown in continuous culture (A. Esteve-Núñez, M. Rothermich, M. Sharma, and D. R. Lovley, submitted for publication) with the electron donor, acetate (5.5 mM), which limited growth at a dilution rate of 0.05 h−1. This culture condition was designed to simulate the most prevalent conditions in anoxic soils and subsurface environments, in which acetate is the key electron donor for Fe(III) reduction (10). When fumarate (30 mM) was provided as the electron acceptor, there was a steady-state accumulation of succinate resulting from fumarate reduction (Fig. 1). The steady-state biomass was 45.5 ± 5 (mean ± standard deviation of results from three replicate experiments) mg of protein per liter. When cultures were grown with the same acetate input, but with 60 mM Fe(III) citrate as the electron acceptor, the culture density was 15.5 ± 3 mg of protein per liter. Thus, fumarate reduction provided significantly more energy to support growth than Fe(III) reduction.
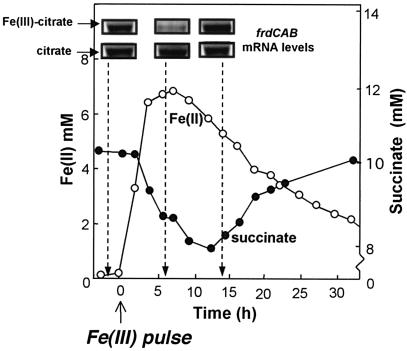
FIG. 1.
A steady-state culture of G. sulfurreducens growing at a dilution rate of 0.05 h−1 with fumarate as the terminal electron acceptor was pulsed with 10 mM Fe(III) citrate (arrow). The response of the cell to the pulse was monitored by analyzing the respective respiration products, succinate (filled circles) and Fe(II) (open circles). All the Fe(II) produced was finally washed out of the chemostat as expected in a continuous culture system. Cells were harvested before the Fe(III) pulse, after the Fe(III) pulse (6 h), and when all the Fe(III) was fully reduced to Fe(II) (14 h). RNA was isolated from those cells, and Northern blot hybridization was performed with a specific oligoprobe for frdC (fumarate reductase). The detected transcript corresponds with the 3.3 kb of the frdCAB operon. As a control, parallel cultures were pulsed with the chelating agent citrate, and Northern analysis was performed as described for the Fe(III) citrate pulse.
In order to examine the interaction between fumarate and Fe(III) reduction, Fe(III) citrate (10 mM) was added to steady-state cultures of cells growing with fumarate as the electron acceptor (Fig. 1). There was an immediate decrease in succinate concentration concurrent with an accumulation of Fe(II) resulting from Fe(III) reduction. The inhibition of fumarate reduction was associated with a dramatic decline in levels of mRNA for the fumarate reductase operon, frdCAB (Fig. 1), measured by Northern analysis (the probe was generated with PCR using the primers frdCfor [5′-GTTCGGTATCCAGCTGAG-3′] and frdCrev [5′-CTTTCAGAATGCCGGTGACG-3′]). Six hours after the addition of Fe(III), the mRNA levels for frdCAB were 17-fold lower than in cells not exposed to Fe(III) (Fig. 1). Furthermore, at this time fumarate reductase-specific activity in the membrane fraction of Fe(III)-amended cultures was fourfold lower than in unamended cultures (13 ± 1 versus 52 ± 4 nmol min−1 mg of protein−1). Once the Fe(III) was completely reduced to Fe(II), the level of mRNA for the fumarate reductase genes returned to the level detected prior to the addition of Fe(III), even though there was still a significant quantity of Fe(II) in the culture (Fig. 1). The finding that the expression of the fumarate reductase was down-regulated in the presence of Fe(III), but not Fe(II), represents a novel instance in which discrimination between iron redox states seems essential for regulating processes that might have an impact on the pathway for electron transfer.
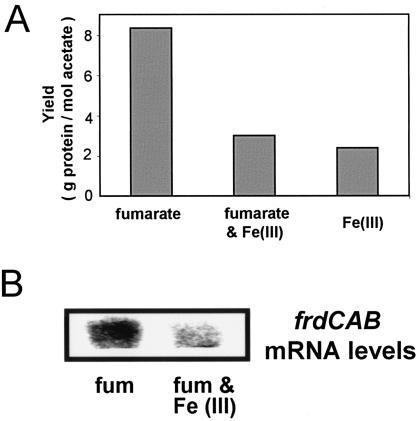
In order to determine whether Fe(III) had a similar effect on fumarate respiration over longer periods of time in which G. sulfurreducens would have time to adapt to the simultaneous presence of high quantities of Fe(III) and fumarate, G. sulfurreducens was grown in continuous culture with acetate as the electron donor and the limiting growth substrate, with both fumarate (35 mM) and Fe(III) (70 mM) as potential electron acceptors (Fig. 2). As was observed in the short-term studies, fumarate reduction was inhibited and levels of fumarate reductase mRNA were lower in the presence of Fe(III) and fumarate than in control cultures growing on fumarate alone. The steady-state biomass concentration in the presence of both fumarate and Fe(III) was slightly higher than that with Fe(III) alone but significantly lower than when fumarate was the sole electron acceptor.
FIG. 2.
(A) Effect of terminal electron acceptors on the growth yield. Cells of G. sulfurreducens were cultured in chemostats in the presence of fumarate, fumarate and Fe(III), or just Fe(III) as the terminal electron acceptor. The results shown are the means of results from triplicate cultures for each treatment. (B) Effect of the terminal electron acceptor on fumarate reductase (frdCAB) expression. Northern blot hybridizations on isolated RNA were performed with a specific oligoprobe for frdC. fum, fumarate.
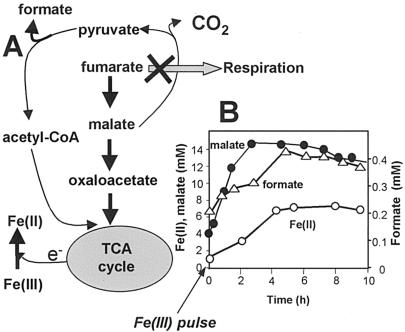
During bacterial growth on fumarate, the succinate that is produced from fumarate reduction is not oxidized in the tricarboxylic acid cycle (4), but it is possible that in the presence of Fe(III), small amounts of fumarate were used as an electron donor or carbon source in a pathway in which fumarate was converted to acetyl coenzyme A via malate and pyruvate with the formation of formate (Fig. 3A). In the presence of Fe(III), high concentrations of malate accumulated, and there was an increased rate of formate production (Fig. 3B). The high levels of malate indicated that conversion of malate to pyruvate might be the limiting step in fumarate utilization. Once Fe(III) was depleted and fumarate reduction resumed, malate accumulation and formate production stopped (Fig. 3B).
FIG. 3.
(A) Scheme of fumarate transformation by G. sulfurreducens in the presence of Fe(III). Fumarate is transformed into malate when fumarate respiration is inhibited. Malate is subsequently converted to acetyl coenzyme A (CoA) via pyruvate with the release of formate. TCA, tricarboxylic acid; e−, electron. (B) Malate (filled circles) and formate (open triangles) formation after a pulse of 10 mM Fe(III) citrate. The reduction of Fe(III) is plotted as Fe(II) production (open circles).
Implications.
The respiration in G. sulfurreducens is clearly not optimized for maximal growth when high concentrations of fumarate as well as Fe(III) are available. However, the down-regulation of fumarate respiration in the presence of Fe(III) may represent an effective adaptation to conditions found in most subsurface environments. Generally, the scarcity of readily metabolizable organic matter severely limits microbial growth in the subsurface. In contrast, Fe(III) is often the most abundant electron acceptor available for microbial respiration (9). Therefore, in typical anoxic subsurface environments in which Geobacter species predominate, if any fumarate becomes available it may be more beneficial to funnel it toward catabolic reactions rather than to use it as an electron acceptor. Thus, although the regulation of respiration in G. sulfurreducens does not fit the paradigm that the most energetically favorable electron acceptors are reduced first, this finding may represent the most adaptive response to the environmental conditions that Geobacter species typically encounter.
Acknowledgments
This research was funded by the Genomes to Life Program, U.S. Department of Energy (grant DE-FC02-02ER63446). A.E.-N. was the recipient of a postdoctoral fellowship from the Secretaría de Estado de Educación y Universidades (Madrid, Spain), cofunded by the European Social Fund. In addition, C.N. was the recipient of a DGAPA/UNAM postdoctoral fellowship.
REFERENCES
- 1.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates, J. D., E. J. P. Phillips, D. J. Lonergan, H. Jenter, and D. R. Lovley. 1996. Isolation of Geobacter species from diverse sedimentary environments. Appl. Environ. Microbiol. 62:1531-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galushko, A. S., and B. Schink. 2000. Oxidation of acetate through reactions of the citric acid cycle by Geobacter sulfurreducens in pure culture and in syntrophic coculture Arch. Microbiol. 174:314-321. [DOI] [PubMed] [Google Scholar]
- 5.Gunsalus, R. P. 1992. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J. Bacteriol. 174:7069-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes, D. E., K. T. Finneran, R. A., O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, H. M., and R. P. Gunsalus. 1987. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J. Bacteriol. 169:3340-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu, P. H., S. A. Svoronos, and B. Koopman. 1998. Experimental and modeling study of diauxic lag of Pseudomonas denitrificans switching from oxic to anoxic conditions. Biotechnol. Bioeng. 60:649-655. [PubMed] [Google Scholar]
- 9.Lovley, D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55:259-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley, D. R., and F. H. Chapelle. 1995. Deep subsurface microbial processes. Rev. Geophys. 33:365-382. [Google Scholar]
- 11.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to simulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 12.Unden, G. 1998. Transcriptional regulation and energetics of alternative respiratory pathways in facultatively anaerobic bacteria. Biochim. Biophys. Acta Bioenerget. 1365:220-224. [Google Scholar]