Abstract
We conducted a screening campaign to investigate fungi as a source for new antimalarial compounds. A subset of our fungal collection comprising Chinese mangrove endophytes provided over 5000 lipophilic extracts. We developed an accelerated discovery program based on small-scale cultivation for crude extract screening and a high-throughput malaria assay. Criteria for hits were developed and high priority hits were subjected to scale-up cultivation. Extracts from large scale cultivation were fractionated and these fractions subjected to both in vitro malaria and cytotoxicity screening. Criteria for advancing fractions to purification were developed, including the introduction of a selectivity index and by dereplication of known metabolites. From the Chinese mangrove endophytes, four new compounds (14–16, 18) were isolated including a new dimeric tetrahydroxanthone, dicerandrol D (14), which was found to display the most favorable bioactivity profile.
Keywords: fungi, endophytes, mangroves, malaria, cytotoxicity, high-throughput
1. Introduction
Mangrove plants and their associated microfauna have been a rich source of bioactive molecules [1,2,3], though only limited [4,5,6] antimalarial screening of this chemodiversity source has been reported. Mangrove forests are fascinating and complex ecosystems [7]. They serve coastal populations worldwide by protecting shorelines from storm surge and erosion, through filtration/remediation of terrestrial runoff, and as nurseries for important fisheries, among other useful roles. Yet these marine margin communities are under constant threat of clearing from real estate development, fish-pond farming, and even for their wood to support cooking hearths in poor, rural communities. The World Wildlife Foundation [8] reports that 35% of global mangrove communities have disappeared in the last two decades alone [9,10], and one in six species are in danger of extinction [11,12]. Similar to the more visible coral reef and rainforest ecosystems, a better understanding of the biotechnological value of marine margin communities may engender respect and enthusiasm for conservation efforts. We report here a screening campaign using mangrove endophytic fungi from the Mai Po Nature reserve, Hong Kong, and Hainan Island, Taiwan, as sources for new antimalarial compounds.
2. Results and Discussion
2.1. Strategy
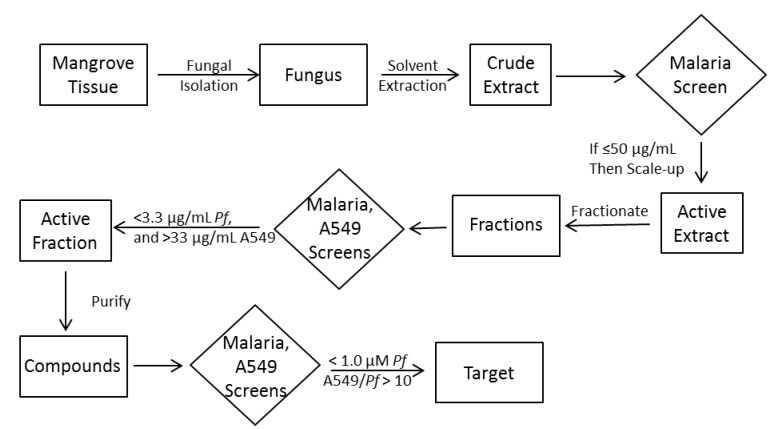
Our strategy to miniaturize fungal cultures provided for an economy of scale that allowed us to rapidly produce small quantities of crude extracts for screening. Using a robust and validated Plasmodium falciparum (3D7, a drug sensitive strain) screen [13], 96-well plated crude extract screening data was available to us weekly. Our workflow (Scheme 1) included decision points based on crude extract activity, leading to scaled-up cultivation, then new decision points based both on IC50 values of parasite and mammalian (A549) cytotoxicity. All purified metabolites were then screened for bioactivity and characterized either by LC/MS dereplication with verification by NMR, or by de novo structure analysis. Over a two year period we cultured, screened and prioritized approximately 50,000 total fungal extracts, and conducted fractionation, re-screening, and purification on approximately 10% of those. Such an intensive effort requires trade-offs; we chose, for example, to conduct a single extract of freeze-dried cultures since it yielded sufficient material for screening, and we chose to forego verification of activity in scaled-up cultures, since fractionation was quick and fraction activity was more important than extract activity. Similarly, dereplication of known compounds was done late in the workflow. We reasoned that previously published compounds were still of interest to us, and even nuisance compounds might mask compounds of interest, so we chose to dereplicate only at the purification step (end) of the workflow. Such sharp focus on a bioactivity criterion (Scheme 1) left many potentially useful fractions along the workflow, fractions which will be deconvoluted in ensuing studies.
Scheme 1.
Sample workflow and decision points (Pf = Plasmodium falciparum).
2.2. Fungal Isolation and Fermentation
Hong Kong and Taiwan have a similar subtropical climate and harbor many species of mangrove trees along the coastline where there is inundation of estuarine waters. Kandelia obovata and Avicennia marina are the dominant tree species in both healthy mangrove areas of Hong Kong and Taiwan while Lumnitzera racemosa is only found in Hong Kong mangrove communities. The endophytic fungal strains used in the study were isolated from surface sterilized plant tissues, using either 4% sodium hypochlorite [14] or 75% ethanol combined with 5% sodium hypochlorite solution [15]. Leaf and bark tissues of A. marina, K. obovata and L. racemosa were studied. A total of 5486 fungi were isolated and cultivated for screening.
2.3. Extraction, Plating and Screening of Miniaturized Cultures
Freeze dried fungal mycelia were extracted with 15 mL of dichloromethane/methanol (1:1) for 24 h then transferred to a 20 mL scintillation vials arranged in 8 × 12 arrays where they were air dried and taken up in DMSO to approximately 30 mg/mL. After transfer of 100 μL aliquots into 96-well plates, screening at two concentrations (5 and 50 μg/mL) using our previously published protocol [13] was conducted. Samples were prioritized as Active if they inhibited 3D7 by ≥67% at 5 µg/mL, and Partially Active if there was >67% inhibition at 50 µg/mL, leading to approximately 0.6% extracts categorized as Active, ~5% Partially Active, and more than 90% inactive. We advanced all Active extracts and 10% of the Partially Active into scale-up cultivation studies.
2.4. Chromatographic Separation, Screening and Structure Elucidation
Freeze-dried biomass from 2 L cultures were exhaustively extracted with dichloromethane/methanol (1:1) and applied to Combiflash® MPLC cartridges based on the manufacturers recommendation of cartridge size to analyte mass. A linear gradient from hexane to ethyl acetate and then methanol was conducted, collecting ten to twelve fractions/extract. Fractions were concentrated and re-submitted for 3D7 screening, and cytotoxicity screening against A549 human lung adenocarcinoma epithelial cells followed. The cytotoxicity screen introduced a new decision point whereby fractions and purified compounds had to exceed a threshold selectivity index (A549 activity/3D7 activity) of 10. Fractions were advanced to HPLC if their 3D7 activity was <3.3 μg/mL and they met the selectivity index criterion, although priority was initially given to fractions with <1.1 μg/mL to focus on the most promising fractions first.
Purified compounds were first dereplicated using mass and 1H NMR data searched against AntiBase and SciFinder databases. For new compounds, full spectroscopic data sets (1H, 13C, DEPT, COSY, HSQC, HMBC, NOESY, HRMS) were acquired and analyzed to arrive at structural assignments.
2.5. Fungal Chemistry and its Bioactivity
Of the 5486 fungi tested against P. falciparum, 266 were Partially Active (~5%) and 34 were Active (~0.6%). Of these, 58 samples were scaled-up and subjected to MPLC fractionation. A total of 103 MPLC fractions were identified for purification, and 18 compounds identified either through dereplication or structure analysis (Figure 1).
Figure 1.
Summary of the screening results.
2.5.1. Mycotoxins
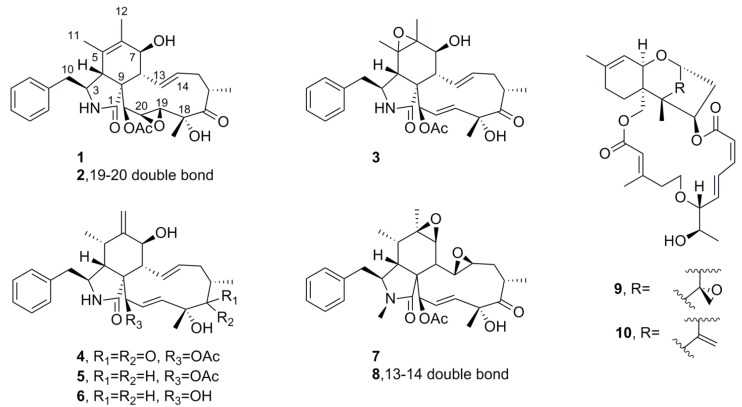
Mycotoxins proved to be the nuisance compounds in this study. Eight cytochalasins (1–8) and two trichothecenes (9, 10) were dereplicated (Figure 2) from eight different fungal strains (Table 1), though others were not pursued to purity. All structures reported here bore spectroscopic data (ESIMS, 1H NMR) that agreed well with published [16,17,18,19,20] data. While often displaying potent antimalarial activity (Table 1), cytostatic activity that was not apparent in cytotoxicity screening precludes use of these toxins as drugs. Nonetheless, and in support of our decision to dereplicate late in the workflow, one trichothecene from another source remains one of the most promising leads of the project (work in progress).
Figure 2.
Mycotoxins from mangrove endophytes.
Table 1.
Compound summary and the associated anti-malarial activity against Plasmodium falciparum (3D7) and cytotoxicity against A549 cells.
| Group | Cpd | IC50 (nM) | S.I. | Source (Taxonomic Identification) | |
|---|---|---|---|---|---|
| 3D7 | A549 | A549/3D7 | |||
| Mycotoxin | |||||
| Cytochalasin | 1 | <20 | ND * | − | CY-5286 (Diaporthe sp.) |
| 2 | 136 | ND * | − | CY-5331 (Xylaria sp.) | |
| 3 | <20 | ND * | − | CY-5368 (Verticillium sp.) | |
| 4 | 25.8 | ND * | − | CY-5286 (Diaporthe sp.), CY-6884 (Xylaria sp.), NTOU-3332 (Xylaria sp.), NTOU-1430 (Xylaria sp.) | |
| 5 | <20 | ND * | − | CY-5286 (Diaporthe sp.) | |
| 6 | <20 | ND * | − | CY-5286 (Diaporthe sp.) | |
| 7 | 290 | ND * | − | CY-5331 (Xylaria sp.) | |
| 8 | 26 | ND * | − | CY-5331 (Xylaria sp.) | |
| Trichothecene | 9 | <20 | <200 | − | CY-3923 † |
| 10 | <20 | <200 | − | CY-3923 † | |
| Polyketide | 11 | 36,000 | >38,700 | − | NTOU-1455 (Xylaria sp.) |
| 12 | >48,500 | >48,500 | − | NTOU-2009 (Phomopsis sp.) | |
| 13 | >25,000 | >25,000 | − | CY-5188 (Diaporthe sp.) | |
| 14 | 600 | 7800 | 13 | CY-5188 (Diaporthe sp.) | |
| 15 | >25,000 | >25,000 | − | CY-5286 (Diaporthe sp.) | |
| 16 | >25,000 | >25,000 | − | CY-5286 (Diaporthe sp.) | |
| Lipid | 18 | >25,000 | >25,000 | − | CY-5331(Xylaria sp.) |
| 19 | >25,000 | >25,000 | − | CY-6884 (Xylaria sp.) | |
| Control | CQ | 4.5 | >25,000 | − | |
| ATO | 0.3 | >25,000 | − | ||
| DHA | 0.8 | >25,000 | − | ||
S.I., selectivity index; * cytotostatic (recorded as lack of cell proliferation without cell death); † Not identified; ND, not determined; CQ, chloroquine; ATO, atovaquone; DHA, dihydroartemisinin.
2.5.2. Polyketides
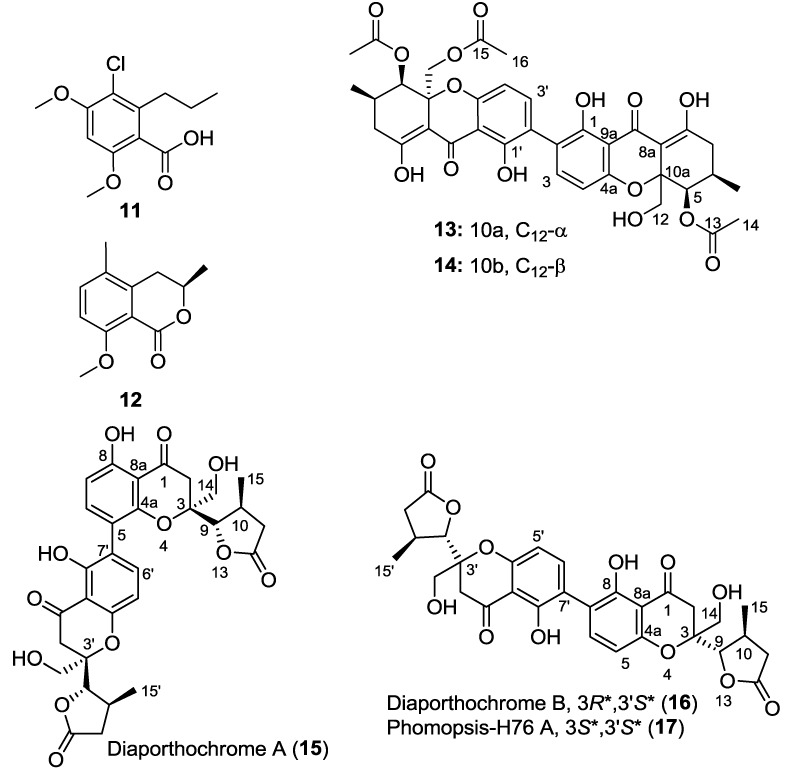
Six polyketides were found in our Chinese endophytic fungi (Figure 3). Two previously reported isolates, acremonisol A (11) [21] and 3,5-dimethyl-8-methoxy-3,4-dihydroisocoumarin (12) [22,23], were devoid of antimalarial activity but derived from fractions found active due to the presence of cytochalasin D (4). Dicerandrol B (13) and a previously unreported derivative, named here as dicerandrol D (14), were found from a strain of Diaporthe sp. (CY-5188). Further work on a related active extract produced two further unreported compounds, 15 and 16 [24], from another strain of Diaporthe (CY-5286). Dicerandrol D (14) displayed nanomolar antimalarial activity and a low cytotoxicity with a selectivity index of 13 (Table 1).
Figure 3.
Polyketide chemodiversity from Chinese mangrove endophytes.
Isomers 13 and 14 shared a molecular formula, C36H37O15 (HRESIMS m/z at [M + H]+: 709.2123 for 13: 709.2137 for 14; calc’d 709.2132). Isolated as bright yellow solids, 13 was dereplicated as the dimer dicerandrol B [25]. Isomer 14 displayed similar chemical shifts in the 1H NMR spectrum with dicerandrol B, and detailed analysis of gHMBC data failed to find a connectivity difference. However, the 2D ROESY spectrum tellingly found no correlation between H-5 (δ 5.82) and hydroxymethyl H2-12 (δ 5.02 and 3.39), leading us to assign a trans relationship between H-5 the hydroxymethine group on C-10a. Optical rotation of the two isomers are divergent, supporting their stereochemical isomerism (14: [α]20D +12.6; 13: [α]20D −8.3 (our isolate, in CHCl3), [α]25D −6.5, reported in CHCl3 for dicerandrol B [25]). Interestingly the epimer 14 showed strong activity against P. falciparum with only moderate cytotoxicity, whereas dicerandrol B (13) failed to show either activity (Table 1). While the modest structural difference is difficult to rationalize as leading to the observed bioactivities, the change in sign of the optical rotation may suggest sufficient atropisomerism to make 14 a better receptor binder than 13.
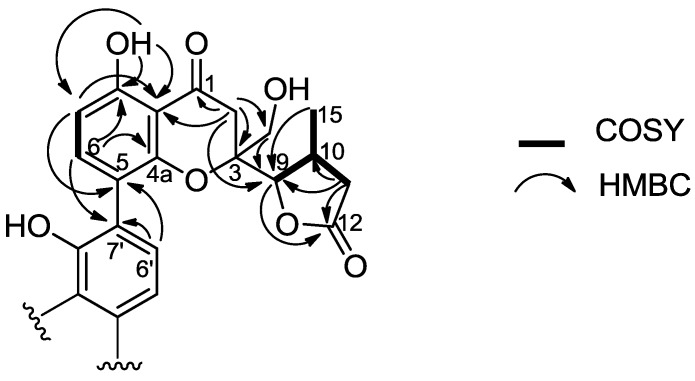
Compound 15 was obtained from strain CY-5286 which was identified as Diaporthe sp., the same genus as that yielding dicerandrols. Like 13 and 14, compound 15 displayed a series of paired resonances in the 13C NMR spectrum, although only 30 resonances were observed (Table 2). The molecular formula of C30H30O12 was determined by HRESIMS ([M + H]+ 583.1842; [M + Na]+ 605.1630; calc’d for C30H31O12 583.1816; calc’d for C30H30NaO12 605.1635). However, 15 appeared to be a chromone, rather than xanthone, based on the presence of phenolic protons (δH 12.28 (8′-OH), 11.73 (8-OH)), but lack of enolic protons, among other differences. It also differed from 13 and 14 in that it lacked acetyl groups. The 1H NMR spectrum of 15 displayed aromatic signals on each of two monomeric units (δH-6 7.25 and δH-7 6.59; δH-5′ 6.50 and δH-6′ 7.29), which correlated in the COSY spectrum and could be assigned as ortho disposed based on the 8.8 and 8.3 Hz coupling constant, respectively. Focusing on one monomeric unit at a time, H-6 showed HMBC correlation to two aromatic carbons bearing oxygen (δC-4a 155.6 and δC-8 161.7), the latter of which proved to be a phenolic oxygen based on HMBC coupling from the δH 11.73 phenolic proton to δC-8 161.7. The C-8 phenolic proton showed further HMBC correlation to C-7 (δC 109.9) and C-8a (δC 107.0), and H-7 (δH 6.59) correlated with C-8a and C-5 (δC 115.2). That aromatic ring partial structure (Figure 4) was further developed by observation of HMBC correlations of H-2a (δH 3.22) to C-8a, the ketone carbon at C-1 (δC 196.9), an oxygenated quaternary carbon (δC-3 83.5), and with an oxygenated methine (δC-9 86.9). That H-9 (δH 4.22) anchored a γ-lactone to the chromone ring system was demonstrated through key HMBC correlations depicted in Figure 4.
Table 2.
NMR data of diaporthochromones A (15), B (16) and phomopsis H76 A (17).
| 15 a | 16 a | 17 b | ||||
|---|---|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (int., J in Hz) | δC | δH | δC |
| 1 | 196.9 | 195.9 | 200.2 | |||
| 2 | 3.22 (1H, d, 17.5) 2.86 (1H, d, 17.6) |
38.3 | 3.05 (1H, dd, 17.6, 0.8) 2.90 (1H, brd, 15.1) |
37.9 | 3.11 2.93 |
39.9 |
| 3 | 83.5 | 82.9 * | 86.6 | |||
| 4a | 155.6 | 158.1 | 161.5 | |||
| 5 | 115.2 | 6.45 * (1H, d, 8.6) | 106.9 | 6.48 | 109.4 | |
| 6 | 7.25 (1H, d, 8.8) | 140.2 | 7.43 * (1H, d, 8.5) | 140.8 | 7.38 | 142.7 |
| 7 | 6.59 (1H, d, 8.8) | 109.9 | 117.2 | 118.8 | ||
| 8 | 161.7 | 159.0 | 160.6 | |||
| 8a | 107.0 | 107.2 * | 109.6 | |||
| 9 | 4.22 (1H, d, 3.9) | 86.9 | 4.40 (1H, dd, 1.5,4.4) | 86.5 * | 4.37 | 89.3 |
| 10 | 2.67 (1H, m) | 29.6 | 2.84 (1H, m) | 29.9 | 2.79 | 31.8 |
| 11 | 2.34 (1H, dd, 17.8, 9.0) 2.00 (1H, dd, 18.1, 5.4) |
36.2 | 2.26 (1H, m) 2.23 (1H, m) |
36.5 | 2.75 2.22 |
38.6 |
| 12 | 176.0 | 175.6 | 178.7 | |||
| 14 | 3.95 (1H, dd, 5.6, 11.6) 3.89 (1H, dd,5.5, 12.3) |
62.9 | 3.94 * (2H, m) | 62.4 | 3.68 | 65.0 |
| 15 | 1.10 (3H, d, 6.8) | 20.5 | 1.27 (3H, d, 7.0) | 20.8 | 1.15 | 22.7 |
| 1′ | 197.0 | 196.7 | 200.2 | |||
| 2′ | 3.24 (1H, d, 17.6) 3.10 (1H, d, 17.5) |
38.8 | 3.22 (1H, d, 17.6) 2.96 (1H, d, 17.6, 1.5) |
38.2 | 3.11 2.93 |
39.9 |
| 3′ | 83.3 | 82.9 * | 86.6 | |||
| 4a′ | 158.5 | 158.1 | 161.5 | |||
| 5′ | 6.50 (1H, d, 8.3) | 107.4 | 6.46 * (1H, dd, 1.5, 8.5) | 106.9 | 6.48 | 109.4 |
| 6′ | 7.29 (1H, d, 8.3) | 140.1 | 7.44 * (1H, dd, 1.5, 8.5) | 140.8 | 7.38 | 142.7 |
| 7′ | 118.6 | 117.2 | 118.8 | |||
| 8′ | 158.4 | 159.0 | 160.6 | |||
| 8a′ | 106.8 | 107.0 * | 109.6 | |||
| 9′ | 4.33 (1H, d, 3.9) | 87.6 | 4.35 (1H, dd, 1.5, 4.3) | 86.4 * | 4.37 | 89.3 |
| 10′ | 2.95 (1H, m) | 29.2 | 2.90 (1H, m) | 29.4 | 2.79 | 31.8 |
| 11′ | 2.88 (1H, dd, 18.0, 10) 2.22 (1H, dd, 17.6, 4.9) |
36.4 | 2.90 (2H, m) | 36.6 | 2.75 2.22 |
38.6 |
| 12′ | 175.3 | 175.8 | 178.7 | |||
| 14′ | 3.82 (2H, m) | 63.2 | 3.94 * (1H, m) 3.86 (1H, brd, 11.7) |
62.8 | 3.68 | 65.0 |
| 15′ | 1.30 (3H, d, 6.8) | 20.8 | 1.30 (3H, d, 6.4) | 20.6 | 1.15 | 22.7 |
| 8-OH | 11.73 (1H, s) | 12.01 * (1H, s) | 11.97 | |||
| 8′-OH | 12.28 (1H, brs) | 12.00 * (1H, d, 1.5) | 11.97 | |||
a recorded in CDCl3 at 500 MHz for 1H and at 125 MHz for 13C; b recorded in DMSO-d6 at 500 MHz for the 1H and at 125 MHz for 13C [24]; * Interchangeable between monomeric units.
Figure 4.
Selected HMBC and COSY correlations of 15.
The second monomeric unit associated with compound 15 displayed largely the same HMBC correlations as that described above. The two units could be connected through observation of both H-6 (δH 7.25) to C-7′ (δC 118.6) and H-6′ (δH 7.29) to C-5 (δC 115.2) HMBC correlations, defining a new dimeric chromone, diaporthochromone A (15). Significant chemical shift differences between γ-lactone chiral centers of each monomeric unit suggested an unsymmetrical nature of diaporthochromone A. The 2D ROESY spectrum clearly defined some stereochemical relationships: for example, both monomeric units show strong ROESY correlation between the H-9 methine and the H3-15 methyl groups, placing them on the same face (cis to one another) of the γ-lactone. That both monomeric units display H-10 to H2-14 correlation in the ROESY requires that H-9 and H3-15 both be on the opposing face from H2-14, reinforcing the H-9/H3-15 cis-relationship. The major difference between the two monomeric units is the relationship between H-9 and H2-2: In the monomeric unit bearing prime distinctions, there is no correlation between the two, while in the other there is, requiring a shift of H-9′ away from the dihydro-γ-pyrone ring system, which can only be achieved by an inverted stereocenter at C-9′ compared to C-9. Thus we propose the relative stereochemistry for diaporthochromone A as 3R*,9S*,10S*,3′S*,9′S*,10′S*.
A second compound was isolated from fungal strain CY-5286 as an isomer of diaporthochromone A (15). Diaporthochromone B (16) was demonstrated to have the molecular formula C30H30O12 based on HRESIMS (m/z at [M + H]+ 583.1837; calc’d for C30H31O12 583.1816). A similar unsymmetrical γ-lactone-substituted chromone dimer was evident from the 1H and 13C NMR spectra (Table 2). Indeed, except for the two carbons C-5 and C-7, the NMR data is nearly superimposable. Distinguishing 16 are HMBC correlations for overlapping quaternary carbons at δC 117.2 (C-7/7′) from δH 12.0 (8/8′-OH), compared to 15 in which two signals are found near 117, quaternary carbons δC-7′ 118.6 and δC-5 115.2, only one of which (δC-7′ 118.6) correlates to a phenolic proton, 8′-OH in this case, at δH 12.28. Instead, 15 displays two methine carbons at δC-7 109.9 and δC-5′ 107.4, in the vicinity of 16’s overlapping C-5/5′ (δC 106.9), and one of them, δC-7 109.0, has HMBC correlation from the phenolic proton 8-OH (δH 11.73). Thus, where 15 bears a phenolic proton that correlates by HMBC to an aromatic methine, 16 only has phenolic protons correlating to quaternary aromatic carbons. Diaporthochromone B (16) therefore bears a linear 7/7′ coupled chromone ring system, analogous to the recently reported phomopsis-H76 A (17) [26]. That diaporthochromone B and phomopsis-H76 A are isomeric is evident from variations in the chemical shifts (Table 2), not all of which can be attributed to solvent effects, and their divergent optical rotations: [α]20D (CHCl3) −24.1 and [α]25D (MeOH) +20, respectively. A ROESY spectrum of 16 secured the cis-relationship of the 15/15′ methyl groups and their corresponding H-9/9′ methines, analogous to diaporthochromone A (described above) and phomopsis-H76 A. While a small sample size rendered the ROESY from 16 less informative from that of 15, the H-10 to H-2 correlation, in the absence of an analogous H-10′ to H-2′ correlation, supports an identical stereochemical assignment for the two.
2.5.3. Lipids
Two inactive lipids (Figure 5) were found in otherwise active extracts. (2E,4E)-Dimethyldeca-2,4-dienoic acid (18) is a previously unreported compound which was found among active antimalarial cytochalasins from Xylaria sp. (strain CY-5331). Similarly, piliformic acid (19) [27] was found in strain CY-6884. Neither 18 nor 19 displayed activity against malaria. HRESIMS of 18 indicated a formula of C12H20O2 (m/z at [M + H]+ 197.1545; calc’d 197.1542), which suggested three unsaturations. The 13C NMR chemical shift data indicated carboxylic acid equivalent carbon at δC 172.5. Sequential olefins followed: H-2 (δH 5.82) showed correlations to the carboxylate, C-1, and to C-4 (δC 126.5) in the HMBC spectrum. Correlations observed in the COSY spectrum between H-3 (δH 7.37) and H-2 and H-4 (δH 6.20), and H-4 to H-5 (δH 6.04), secured the C-1 through C-5 dienoate. H-5, in turn, showed HMBC correlation to C-3 (δC 147.7), C-4 (δC 126.5), C-6 (δC 35.0), C-7 (δC 43.8), and C-11 (δC 18.9). C-12 (δC 20.9) is a methyl group substituted on C-8, as suggested by COSY correlation between H-12 (δH 0.85) and H-8 (δH 1.31). The terminal methyl protons H-10 (δH 0.83) correlated to C-8 (δH 1.31) and C-9 (δH 1.14) by HMBC. The olefinic stereochemistry of 18 was deduced by analysis of coupling constants (J2,3 = 15.3 Hz and J4,5 = 15.2 Hz), supporting the trans,trans-relationship. The stereochemistry of the methyl substituents is unknown.
Figure 5.

Lipids from Chinese mangrove endophytes.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Rudolph Research Analytical AUTOPOL IV digital polarimeter. IR and UV spectra were measured on Nicolete Avatar 320FT infrared and Hewlett-Packard 8452A diode array instruments, respectively. Medium pressure liquid chromatography was carried out on a Teledyne Isco Combiflash Companion using normal and reverse phase silica gel or C18 cartridges, respectively, purchased from Teledyne Isco. High performance liquid chromatography was carried out on semipreparative Phenomenex Luna C18(2) reverse phase (250 × 10 mm) and analytical (250 × 4.6 mm) columns using a LC-20A Shimadzu multi-solvent delivery system, a CBM-20A Shimadzu system controller, and a SPD-M20A Shimadzu PDA detector. Low resolution mass spectra were recorded on an Agilent Technologies LC/MSD VL electrospray ionization mass spectrometer. High resolution mass spectra were recorded on an Agilent Technologies LC/MSD TOF electrospray ionization spectrometer. 1H and 13C NMR spectra were recorded on a Varian Inova instrument operating at 500 MHz for 1H, 125 MHz for 13C, except 2D ROESY, which were acquired at 600 MHz on a Varian Inova, using residual protonated solvent as 1H internal standard or 13C absorption lines of solvents for 13C internal standard.
3.2. Biological Materials
Fungi were isolated from samples collected in mangroves of the South China Sea coast at Mai Po Nature Reserve, or from Hainan Island coastal regions. Segments of approximately 1–2 mm2 were plated on malt extract freshwater agar. Fungal growth was examined every day for two weeks and then twice a week for at least one month. A hypha growing out from the incubated plant tissue was cut out and transferred to a fresh malt extract agar plate [15]. Pure axenic endophytic fungal cultures were grown in the commonly used liquid medium which contained 1% w/v glucose, 0.1% w/v yeast extract and 0.2% w/v peptone for 3 weeks for the production of secondary metabolites; samples for crude extract preparation were 30 mL total volume each, while high priority samples were scaled up to 2 L total volume to support fractionation and characterization studies. Cultures were freeze dried and shipped expedited to USF.
3.3. Dicerandrol D (14)
From 6.4 g of freeze dried mycelia from Diaporthe sp. (strain CY-5188), a lipophilic (1:1 CH2Cl2/CH3OH) extract was prepared and fractionated by MPLC and HPLC, using 2% CH3OH in CH2Cl2 on silica gel, to yield 1.6 mg of dicerandrol D (14). Yellow amorphous solid. [α]20D +12.6 (c 0.02 CHCl3); 1H NMR (500 MHz, CDCl3) δH 14.23 (enol, s, 1H), 13.98 (enol, s, 1H), 11.69 (phenol, s, 1H), 11.66 (phenol, s, 1H), 7.41 (H-3, d, J = 8.9 Hz, 1H), 7.31 (H-3′, J = 8.8 Hz, 1H), 6.57 (H-4, d, J = 8.9 Hz, 1H), 6.52 (H-4′, d, J = 8.8 Hz, 1H), 5.83 (H-5, s, 1H), 5.61 (H-5′, s, 1H), 5.03 (H-12, d, J = 12.6 Hz, 1H), 4.01 (H-12′, dd, J = 13.6, 5.1 Hz, 1H), 3.62 (H-12, d, J = 12.8 Hz, 1H), 3.39 (H-12′, dd, J = 13.5, 10.0 Hz, 1H), 2.56-2.29 (H-6/6′, H-7/7′, m, 6H), 2.21 (H-14, s, 3H), 2.18 (H-14′, d, 3H), 1.63 (H-16′, s, 3H), 1.07 (H-11, d, J = 6.2 Hz, 3H), 1.04 (H-11′, d, J = 5.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δC 187.9, 187.5 (C-9/9′), 177.6, 177.1 (C-8/8′), 170.8, 169.9 (C-13/13′), 169.8 (C-15′), 161.5, 161.3 (C-1/1′), 154.1, 154.1 (C-4a/4a′), 141.6, 141.1 (C-3/3′), 115.1, 115.0 (C-2/2′), 109.8, 108.8 (C-4/4′), 106.2, 106.0 (C-9/9′), 101.0, 99.7 (C-8a/8a′), 82.2, 80.9 (C-10a/10a′), 70.0, 70.0 (C-5/5′), 65.2, 65.4 (C-12/12′), 33.4, 33.4 (C-7/7′), 28.1, 27.7 (C-6/6′), 21.1, 21.0 (C-14/14′), 17.7, 17.6 (C-11/11′), 20.1 (C-16′); HRESIMS m/z: [M + H]+ 709.2137, [M + Na]+ 731.1967 (calc’d for C36H37O15 709.2132, C36H36NaO15, 731.1952).
3.4. Diaporthochromones (15 and 16)
From 4.9 g of freeze dried mycelia from Diaporthe sp. (strain CY-5286), 2.1 g of lipophilic extract (1:1 CH2Cl2/CH3OH) was fractionated first on normal phase MPLC using a CH2Cl2 gradient with increasing CH3OH, then repeated HPLC (Figure S8) to achieve separation of 15 and 16, finally yielding 1.2 and 1.0 mg, respectively.
Diaporthochromone A (15): White amorphous solid. [α]20D −84.6 (c 0.1 CHCl3); CD (c 0.01, MeOH) λmax nm (ε) 245 (−30.5), 286 (−22.7); 1H NMR and 13C NMR (Table 2); HRESIMS m/z: [M + H]+ 583.1842, [M + Na]+ 605.1630 (calc’d for C30H31O12 583.1816; C30H30NaO12 605.1635).
Diaporthochromone B (16): Yellow amorphous solid. [α]20D −24.1 (c 0.1 CHCl3); CD (c 0.01, MeOH) λmax nm (ε) 250 (+14.1), 293 (4.2); 1H NMR and 13C NMR (Table 2); HRESIMS m/z: [M + H]+ 583.1837 (calc’d for C30H31O12 583.1816).
3.5. (2E,4E)-6,8-Dimethyldeca-2,4-dienoic Acid (18)
From 4.89 g of freeze dried CY-5331 mycelia, 2.06 g of lipophilic (1:1 CH2Cl2/CH3OH) extract were obtained. Silica gel MPLC using 50% EtOAc/hexane yielded 12 fractions, including a 226 mg fraction 2. Fraction 2, after further MPLC (30% EtOAc/hexane) and HPLC using 20% EtOAc/hexane, yielded 7 mg of 18. Yellow oil. [α]20D −20.1 (c 0.1 CHCl3); 1H NMR (500 MHz, CDCl3) δH 7.37 (H-3, dd, J = 15.3, 10.9 Hz, 1H), 6.20 (H-4, dd, J = 15.2, 10.9 Hz, 1H), 6.04 (H-5, dd, J = 15.2, 8.3 Hz, 1H), 5.82 (H-2, d, J = 15.4 Hz, 1H), 2.38 (H-6, m, 1H), 1.31 (H2-7, H-8, m, 3H), 1.14 (H-9, m, 2H), 1.03 (H-11, d, J = 6.6 Hz, 3H), 0.85 (H-12, d, J =7.3 Hz, 3H), 0.83 (H3-10, m, 3H); 13C NMR (125Hz, CDCl3) δC 172.5 (C-1), 152.0 (C-5), 147.7 (C-3), 126.5 (C-4), 118.2 (C-2), 43.8 (H-7), 35.0 (C-6), 32.0 (C-8), 29.9 (C-9), 20.9 (C-12), 18.9 (C-11), 11.2 (C-10); HRESIMS m/z: [M + H]+ 197.1545, [M + Na]+ 219.1365 (calc’d for C12H21O2, 197.1542; C12H20NaO2, 219.1361).
3.6. Malaria Assay
Malaria screening was conducted as previously reported [13].
3.7. In Vitro Toxicity Assay
Cell line A-549 (adenocarcinomic human alveolar epithelial cells) was cultured in F-12K Nutrient Mixture (Kaighn’s Modification) media containing L-glutamine, supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. For the assay, A549 cells were diluted to 1.33 × 105 cells/mL in DMEM F12 media with l-glutamine, without HEPES or phenol red, and supplemented with 2% fetal bovine serum and 1% penicillin-streptomycin. Test compounds at 2 mg/mL in DMSO were diluted 1:200 then serially diluted in duplicate over 11 concentrations. In 96-well plates, a volume of 90 µL/well of A549 cells was added on top of 25 µL/well of the test compound. Final concentration of A549 cells was 12,000 cells per well and the final starting concentration for test compounds was 10 µg/mL. A Beckman-Coulter Biomek 3000 was used to dispense cells and prepare and dispense test compounds to the 96 well plates. Positive and negative controls were included on each assay plate. Plates were incubated for 72 h at 37 °C and 5% CO2. After the incubation period, cell proliferation was assessed using Promega’s CellTiter 96 Aqueous One Solution Cell Proliferation Assay reagent. Into each well 20 µL of reagent was added followed by incubation for 3.5 h at 37 °C and 5% CO2. A Molecular Devices Spectramax M2e plate reader was used to read absorbance at 490 nM. IC50 values were determined using a custom database manager (Dartaspects Corporation, Glencoe, CA, USA) by the use of nonlinear regression analysis.
4. Conclusions
A significant number of endophytic fungal extracts have been evaluated for antimalarial activity. The stringent bioassay restrictions used to advance extracts and fractions, using both malaria and cytotoxicity data, limited resource intensive scale-up and fractionation studies to roughly 1% of fungi studied. Nonetheless, the subset of fungal extracts described in this paper, derived from Hong Kong and Taiwan mangroves, yielded several new compounds, one of which, dicerandrol D (14), met our target criteria of nanomolar malaria activity with at least 10-fold less cytotoxicity.
Acknowledgments
This research was supported by Medicines for Malaria Venture, MMV08/0105. NMR and mass spectrometric facilities in the Center for Drug Discovery and Innovation were supported by a State of Florida Center of Excellence award. None of this work would have been possible without the relentless assistance of a number of dedicated undergraduate researchers, to whom we are indebted.
Supplementary Files
Supplementary Information (PDF, 1713 KB)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rateb M.E., Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290–344. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 2.Xu J. Biomolecules produced by mangrove-associated microbes. Curr. Med. Chem. 2011;18:5224–5266. doi: 10.2174/092986711798184307. [DOI] [PubMed] [Google Scholar]
- 3.Wu J., Xiao Q., Xu J., Li M.Y., Pan J.Y., Yang M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008;25:955–981. doi: 10.1039/b807365a. [DOI] [PubMed] [Google Scholar]
- 4.Omar S., Godard K., Ingham A., Hussain H., Wongpanich V., Pezzuto J., Durst T., Eklu C., Gbeassor M., Sanchez-Vindas P., et al. Antimalarial activities of gedunin and 7-methoxygedunin and synergistic activity with dillapiol. Ann. Appl. Biol. 2003;143:135–141. doi: 10.1111/j.1744-7348.2003.tb00279.x. [DOI] [Google Scholar]
- 5.Castillo U., Harper J.K., Strobel G.A., Sears J., Alesi K., Ford E., Lin J., Hunter M., Maranta M., Ge H., et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL, 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol. Lett. . 2003;224:183–190. doi: 10.1016/S0378-1097(03)00426-9. [DOI] [PubMed] [Google Scholar]
- 6.Isaka M., Suyarnsestakorn C., Tanticharoen M., Kongsaeree P., Thebtaranonth Y. Aigialomycins A–E, new resorcylic macrolides from the marine mangrove fungus Aigialus parvus. J. Org. Chem. 2002;67:1561–1566. doi: 10.1021/jo010930g. [DOI] [PubMed] [Google Scholar]
- 7.Feller I.C., Lovelock C.E., Berger U., McKee K.L., Joye S.B., Ball M.C. Biocomplexity in mangrove ecosystems. Annu. Rev. Mar. Sci. 2010;2:395–417. doi: 10.1146/annurev.marine.010908.163809. [DOI] [PubMed] [Google Scholar]
- 8.Mangrove Forest Threats. [(accessed on 8 September 2013)]. Available online: http://wwf.panda.org/about_our_earth/blue_planet/coasts/mangroves/mangrove_threats/
- 9.Alongi D.M. Present state of future and the world’s mangrove forests. Environ. Conserv. J. 2002;29:331–349. [Google Scholar]
- 10.Valiela I., Bowen J.L., York J.K. Mangrove forests: one of the world’s threatened major tropical environments. BioScience. 2001;51:807–915. doi: 10.1641/0006-3568(2001)051[0807:MFOOTW]2.0.CO;2. [DOI] [Google Scholar]
- 11.Polidoro B.A., Carpenter K.E., Collins L., Duke N.C., Ellison A.M., Farnsworth E.J., Fernando E.S., Kathiresan K., Koedam N.E., Livingstone S.R., et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS One. 2010;5:e10095. doi: 10.1371/journal.pone.0010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mace G.M., Norris K., Fitter A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2011;27:19–26. doi: 10.1016/j.tree.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Lebar M.D., Hahn K.N., Mutka T., Maignan P., van Olphen A., Kyle D.E., McClintock J.B., Amsler C.D., Baker B.J. CNS and antimalarial activity of synthetic meridianin and psammopemmin analogs. Bioorg. Med. Chem. 2011;19:5756–5762. doi: 10.1016/j.bmc.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski T., Kehr R.D. Endophytic fungal colonization of branch bases in several forest tree species. Sydowia. 1992;44:137–168. [Google Scholar]
- 15.Pang K.L., Vrijmoed L.L.P., Goh T.K., Plaingam N., Jones G.E.B. Fungal endophytes associated with Kandelia candel (Rhizophoraceae) in Mai Po Nature Reserve, Hong Kong. Bot. Mar. 2008;51:171–178. [Google Scholar]
- 16.Edwards R.L., Maitland J., Whalley A.J.S. Metabolites of the higher fungi. Part 24. Cytochalasin N, O, P, Q, and R. New cytochalasins from the fungus Hypoxylon terricola Mill. J. Chem. Soc. Perkin Trans. I. 1989:57–65. [Google Scholar]
- 17.Liu J., Jianwen T., Dong Z., Ding Z., Wang X., Liu P. Neoengleromycin, a novel compound from Engleromyces goetzii. Helv. Chem. Acta. 2002;85:1439–1442. doi: 10.1002/1522-2675(200205)85:5<1439::AID-HLCA1439>3.0.CO;2-X. [DOI] [Google Scholar]
- 18.Espada A., Rivera-Sagredo A., de la Fuente J.M., Hueso-Rodriguez J.A., Elson S.W. New cytochalasins from the fungus Xylaria hypoxylon. Tetrahedron. 1997;53:6485–5492. doi: 10.1016/S0040-4020(97)00305-0. [DOI] [Google Scholar]
- 19.Konig G.M., Wright A.D., Angerhofer C.K. Antimalarial diterpene isonitriles, isothiocyanates and isocyanates from the tropical marine sponge Cymbastela hooperi. J. Org. Chem. 1996;61:3259–3267. doi: 10.1021/jo952015z. [DOI] [Google Scholar]
- 20.Namikoshi M., Akano K., Meguro S., Kasuga I., Mine Y., Takahashi T., Kobayashi H. A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine-derived fungus Myrothecium roridum collected in Palau. J. Nat. Prod. 2001;64:396–398. doi: 10.1021/np000443g. [DOI] [PubMed] [Google Scholar]
- 21.Pontius A., Mohamed I., Krick A., Kehraus S., Konig G.M. Aromatic polyketides from marine algicolous fungi. J. Nat. Prod. 2008;71:272–274. doi: 10.1021/np0704710. [DOI] [PubMed] [Google Scholar]
- 22.Kokubun T., Bridge P.D., Simmonds M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry. 2003;62:779–782. doi: 10.1016/S0031-9422(02)00606-4. [DOI] [PubMed] [Google Scholar]
- 23.Kamisuki S., Ishimaru C., Onoda K., Kuriyama I., Ida N., Sugawara F., Yoshida H., Mizushina Y. Nodulisporol and nodulisporone, novel specific inhibitors of human DNA polymerase lambda from a fungus, Nodulisporium sp. Bioorg. Med. Chem. 2007;15:3109–3114. doi: 10.1016/j.bmc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Ma W.S. Ph.D. Thesis. Department of Chemistry, College of Arts and Sciences, University of South Florida; Tampa, FL, USA: Nov 11, 2011. Natural Product Drug Discovery against Tropical Diseases. [Google Scholar]
- 25.Wagenaar M.M., Clardy J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 2001;64:1006–1009. doi: 10.1021/np010020u. [DOI] [PubMed] [Google Scholar]
- 26.Yang J.F., Xu F., Huang C.H., Li J., She Z.G., Pei Z., Lin Y.C. Metabolites from the mangrove endophytic fungus Phomopsis sp. (#zsu-H76) Eur. J. Org. Chem. 2010;2010:3692–3695. doi: 10.1002/ejoc.201000329. [DOI] [Google Scholar]
- 27.Anderson J.R., Edward R.L., Whalley A.C.J. Metabolites of the higher fungi. Part 22. 2-Butyl-3-methylsuccinic acid and 2-hexylidene-3-methylsuccinic acid from xylariaceous fungi. J. Chem. Soc. Perkin Trans. I. 1985:1481–1485. doi: 10.1039/p19850001481. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1713 KB)