Abstract
Objective
7-Hydroxymaitairesinol (7-HMR) is a naturally occurring plant lignan found in whole grains and the Norway spruce (Piciea abies). The purpose of this study was to evaluate the bioavailability of a proprietary 7-HMR product (HMRlignan, Linnea SA, Locarno, Switzerland) through measurement of lignan metabolites and metabolic precursors.
Methods
A single-blind, parallel, pharmacokinetic and dose-comparison study was conducted on 22 post-menopausal females not receiving hormone replacement therapy. Subjects were enrolled in either a 36 mg/d (low-dose) or 72 mg/d dose (high-dose) regimen for 8 weeks. Primary measured outcomes included plasma levels of 7-HMR and enterolactone (ENL), and single-dose pharmacokinetic analysis was performed on a subset of subjects in the low-dose group. Safety data and adverse event reports were collected as well as data on hot flash frequency and severity.
Results
Pharmacokinetic studies demonstrated 7-HMR Cmax = 757.08 ng/ml at 1 hour and ENL Cmax = 4.8 ng/ml at 24 hours. From baseline to week 8, plasma 7-HMR levels increased by 191% in the low-dose group (p < 0.01) and by 1238% in the high-dose group (p < 0.05). Plasma ENL levels consistently increased as much as 157% from baseline in the low-dose group and 137% in the high-dose group. Additionally, the mean number of weekly hot flashes decreased by 50%, from 28.0/week to 14.3/week (p < 0.05) in the high-dose group. No significant safety issues were identified in this study.
Conclusion
The results demonstrate that HMRlignan is quickly absorbed into the plasma and is metabolized to ENL in healthy postmenopausal women. Clinically, the data demonstrate a statistically significant improvement in hot flash frequency. Doses up to 72 mg/d HMRlignan for 8 weeks were safe and well tolerated in this population.
Keywords: lignans, 7-Hydroxymatairesinol, enterolactone, pharmacokinetics, bioavailability, hot flashes, postmenopausal women
INTRODUCTION
Lignans are naturally occurring diphenolic compounds that are classified as phytoestrogens. They are commonly found in whole grains, seeds, nuts, legumes, fruits, and vegetables [1,2]. Among commercially available products, the highest concentrations of lignans are found in flaxseed powder extract standardized to secoisolariciresinal and a 7-hydroxymatairesinol (7-HMR) extract from the knot wood of Norwegian spruce (Picea abies) [1,3]. HMRlignan (Linnea SA, Switzerland), an extract derived from this plant, is a pure lignan in the aglycone form. In humans, dietary lignans are converted by enteric bacteria to enterolactone (ENL) and enterodiol and have been shown in vitro as well as in rat studies to preferentially bind alpha-estrogen receptors and demonstrate weak estrogenic activity [1,4]. The effects of these plant-derived compounds are primarily mediated through 2 estrogen receptors, ER-α and ER-β, which affect transcription of genes involved in cell growth, proliferation, and differentiation [5]. Members of the lignan family have variable effects on these 2 receptors, with some preferentially binding ER-α and others preferentially binding ER-β. Phytoestrogens also have mixed agonist–antagonist activity. ENL, specifically, has been shown to act as either as a partial agonist or antagonist, depending on estrogen concentration [5].
In postmenopausal women, lignans and increased ENL have been associated with a reduction of hot flashes [11], improvement in bone mineral density [12], and enhanced cognition [13]. Both consumption of dietary lignans as well as elevated plasma ENL are associated with a reduced risk of breast cancer in pre-and postmenopausal women [14–20]. A variety of mechanisms have been proposed to mediate these effects. In mammals, lignans may bind to estrogen receptors and serve as weak estrogen receptor agonists [1,21]. Increased intake of dietary lignans is also associated with increased serum levels of sex hormone-binding globulin (SHBG) [10,22–24] in postmenopausal women. In addition, lignans, particularly 7-HMR and ENL, demonstrate antioxidant properties [8,25] as well as antiproliferative activity in cancer cell lines [25]. An elevated level of 7-HMR has been described in the rat dimethylbenz[a]-anthracene-induced breast cancer model to reduce tumor volume [25].
The dietary supplement 7-HMR (HMRlignan) has been shown to be metabolized to ENL in healthy male volunteers [25]. The primary purpose of this study was to (1) measure the pharmacokinetics and bioavailability of HMRlignan and its metabolic product ENL; (2) secondarily assess the safety of HMRlignan in part by measuring its effect on estrogen (E1 and E2), estrogen metabolites 2-hydroxyestrone (2-OHE), 16-alphahydroxyestrone (16-OHE; measured as the ratio of 2-OHE/16-OHE), and SHBG; and (3) evaluate the effect of HMRlignan in decreasing the frequency and severity of hot flashes in healthy postmenopausal women.
METHODS
Study Subjects
Postmenopausal women (n = 22) not currently receiving hormone replacement therapy were recruited from existing patient populations, physician referrals, subject databases, and community-based local advertising to participate in this single-blind, parallel dose-comparison study.
The study enrolled subjects who were 50–75 years of age, had natural or surgical menopause for at least one year (confirmed by a follicle-stimulating hormone ≥23 mIU/mL), a body mass index between 20 and 35 kg/m2, and a negative cancer screening mammogram within one year of visit. The presence of hot flashes was not required for participation. Exclusion criteria included the following: Women who were pregnant or lactating, at increased risk for breast cancer either through positive BRCA testing or having a first-degree relative with a positive breast cancer history, use of probiotics within past 3 months, weight loss of ≥9.1 kg in last 3 months, untreated or unstable hypothyroidism, active eating disorder, a known sensitivity to the spruce tree, untreated psychiatric disease or heart disease, anticoagulation therapy, or brain and/or spinal cord injury. Other exclusion criteria included a history of fibrocystic breast disease, breast cancer or any other cancer in the past 5 years (except nonmelanoma skin cancer), irritable bowel syndrome, Crohn's disease, ulcerative colitis, gastrointestinal surgery, seizures, perforation of the stomach or intestine, and drug or alcohol abuse. Subjects taking hormone replacement therapy were also excluded from the study. All subjects signed an informed consent form (including HIPAA authorization) and the California Bill of Rights document before any procedure was performed. The study was approved by the Copernicus Group institutional review board (Cary, NC) and adhered to the tenets of the Declaration of Helsinki.
Investigational Product
The test product, HMRlignan, is derived from Norwegian spruce knotwood and contains lignans standardized to 70% 7-HMR. Each capsule of HMRlignan contained 36 mg of product. The 36 mg/d dose contained 25 mg/d 7-HMR and the 72 mg/d dose contained 50 mg/d of 7-HMR. The placebo, dispensed in a sensory-identical, opaque capsule, was a combination of magnesium stearate, microcrystalline cellulose, and other inactive components. Subjects enrolled into the pharmacokinetic study were blinded to the dosage used and were administered one capsule of placebo and one capsule of 36 mg product.
Study Design
The initial screening baseline visit included a medical history, physical examination (including breast examination), blood draw for follicle-stimulating hormone level, urine pregnancy test, vital signs, demographic assessment, and reviews of inclusion/exclusion criteria as well concomitant use of medications or supplements.
Subjects underwent a one-week washout period where they adhered to a lignan-poor diet by avoiding the following foods: flaxseed, sesame seed, cruciferous vegetables such as broccoli or kale, whole grains, peanuts, hazelnuts, cashew nuts, pistachio nuts, cranberries, strawberries, and soy. All subjects were given one week's worth of single-blind placebo run-in product. These subjects were also given a hot flash diary to record the daily number of hot flashes at baseline and throughout the study period as well as a diet diary during the washout phase to document adherence to a lignan-poor diet. They continued the lignan-poor diet for the duration of the study, and they continued the daily diet diary to ensure compliance. All subjects received a single-blind placebo during the washout period.
Subjects arrived at visit 1 at least 7 days after the initial screening visit, having fasted for at least 10 hours. At this time, their interim medical history was reviewed, the placebo run-in dosing diary and diet diary were assessed for compliance, and concomitant medication and supplement usage was reviewed. Vital signs were completed and blood was drawn for baseline 7-HMR, ENL, estradiol, SHBG, complete blood count, high-sensitivity C-reactive protein (hs-CRP), and comprehensive metabolic panel. The first morning urine was collected for the baseline measurements of 2-OHE/16-OHE.
Pharmacokinetic Assessment
A subset of subjects (n = 12) participated in a pharmacokinetic assessment on visit 1. These subjects received a single dose of 36 mg HMRlignan followed by blood draws at 0, 0.5, 1, 2, 4, 8, 12, 24, 48, and 72 hours for 7-HMR and ENL analysis. Subjects were allowed to leave the facility after the initial 12 hours and returned daily for the 24-, 48-, and 72-hour blood draws.
Longitudinal Assessment
Subjects who consented to participation in a single-dose pharmacokinetic assessment were enrolled in the 36 mg/d (low-dose) group. Subjects declining the pharmacokinetic study were enrolled in the 72 mg/d (high-dose) group. For a course of 8 weeks, subjects in both groups consumed 2 capsules of study product daily in the morning: utilizing the double-dummy blender method, either both capsules contained active product consisting of 36 mg of HMRlignan or one capsule contained active product and the other was an identical capsule containing placebo. On visit 1, subjects consumed the first dose of treatment. Subjects returned at week 4 (visit 2) and week 8 (visit 3) for collection of vital signs, urine, and blood. Treatment compliance was assessed every 2 weeks via a telephone call, and diet compliance was assessed via the diet diary. Plasma 7-HMR and ENL concentrations were measured at baseline as well as during subsequent visits at week 4 and week 8.
Safety-Related Procedures
An evaluation of safety was performed based on the frequency of adverse events as well as vital signs or lab values (complete blood count and comprehensive metabolic panel) that fell outside of predetermined ranges. Estrogen metabolites were assessed in urine and serum using stable isotope dilution gas chromatography–mass spectrometry (GC-MS), and SHBG was measured via ELISA. The change in estrogen metabolites was used as a safety endpoint. Participants were instructed to report serious adverse events within 24 hours of occurrence, even if not felt to be product related. Each adverse event was to be recorded in source documents.
Endpoint Analyses
The primary efficacy end point was the change in plasma 7-HMR and ENL levels. Lignan levels in plasma were assessed by measuring ENL and HMR using stable isotope dilution GC-MS. The secondary efficacy end point was the frequency of hot flash symptoms, which were recorded in a daily dairy. For each hot flash, severity was rated as mild (sensation of heat without sweating), moderate (sensation of heat with sweating, able to continue activity), or severe (sensation of heat with sweating, causes cessation of activity). Safety end points included measurement of changes in hs-CRP and cholesterol using dilution GC-MS.
Statistical Methods
Laboratory test assessments were analyzed using within-group (baseline compared to subsequent data collection points: week 4 and week 8) and between low-dose and high-dose group means. Numerical variables are presented as mean ± SD to describe each of the variables at baseline and at visit 2 (week 4) and visit 3 (week 8). Percentage changes are used to quantify an increase or decrease in end points versus baseline. For dichotomous data, the difference between proportions for each treatment group was tested using Fisher's exact test. For each continuous variable, the difference from baseline to each subsequent visit was tested for nominal significance by the paired Student's t test or by the nonparametric sign test upon violation of the normality assumption of the paired Student's t test. At each time point, the mean difference of the treatment groups for each variable was tested for significance using the independent t test if normally distributed or by using the nonparametric Wilcoxon Mann-Whitney test once the assumptions of the independent t test were violated. The main efficacy analysis was performed using the intent-to-treat methodology. If any of the subjects had missing data during the study, last observation carried forward was used in the intent-to-treat methodology. Data on the frequency of hot flashes were recorded by subjects in diaries documenting the number of hot flashes experienced each day. Weekly totals were calculated for each subject.
Analysis of covariance (ANCOVA) was used for all laboratory data, and odds ratios were used to determine the likelihood of having hot flashes for the treatment group. In the ANCOVA analysis, the value of the efficacy variable at every visit was modeled as a function of the treatment group (predictor variable of interest) and of the value of that efficacy variable at baseline (covariate). The analysis resulted in significant efficacy if the coefficient of the treatment group variable was significantly different from zero and in the right direction. The ANCOVA approach was used to mathematically compensate for the subject's baseline characteristics that happened to be substantially unbalanced between the 2 treatment groups. The significance level for all analyses was set at p < 0.05.
RESULTS
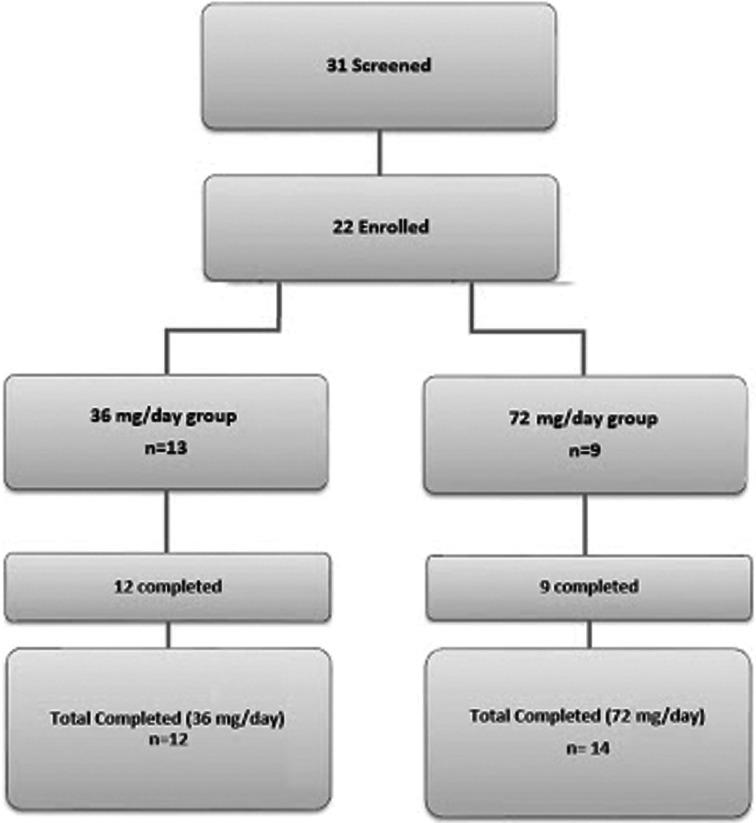
A total of 31 women were screened and 22 were enrolled in the study. Figure 1 demonstrates the study attrition data. Thirteen subjects were selected to receive 36 mg/d of HMRlignan, and 9 were selected to receive 72 mg/d of the study product. The mean age of all subjects was 54.5 ± 4.5 years and the mean body mass index was 26.6 ± 3.5 kg/m2. One subject withdrew from the study due to noncompliance. Twenty-one subjects completed the study and data were evaluated for the remaining 12 subjects in the low-dose group and 9 subjects in the high-dose group.
Fig. 1.
Study population. (Color figure available online.)
The 12 subjects in the low-dose group also participated in the single-dose pharmacokinetic study at their baseline visit to evaluate plasma concentrations of 7-HMR and ENL after administration of a single dose of 36 mg HMRlignan. Plasma concentrations were measured at multiple time points and compared against the baseline measurement prior to administration of any study product.
Single Dose Pharmacokinetics
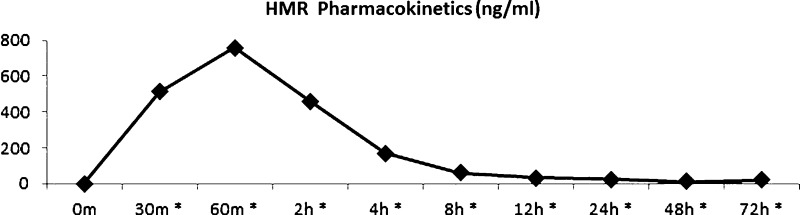
Plasma 7-HMR concentration reached Cmax at 1 hour after administration of 36 mg HMRlignan (757.08 ± SE 41.24 ng/ml). A statistically significant increase in plasma 7-HMR concentration was noted at all time points compared to the baseline measurement, including at 30 minutes (p = 0.001), 60 minutes (p < 0.001), 2 hours (p < 0.001), 4 hours (p < 0.001), 8 hours (p < 0.001), 12 hours (p = 0.001), 24 hours (p = 0.001), 48 hours (p < 0.001), and 72 hours (p = 0.003; Fig. 2).
Fig. 2.
Pharmacokinetic profile of plasma 7-HMR (ng/mL) after supplementation of 36 mg HMRlignan. Cmax was reached 1 hour after administration (757.1 ng/mL). *All time points from 30 minutes to 72 hours demonstrated a statistically significant increase compared to baseline. (Color figure available online.)
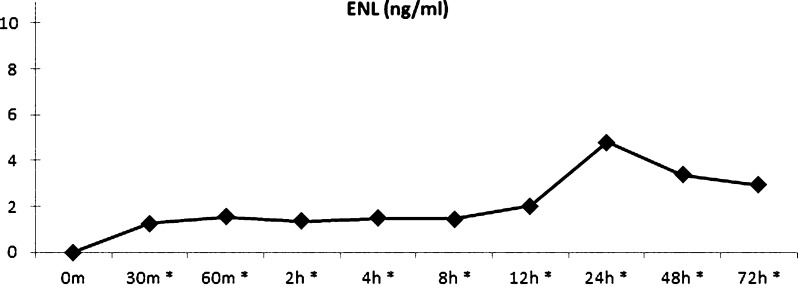
Plasma ENL concentration reached Cmax at 24 hours after administration of 36 mg HMRlignan (4.80 ± SE of 1.64 ng/ml). A statistically significant increase in plasma ENL concentration was noted at all time points compared to the baseline measurement, including at 30 minutes (p < 0.001), 60 minutes (p = 0.002), 2 hours (p = 0.001), 4 hours (p < 0.001), 8 hours (p = 0.001), 12 hours (p = 0.008), 24 hours (p = 0.015), 48 hours (p = 0.039), and 72 hours (p = 0.027; Fig. 3).
Fig. 3.
Pharmacokinetic profile of plasma ENL (ng/mL) after supplementation of 36 mg HMRlignan. Cmax was reached 24 hours after administration of 36 mg HMRlignan (4.8 ng/ml). *All time points from 30 minutes to 72 hours demonstrated a statistically significant increase compared to baseline. (Color figure available online.)
Bioavailability Data
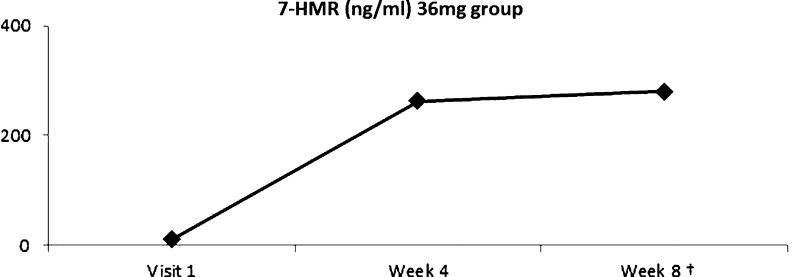
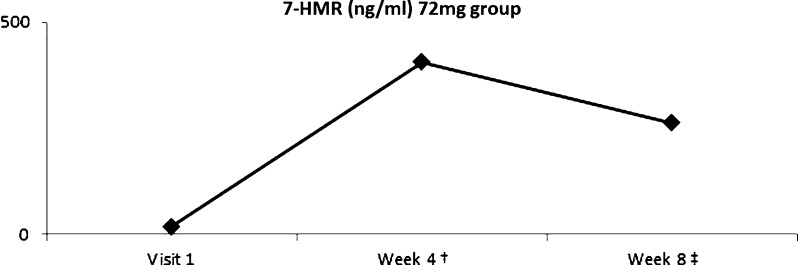
Among subjects in the low-dose group, plasma 7-HMR levels increased by 191% at week 8 (p = 0.006) compared to baseline (Fig. 4). Subjects in the high-dose group were noted to have an increase of 1928% from baseline to week 4 (p = 0.004) and an increase of 1238% from baseline to week 8 (p = 0.038; Fig. 5).
Fig. 4.
Plasma 7-HMR level after supplementation of 36 mg HMRlignan. 7-HMR level demonstrated an increase of 191% above baseline by week 8 (†p = 0.006). (Color figure available online.)
Fig. 5.
Plasma HMR level after supplementation of 72 mg HMRlignan. 7-HMR level increased by 1928% from baseline to week 4 (†p = 0.004) and by 1238% from baseline to week 8 (‡p = 0.038). (Color figure available online.)
From baseline to week 8, ENL levels increased by 157% in the low-dose group. Among subjects in the high-dose group, ENL levels increased by 70% from baseline at week 4 and by 137% from baseline at week 8. Despite large increases in ENL over these time points, statistical significance was not reached (data not presented).
Hot Flashes
From baseline to week 8 of the study, subjects in the low-dose group experienced a 44% decrease in the average number of hot flashes per week (p = 0.029). Subjects in the high-dose group experienced a 55% decrease in hot flashes from baseline to week 4 (p = 0.009) and a 50% decrease from baseline to week 8 (p = 0.025).
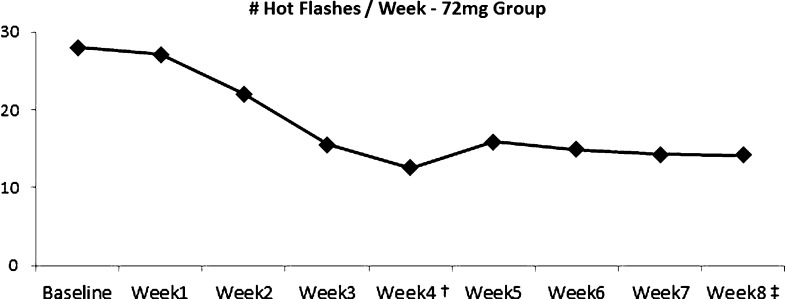
In the high-dose group, statistically significant decreases in frequency of hot flashes per week were noted at all time points subsequent to week 2, including at 3 weeks (p = 0.04), 4 weeks (p = 0.009), 5 weeks (p = 0.02), 6 weeks (p = 0.13), 7 weeks (p = 0.012), and the end of the study at 8 weeks (p = 0.025; Fig. 6). The mean number of weekly hot flashes decreased from 28 per week at baseline to 14.3 per week at week 8.
Fig. 6.
Hot flash frequency decreased by 55% from baseline to week 4 (†p = 0.009) and by 50% from baseline to week 8 (‡p = 0.025). (Color figure available online.)
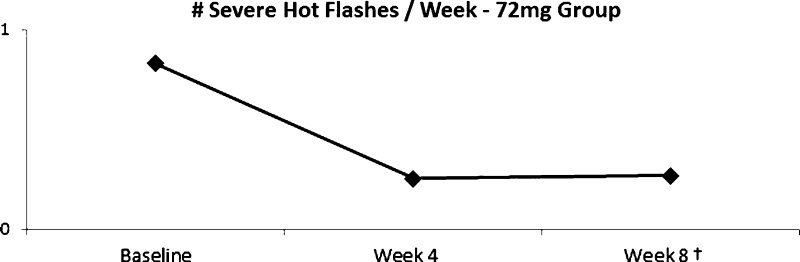
Severity data revealed that subjects in the low-dose group had 88% fewer severe hot flashes at week 4 compared to baseline (p < 0.001) and 34% fewer at week 8 compared to baseline (p = 0.035). Subjects in the high-dose group noted 79% fewer severe hot flashes compared to baseline at week 4 (p < 0.001) and 80% fewer at week 8 (p < 0.001; Fig. 7).
Fig. 7.
Frequency of severe hot flashes decreased by 79% from baseline to week 4 (†p < 0.001) and by 80% from baseline to week 8 (‡p < 0.001). (Color figure available online.)
Safety Assessments
No adverse events were reported during the study. There were no clinically significant changes in blood counts (hemoglobin, hematocrit, white blood count, and platelets), blood chemistry, or liver and kidney function tests. There were no statistically significant changes in levels of serum estradiol, SHBG, 2-OHE, 16-OHE, hs-CRP, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, or the LDL:HDL ratio compared to baseline.
DISCUSSION
The hormonal changes occurring with menopause have been described as associated with an increased risk of several health conditions, including osteoporosis, cardiovascular diseases, and breast cancer [32]. These changes also lead to symptoms that affect quality of life, such as hot flashes, night sweats, vaginal dryness, sleeping difficulty, mood changes, and difficulty with memory.
Hormone replacement therapy (HRT) is a commonly used treatment for relief of menopausal symptoms. Other beneficial effects of HRT include maintenance of bone density [33]. HRT, however, is not without risk. Recently, the Women's Health Initiative reported that long-term use of these hormones can lead to increased risk of breast cancer, stroke, heart attack, and blood clots [32]. These deleterious adverse effects have made phytoestrogen supplementation a popular alternative to hormone replacement therapy [2].
Phytoestrogens have chemical structures similar to those of estrogens, enabling them to bind to estrogen receptors [1,5]. The majority of phytoestrogens can be categorized into 3 classes: isoflavonoids, lignans, and coumestans. The predominant plant lignans found in the diet are matairesinol, hydroxymatairesinol, pinoresinol, and secoisolariciresinol. Matairesinol and hydroxymatairesinol are converted to enterolactone, and secoisolariciresinol is a precursor of enterodiol, which subsequently converts to enterolactone [1,5,6]. These conversions are facilitated by microbial flora in the gastrointestinal tract [1].
Numerous health benefits have been linked to phytoestrogen intake. Studies have suggested that supplementation of phytoestrogens is effective in maintaining bone mineral density and bone metabolism in postmenopausal women [12]. Phytoestrogens have also been described to help lower total cholesterol and LDL cholesterol and to raise HDL cholesterol [10]. Additionally, increased concentrations of enterolactone have been associated with a decreased risk of cardiovascular disease [6–9].
In this study, HMRlignan, a standardized product containing the plant lignan 7-HMR, was investigated to determine bioavailability and metabolism to ENL as well as any clinical improvement in hot flashes. The pharmacokinetic data indicate that 7-HMR is rapidly absorbed into the bloodstream and effectively metabolized to ENL in postmenopausal women. These findings are corroborated with previously published results in a population of healthy men [25]. In the present study, ENL levels increased by over 100% from baseline in women administered 36 mg/d HMRlignan as well as those administered 72 mg/d. Of note, ENL levels were shown to be higher in the group receiving the lower dose of 36 mg/d. Unfortunately, because the pharmacokinetic study was only performed on the 36 mg/d dose, we cannot make conclusions on a dose-dependent relationship between consumption of HMRlignan and ENL levels. Despite these large increases in ENL over these time points, statistical significance was not reached. This is likely related to the sample size of the study. Future studies may recruit a larger sample of subjects to gain enough power to reveal statistically significant increases in ENL after chronic ingestion of HMRlignan. Regardless, demonstration of increased ENL levels after administration of the study product as well as the reduction in hot flashes hold promise for the potential of HMRlignan to aid in management of both quality of life and disease risks related to menopause.
Specifically with regard to symptoms of hot flashes, HMRlignan was found to have a statistically significant effect on reducing the frequency and severity of hot flashes in post-menopausal women. Subjects in the group receiving 72 mg/d HMRlignan experienced statistically significant reductions in frequency of total hot flashes at week 4 (55% from baseline) and week 8 (50% from baseline). When assessing the frequency of severe hot flashes in particular, the 72 mg group also experienced statistically significant reductions in the frequency of severe hot flashes at week 4 (79% from baseline) and week 8 (80% from baseline).
These findings are supported by a pilot study describing a 50% decrease in hot flash frequency associated with consumption of lignans from flaxseed [11]. Both groups in the present study experienced a statistically significant improvement in frequency of hot flash symptoms, with a 44% reduction from baseline to week 8 in the low-dose group compared to a 50% reduction in the high-dose group. This finding supports a possible dose–effect relationship of HMRlignan on relieving hot flashes. The specific effect of HMRlignan on reducing severity of hot flashes compared to placebo was not evaluated, which is a limitation of the study.
In the average Western diet, lignans are consumed primarily from whole grains, seeds, nuts, legumes, fruits, vegetables tea, coffee, and wine [26,27]. Dietary lignan intake typically falls below 1 mg/d in Western populations [28]. A nationwide study of dietary recall in Finland reported a mean intake of dietary lignans among 1493 Finnish women of 151 μg/d, with matairesinol contributing only 20% of the intake [29]. The Framingham Offspring Study reported that the mean intake of lignans in 964 healthy postmenopausal females was 578 μg/d [26]. The Dutch Food Consumption Study reported that total lignan intake in a sample of 4660 females was 979 μg/d, with matairesinol contributing only 1% of the total lignan intake [27]. These rates fall substantially below the dosages of 36 and 72 mg/d described in this study, which explains the significant increases in plasma HMR levels following administration of HMRlignan, in both the acute and chronic phases. Though such a drastic increase in lignan intake may raise concerns for safety, there were no adverse events related to consumption of either dosage used throughout the course of this study.
The pharmacokinetic study demonstrated no significant changes in plasma estradiol, SHBG, 2-OHE, or 16-OHE compared to baseline. Other studies have reported an increase in the 2-OHE:16-OHE ratio after lignan supplementation [23,30]. This may be related to the acuity of measurement of these metabolites because data were not collected on these components in the subsequent 4-week and 8-week visits. Additionally, diet has been described to influence the 2-OHE:16-OHE ratio [30]; in particular, one high in fat and low in cruciferous vegetables (e.g., broccoli, cabbage, and cauliflower) can decrease the 2-OHE:16-OHE ratio [30,31]. Although a daily diet diary was monitored as part of this study, the focus was on ensuring compliance with a lignan-free diet rather than one that would affect the 2-OHE:16-OHE ratio.
CONCLUSION
The findings in this study indicate that HMRlignan is quickly absorbed into the plasma and is metabolized to ENL in healthy postmenopausal women. Doses up to 72 mg/d HMRlignan for 8 weeks were safe and well tolerated in this population. Clinical efficacy was demonstrated via an overall decrease in hot flash frequency and severity. Further controlled studies are needed to corroborate these findings.
REFERENCES
- 1.Aldercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–425. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 2.Smeds AI, Eklund PC, Sjoholm RE. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J Agr Food Chem. 2007;55:1337–1346. doi: 10.1021/jf0629134. [DOI] [PubMed] [Google Scholar]
- 3.Horn-Ross PL, Lee M, John EM, Koo J. Sources of phytoestrogen exposure among non-Asian women in California, USA. Cancer Causes Control. 2000;11:299–302. doi: 10.1023/a:1008968003575. [DOI] [PubMed] [Google Scholar]
- 4.Saarinen NM, Warri A, Airio M, Smeds A, Makela S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. 2007;51:857–866. doi: 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 6.Vanharanta M, Voutilainen S, Rissanen TH, Adlercreutz H, Salonen JT. Risk of cardiovascular disease-related and all-cause death according to serum concentrations of enterolactone: Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med. 2003;163:1099–1104. doi: 10.1001/archinte.163.9.1099. [DOI] [PubMed] [Google Scholar]
- 7.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. women: the Framingham study. J Nutr. 2002;132:276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 8.Vanharanta M, Voutilainen S, Nurmi T. Association between low serum enterolactone and increased plasma F2-isoprostanes, a measure of lipid peroxidation. Atherosclerosis. 2002;160:465–469. doi: 10.1016/s0021-9150(01)00603-7. [DOI] [PubMed] [Google Scholar]
- 9.Vanharanta M, Voutilainen S, Lakka TA, van der Lee M, Adlercreutz H, Salonen JT. Risk of acute coronary events according to serum concentrations of enterolactone: a prospective population-based case-control study. Lancet. 1999;354:2112–2115. doi: 10.1016/S0140-6736(99)05031-X. [DOI] [PubMed] [Google Scholar]
- 10.Peñalvo JL, López-Romero Urinary enterolignan concentrations are positively associated with serum HDL cholesterol and negatively associated with serum triglycerides in U.S. adults. J Nutr. 2012;142:751–756. doi: 10.3945/jn.111.150516. [DOI] [PubMed] [Google Scholar]
- 11.Pruthi S, Thompson SL, Novotny PJ. Pilot evaluation of flaxseed for the management of hot flashes. J Soc Integr Oncol. 2007;5:106–112. doi: 10.2310/7200.2007.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim MK, Chung BC, Yu VY. Relationships of urinary phytooestrogen excretion to BMD in postmenopausal women. Clin Endocrinol (Oxf) 2002;56:321–328. doi: 10.1046/j.1365-2265.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 13.Franco OH, Burger H, Lebrun CE. Higher dietary intake of lignans is associated with better cognitive performance in postmenopausal women. J Nutr. 2005;135:1190–1195. doi: 10.1093/jn/135.5.1190. [DOI] [PubMed] [Google Scholar]
- 14.McCann SE, Hootman KC, Weaver AM. Dietary intakes of total and specific lignans are associated with clinical breast tumor characteristics. J Nutr. 2012;142:91–98. doi: 10.3945/jn.111.147264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCann SE, Kulkarni S, Trevisan M. Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res Treat. 2006;99:309–311. doi: 10.1007/s10549-006-9196-x. [DOI] [PubMed] [Google Scholar]
- 16.Olsen A, Knudsen KE, Thomsen BL. Plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol Biomarkers Prev. 2004;13:2084–2089. [PubMed] [Google Scholar]
- 17.Pietinen P, Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol Biomarkers Prev. 2001;10:339–344. [PubMed] [Google Scholar]
- 18.Piller R, Chang-Claude J, Linseisen J. Plasma enterolactone and genistein and the risk of premenopausal breast cancer. Eur J Cancer Prev. 2006;15:225–232. doi: 10.1097/01.cej.0000197449.56862.75. [DOI] [PubMed] [Google Scholar]
- 19.Touillaud MS, Thiebaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. Dietary lignan intake and post-menopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–486. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velentzis LS, Cantwell MM, Cardwell C, Keshtgar MR, Leathem AJ, Woodside JV. Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer. 2009;100:1492–1498. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino M, Marino F, Ferrari M. Estrogenic activity of 7-hydroxymatairesinol potassium acetate (HMR/lignan) from Norway spruce (Picea abies) knots and of its active metabolite enterolactone in MCF-7 cells. Pharmacol Res. 2007;56:140–147. doi: 10.1016/j.phrs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Low YL, Kardinaal AF, Morton MS, Brüggemann-Rotgans IE, van Beresteijn EC. Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol Biomarkers Prev. 2007;16:1009–1016. doi: 10.1158/1055-9965.EPI-06-0899. [DOI] [PubMed] [Google Scholar]
- 23.Haggans CJ, van der Schouw YT, Sampson L, Willett WC, Rimm EB. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr Cancer. 1999;33:188–195. doi: 10.1207/S15327914NC330211. [DOI] [PubMed] [Google Scholar]
- 24.Laidlaw M, Cockerline CA, Sepkovic DW. Effects of a breasthealth herbal formula supplement on estrogen metabolism in pre-and post-menopausal women not taking hormonal contraceptives or supplements: a randomized controlled trial. Breast Cancer (Auckl) 2010;4:85–95. doi: 10.4137/BCBCR.S6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kangas L, Saarinen N, Mutanen M. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur J Cancer Prev. 2002;11(suppl 2):S48–S57. [PubMed] [Google Scholar]
- 26.de Kleijn MJ, van der Schouw YT, Wilson PW. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study. J Nutr. 2001;131:1826–1832. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 27.Milder IE, Feskens EJ, Arts IC, Bueno de Mesquita HB, Hollman PC, Kromhout D. Intake of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in Dutch men and women. J Nutr. 2005;135:1202–1207. doi: 10.1093/jn/135.5.1202. [DOI] [PubMed] [Google Scholar]
- 28.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. 2010;68:571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilkkinen A, Valsta LM, Virtamo J, Stumpf K, Adlercreutz H, Pietinen P. Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J Nutr. 2003;133:1830–1833. doi: 10.1093/jn/133.6.1830. [DOI] [PubMed] [Google Scholar]
- 30.Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7:112–129. [PubMed] [Google Scholar]
- 31.Longcope C, Gorbach S, Goldin B. The effect of a low fat diet on estrogen metabolism. J Clin Endocrinol Metab. 1987;64:1246–1250. doi: 10.1210/jcem-64-6-1246. [DOI] [PubMed] [Google Scholar]
- 32.Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women's Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172–176. doi: 10.1097/aog.0b013e31827a08c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowring CE, Francis RM. National Osteoporosis Society's position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int. 2011;17:63–65. doi: 10.1258/mi.2011.011012. [DOI] [PubMed] [Google Scholar]