Summary
Tumor infiltrating T cells have recently been found to upregulate immunosuppressive pathways, such as PD-1 ligand (PD-L1), in a paracrine fashion on tumor cells, but tumor cell intrinsic regulation of PD-L1 is another potential mechanism. In this issue of Cancer Discovery, Akbay and colleagues show that signaling via mutant EGFR in murine lung tumor cells directly upregulates tumor PD-L1 and that therapeutic blockade of this pathway improves survival in EGFR-driven preclinical models – highlighting the dynamic interplay and therapeutic opportunities of cancer cell biology and immune biology.
The immune system can inhibit tumor growth for long periods by destroying tumor cells; however, this inhibition necessarily selects for tumor cells that escape immune-mediated destruction. This ‘immune escape’ can occur because the tumor cell population changes after elimination of susceptible tumor cell clones, or because immune checkpoint pathways emerge that suppress the anti-tumor immune response (1). Several immune checkpoint pathways are mediated by receptor-ligand interactions and can therefore be blocked with antibodies. The success of one such antibody in melanoma, the U.S. Food and Drug Administration-approved CTLA-4-blocking antibody ipilimumab, has catalyzed intense efforts to leverage the blockade of additional immune checkpoint proteins to produce durable clinical responses in a variety of cancers (2).
Leading this class of emerging immune therapies is blockade of programmed cell death protein 1 (PD-1). Therapeutic antibodies against PD-1 or PD-L1 have produced objective responses in a fraction of patients with melanoma, non-small cell lung cancer (NSCLC) and other malignancies (3,4). Like CTLA-4, PD-1 is expressed principally on T cells. Unlike CTLA-4, which reduces the amplitude of T cell activation, PD-1 limits the function of already activated T cells (2). The PD-1 ligands, PD-L1 and PD-L2, are upregulated on a plethora of cells during inflammation – including tumor cells – thereby limiting anti-tumor immune responses. The PD-1/PD-L1 pathway also augments suppressive regulatory T cells, representing a second mechanism by which this pathway influences immune responses. Early clinical results from patients with advanced NSCLC suggest that PD-L1 expression on tumor cells may correlate with response to PD-1-blocking antibody (2), which raises a poorly understood question of etiology: what drives the induction of PD-L1 on tumor cells in the first place?
In this issue of Cancer Discovery, Akbay and colleagues (5) help answer this question by showing that signaling via mutant EGFR triggers murine lung tumor cells to upregulate PD-L1. They also show that therapeutic blockade of PD-1 improves survival in the same EGFR-driven models. Prior to this work, studies showed that EGFR signals in murine melanoma increased the suppressive function of regulatory T cells (6) and decreased the accumulation of effector T cell chemoattractants (7). Based on these findings and the fact that EGFR is a commonly mutated oncogene in NSCLC, Akbay and colleagues (5) investigated immune dynamics in the tumor microenvironment of three murine lung tumors with different EGFR mutations. These models are based on the well-studied EGFR-activating and erlotinib-resistance mutations identified in NSCLC patients, making their investigation clinically relevant.
The authors found increased markers of immunosuppression in the lung tumor microenvironment in all three models. Compared to control lung tissue, EGFR-driven lung tumor samples contained increased expression of CTLA-4, PD-1, and PD-L1. Other immune suppressive pathways were also increased in the tumor microenvironment, e.g. IL-6 and TGF-β. Correspondingly, tumor infiltrating CD8 T cells were significantly reduced relative to Foxp3+ regulatory T cells when compared to normal tissue. Originally described as an important biomarker in preclinical melanoma models, the ‘CD8+/Foxp3+ ratio correlates with the degree of immune activation and is associated with improved patient survival in several malignancies. Although the complete dynamics of these immune infiltrates over time remains unknown, these data suggest that the EGFR-driven lung tumor microenvironment is immunosuppressive.
To explore whether increased levels of PD-L1 is an immune escape pathway in EGFR-driven lung tumors, Akbay and colleagues (5) treated the same models with therapeutic PD-1-blocking antibody. At clinically relevant doses, repeated administration resulted in reduced tumor growth rates, corresponding to a 7-week survival advantage (a 50% improvement from the time of therapy initiation). Tumor cell apoptosis increased, consistent with an augmented CD8 T cell anti-tumor response. Furthermore, a greater number of CD8 T cells were present in treated vs. control tumors, and an increased percentage of these cells produced IFN-γ when stimulated ex vivo, an assay of T cell effector function. IL-6 and TGF-β levels decreased in PD-1 antibody-treated tumors, important because both IL-6 and TGF-β inhibit CD8 T cell effector function and recruit additional non-cell autonomous inhibitory mechanisms. Direct evidence that CD8 T cells mediate the effects of PD-1-blocking antibody in these models remains to be provided, but could be readily investigated by the use of CD8 T cell-depleting antibodies administered prior to tumor initiation. Nevertheless, this study supports the notion that therapeutic blockade of the PD-1 immune checkpoint pathway broadly reverses immunosuppression in the tumor microenvironment and augments T cell anti-tumor immunity.
In expanding their findings to human cells, the authors demonstrated that EGFR can induce Pd-l1/2 expression in patient-derived NSCLC cell lines. Another prevalent driving mutation in NSCLC is KRAS; EGFR and KRAS mutations are inversely correlated in patient tumors and associated with divergent tumor pathophysiology (8). Ectopic expression of mutant EGFR in immortalized bronchial epithelial cells led to increased PD-L1 expression by flow cytometry, whereas ectopic expression of mutant KRAS did not lead to increased PD-L1 expression. Additionally, human NSCLC cell lines with EGFR-activating mutations treated with EGFR inhibitors decreased PD-L1 expression, confirming the correlation between EGFR activity and PD-L1 expression. Thus, EGFR but not KRAS induces PD-L1 expression on tumor cells in these models. Because these findings relate to EGFR mutations known to facilitate erlotinib resistance, PD-L1 upregulation may be a tumor escape pathway in patients receiving EGFR inhibitors.
The authors also demonstrate that EGFR-driven lung tumors retained a high prevalence of CTLA-4-expressing regulatory T cells regardless of treatment. Recent preclinical work in melanoma has suggested that regulatory T cells are of importance to the mechanism of action of CTLA-4-blocking antibodies, and the combination of PD-1- and CTLA-4-blocking antibodies in melanoma patients may be more effective than either agent alone (9). Additional work should therefore evaluate the combination of these therapies in EGFR-driven lung tumors.
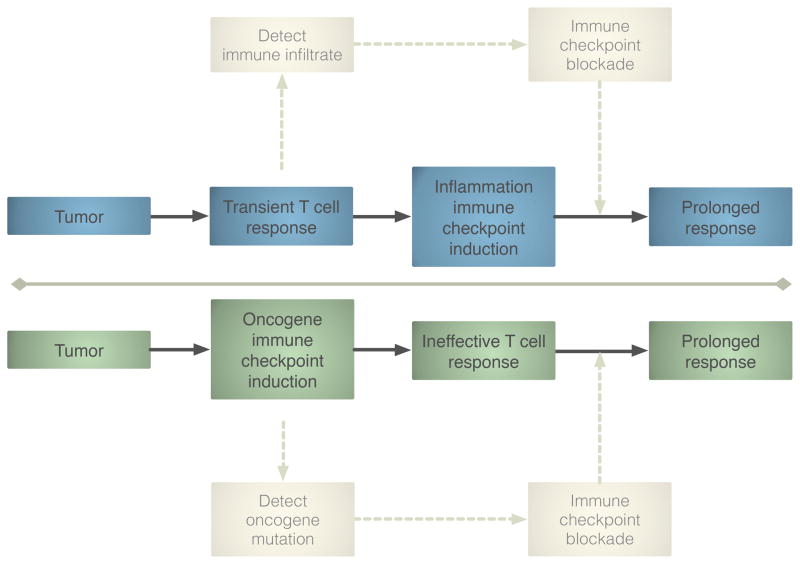
In contrast to this study demonstrating a tumor cell-intrinsic mechanism of PD-L1 upregulation in lung cancer, recent work in melanoma shows that the induction of immune checkpoint pathways is a consequence of CD8 T cell infiltration (10). In this melanoma model, mechanistic studies demonstrated that upregulation of PD-L1 by tumor cells was dependent on the presence of CD8 T cells and IFN-γ – a negative feedback loop intrinsic to immune activation and independent of oncogene signaling. Of course, oncogene-driven vs. T cell-driven regulation of tumor PD-L1 is not mutually exclusive, and future studies may resolve the contribution of each mechanism in these malignancies. In a different murine melanoma model, previous work has already demonstrated a role for the EGFR pathway in immune suppression via upregulation of the cytokine CCL27 (7). Nevertheless, there may be important differences between tumors expressing PD-L1 due to oncogenes and those in which PD-L1 is induced due to the infiltration of anti-tumor T cells (Figure 1). The former are less likely to have undergone selective pressure exerted by the immune system, and may therefore respond more favorably to additional immune therapies. These investigations also generate hypotheses for how to identify patients who are likely to respond to PD-1/PD-L1 blocking antibodies. For instance, we can speculate that EGFR-driven NSCLC tumors may benefit from PD-1 blockade regardless of preexisting immune infiltrate. Alternatively, melanomas harboring a T cell-inflamed tumor microenvironment may respond regardless of driving mutations (such as BRAF, the status of which does not appear to predict response to ipilimumab).
Figure 1.
Oncogene vs. T cell driven immune checkpoint pathway upregulation. Tumor cells can induce PD-L1 expression in a cell autonomous manner via the oncogene EGFR. Alternatively, PD-1 pathway upregulation can be the consequence of CD8 T cell infiltration. These models generate different hypotheses to identify patients who will benefit from immune checkpoint blocking therapies. Future studies may resolve the contribution of each mechanism in various malignancies.
Data from Akbay and colleagues add to increasing evidence that oncogenes impact the tumor microenvironment to promote immune escape (11). Dissecting the crosstalk between oncogene-driven networks of immune suppression and the dynamic regulation of tumor infiltrating T cells will no doubt be a critical area of investigation going forward. In particular, there is likely to be great clinical value in integrating tumor genomic profiling with profiling of the immune response and the tumor microenvironment as a next step in personalized medicine for cancer.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaiss DMWD, van Loosdregt JJ, Gorlani AA, Bekker CPJC, Gröne AA, Sibilia MM, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–84. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pivarcsi A, Müller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, et al. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci. 2007;104:19055–60. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imielinski MM, Berger AHA, Hammerman PSP, Hernandez BB, Pugh TJT, Hodis EE, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–20. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley JL. Combination checkpoint blockade - taking melanoma immunotherapy to the next level. N Engl J Med. 2013;369:187–9. doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
- 10.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment Is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]