Abstract
We previously reported that treatment with the cholinesterase inhibitor rivastigmine (3 mg, PO for 5 days) significantly attenuated “Desire for METH”. Given that higher dosages of rivastigmine produce greater increases in synaptic ACh, we predicted that 6 mg should have more pronounced effects on craving and other subjective measures. In the current study, we sought to characterize the effects of short-term exposure to rivastigmine (0, 3 or 6 mg) on the subjective and reinforcing effects produced by administration of methamphetamine (METH) in non-treatment-seeking, METH-dependent volunteers. This was a double-blind, placebo-controlled, crossover study. Participants received METH on day 1, and were then randomized to placebo or rivastigmine on day 2 in the morning and treatment continued through day 8. METH dosing was repeated on day 6. The data indicate that METH (15 and 30 mg), but not saline, increased several positive subjective effects, including “Any Drug Effect”, “High”, “Stimulated”, “Desire METH”, and “Likely to Use METH” (all p’s<0.0001). In addition, during self-administration sessions, participants were significantly more likely to choose METH over saline (p<0.0001). Evaluating outcomes as peak effects, there was a trend for rivastigmine to reduce “Desire METH” (p=0.27), and rivastigmine significantly attenuated “Likely to Use METH” (p=0.01). These effects were most prominent for rivastigmine 6 mg when participants were exposed to the low dose (15 mg, IV), but not high dose (30 mg, IV), of METH. The self-administration data reveal that rivastigmine did not alter total choices for METH (5 mg, IV/choice). Overall, the results indicate some efficacy for rivastigmine in attenuating key subjective effects produced by METH, though additional research using higher doses and longer treatment periods is likely needed. These data extend previous findings and indicate that cholinesterase inhibitors, and other drugs that target acetylcholine systems, warrant continued consideration as treatments for METH dependence.
Keywords: methamphetamine, treatment, addiction, rivastigmine, acetylcholine
Introduction
Methamphetamine (METH) is a highly addictive stimulant. According to the 2010 United States National Survey on Drug Use and Health (http://oas.samhsa.gov/nsduh.htm), the number of past month METH users was 353,000, and the number of recent new users of METH among persons aged 12 or older was 105,000. This latter value is disconcerting since it reflects ~29 new users of METH each day! Our research group and others have completed several safety and preliminary efficacy trials of potential medications for treating METH dependence. Despite these efforts, much work remains in identifying an effective pharmacotherapy. Recent review articles provide summaries of laboratory-based and outpatient trials conducted to date, and include lists of novel medications on the horizon (Elkashef et al., 2008, Haile et al., 2009, Kampman, 2008, Karila et al., 2010, Moeller et al., 2008, Rose and Grant, 2008, Vocci and Appel, 2007), as well as more recent efforts to develop a vaccine for METH dependence (Shen et al., 2012).
Although changes in the dopamine (DA) system have been most extensively studied, cholinergic transmission is also altered by drugs of abuse and both DA and acetylcholine (ACh) may contribute to psychostimulant reinforcement (Hurd et al., 1990, Mark et al., 1999a, Mark et al., 1999b). DA neurons express multiple types of muscarinic and nicotinic ACh receptors and an interplay of dopaminergic and cholinergic neurons in the nucleus accumbens allows coordinated functioning of these neurotransmitter systems. The role of nucleus accumbens cholinergic interneurons in the effects produced by cocaine were elucidated by recent optogenetic studies in rats (Witten et al., 2010), and the importance of ACh systems in the effects produced by cocaine have been thoroughly summarized by Adinoff and colleagues (Adinoff et al., 2010, Williams and Adinoff, 2008).
Postmortem data indicate that METH users exhibit reduced levels of the ACh synthetic enzyme choline acetyltransferase (Kish et al., 1999, Siegal et al., 2004), but normal or increased expression of the vesicular ACh transporter (Siegal, Erickson, 2004). This may indicate compensatory neuroadaptive changes of the cholinergic system, and suggests that METH users in particular may benefit from treatment with ACh enhancing agents. Pharmacological interactions between nicotine and METH have been demonstrated by several investigators. In rats, nicotine acutely reduced METH self-administration and during reinstatement nicotine exposure significantly increased METH-seeking behavior (Neugebauer et al., 2010). Of interest, both nicotine and donepezil, but not the noncompetitive nicotinic receptor antagonist mecamylamine, reduced reinstatement induced by exposure to conditioned cues and by administration of priming doses of METH (Hiranita et al., 2006). Reinstatement of drug-seeking behavior is a preclinical model for craving in human subjects (Shaham et al., 2003), so these data suggest that enhancing ACh activity may reduce METH craving in humans. Additional data in rodents indicate that nicotine and nicotine agonists fully substitute for METH in a drug discrimination task, and that mecamylamine reduced the discriminative stimulus effects of the training dose of METH (Desai and Bergman, 2010, Gatch et al., 2008). These data indicate that compounds that increase ACh, including cholinesterase inhibitors, may attenuate subjective effects produced by METH in humans.
Given the above evidence, our research group completed a double-blind, placebo-controlled study to determine the effects of the cholinesterase inhibitor rivastigmine on the acute subjective effects produced by METH in METH-dependent human volunteers (De La Garza et al., 2008a, De La Garza et al., 2008b). We showed that METH (30 mg, IV) administration significantly increased self-reported “Desire for METH”, and treatment with rivastigmine (3 mg, PO for 5 days) significantly attenuated this response. Knowing that higher dosages of rivastigmine produce greater increases in synaptic ACh, it is reasonable to predict that 6 mg will have more pronounced effects on craving and other subjective measures. As such, we sought to characterize the effects of short-term treatment with a higher dose of rivastigmine (6 mg) on the cardiovascular, subjective and reinforcing effects produced by a broader range of METH doses in non-treatment-seeking, METH-dependent volunteers. This human laboratory study is a critical next step in the evaluation of rivastigmine as a potential treatment for METH dependence.
Methods
This double-blind, placebo-controlled, crossover study was sponsored by the National Institute on Drug Abuse, and approved by the Baylor College of Medicine (BCM) and Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) Institutional Review Boards. All volunteers provided written informed consent after being apprised of potential risks of study participation.
Sample
Participants were English-speaking volunteers who were not seeking abstinence-focused treatment at the time of the study, between 18–55 years of age, met DSM-IV-TR (DSM-IV-TR 2000) criteria for METH dependence, had a breathalyzer test indicating an undetectable blood alcohol level upon admission, a medical history and brief physical examination demonstrating no clinically significant contraindications for study participation, and a negative urine drug screen, with the exception of METH or marijuana. Participants were not-seeking treatment at the time of enrollment, and were not seeking to remain abstinent either since they all tested positive for recent methamphetamine use during screening and also agreed (during the informed consent process) to participate in a study in which methamphetamine would be administered as part of the protocol. Exclusion criteria included having neurological or psychiatric disorders, as assessed by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998), such as episode of major depression within the past 2 years, lifetime history of schizophrenia, other psychotic illness, or bipolar illness, current organic brain disease or dementia assessed by clinical interview, history of or any current psychiatric disorder which would require ongoing treatment or which would make study compliance difficult, history of suicide attempts within the past three months and/or current suicidal ideation/plan, or history of psychosis occurring in the absence of current METH use, or meet DSM-IV TR criteria for dependence on alcohol or other drugs, other than nicotine or marijuana.
Procedure
Participants resided for 9 days on the Research Commons of the MEDVAMC (Table 1). On Day 1 (pre-randomization), participants received 3 infusions in which placebo was single-blind (administered at 9 AM), and 15 and 30 mg METH doses were double-blind and randomized (administered at either 10 AM or 2 PM). Saline and METH infusions were administered IV by a study physician over 2 min. All participants were randomly assigned to placebo or rivastigmine for days 2–8. METH dosing was repeated on day 6 (post-randomization). On day 7 and 8, participants completed sample and self-administration sessions. On day 9, participants were discharged from the study and returned for enrollment and randomization to alternate rivastigmine dosing conditions (crossover study design; i.e., all participants were exposed to all doses of rivastigmine).
Table 1.
Study Overview
|
Randomize to Rivastigmine (0, 1.5, or 3 mg, BID) Day 2 (AM) through Day 8 (AM) |
Day 1 | Pre-Rand METH (0, 15 and 30 mg, IV) |
| Day 6 | Post-Rand METH (0, 15 and 30 mg, IV) | |
| Day 7–8 | Self-administration sessions | |
| Day 9 | Discharge |
Cardiovascular and Safety Measures
All adverse events, mood, heart rate and blood pressure data have been submitted for publication elsewhere.
Subjective effects
On days 1 and 6, visual analogue scale (VAS) forms were completed at baseline (T=−15 min) and several time points following infusions of 15 and 30 mg METH and saline. VAS adjectives included ratings of ‘Any drug effect’, ‘High’, ‘Desire METH’, ‘Stimulated’, and ‘Likely to Use METH if Given Access’. VAS effects were recorded on a continuous scale digitized between 0 (not at all) to 100 (strongest ever).
Reinforcing Effects
Self-administration sessions were conducted on days 7 and 8. On Day 7 (at 10 AM and 2 PM), participants were provided with 10 consecutive non-contingent sample doses of METH (0 or 5 mg, IV). This session served to inform participants of what was in the syringe and should be expected during choice sessions held on the subsequent day. Participants were told to take notes on the subjective effects (positive and/or negative) associated with each session which was randomly assigned the color RED or BLUE. On Day 8, participants made a series of 10 choices between receiving the infusion of METH (0 or 5 mg) versus receiving no-infusion. Participants made these decisions on the basis of the RED or BLUE color coding provided on the previous day. Infusion choices were made using a patient-controlled analgesia pump and occurred over 2 min followed by a 13 min time-out period. Participants received infusions immediately after indicating that choice, providing vital signs remained within preset limits.
Rivastigmine
Rivastigmine inhibits both acetylcholinesterase (AChE) and butyrylcholinesterase with equal potency, and has selectivity for central activity. Following oral dosing, the plasma half-lives of rivastigmine and its primary metabolite are roughly 1 h and 2 h, respectively; however, cholinesterase inhibition lasts much longer (~10 h) than the plasma half-life indicates. Commercially available rivastigmine tablets (3 mg) were encapsulated in gelatin capsules by the MEDVAMC research pharmacy, and placebo was prepared in a similar manner. Two rivastigmine or matched placebo pills were administered orally at 7:00 AM and 7:00 PM (i.e., 0+0 mg, 3+0 mg, or 3+3 mg) beginning on day 2 (in the AM) and continuing through day 8 (in the AM) of the protocol.
Methamphetamine
A NIDA contractor (RTI International, Research Triangle Park, NC) provided sterile METH solution for human use and a saline solution of equal volume and appearance was used as the control. An IND was obtained from the FDA for the use of rivastigmine and METH in this study. The half-life of METH is ~11–12 h. Single bolus infusions of 15 and 30 mg METH were used since they have been previously associated with significant increases in positive subjective effects, though these changes are not accompanied by adverse events greater than observed after saline administration.
Data Analyses
Data were analyzed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA) and missing values were substituted by single-point multiple imputation using NORM version 2.03 (J. Schafer, The Pennsylvania State University). Across dozens of measures, multiple time-points for each participant, and across several days, only 31 missing values required imputation.
Given that this was a within-subjects study, descriptive statistics were compiled for demographic and drug use variables.
Within-session (post-randomization) Measures
Post-randomization (day 6) VAS data were analyzed by individual METH dose (0, 15 or 30 mg) using repeated-measures ANOVA as a function of rivastigmine dose (0, 3 or 6 mg) and time (in min). Time-courses reflect within-session change from baseline (value at a given time point minus value at T=−15 min). These data were also analyzed with respect to peak effects (maximum change from baseline) using one-way ANOVA. Self-administration data (day 8) were analyzed using a 2-way ANOVA as a function of METH (0 or 5 mg) and rivastigmine dose (0, 3 or 6 mg).
All data, except time, were analyzed as between-subjects factors. Time (in days or min) was analyzed as a within-subject factor. For all measures, statistical significance was set at p<0.05. All data are presented as mean ± standard error of the mean (S.E.M.).
Results
Sample
Twenty eligible subjects were enrolled and 17 participants completed all three portions of the protocol. Two participants were excluded from the final analyses because they did not “respond” (exhibit changes from baseline >20 points) to either the 15 or 30 mg doses of METH. The decision to excluded non-responders was deemed critical since it is unreasonable to expect medication effects if participants do not first demonstrate some change after acute METH exposure. This strategy has been utilized by several researchers in the past (e.g., Stoops et al. 2010). Among non-completers, two of the three chose not to complete subsequent portions of the protocol due to personal reasons. The third non-completer was discharged after experiencing nausea and headaches, which are known side-effects associated with rivastigmine dosing.
The final sample consisted of 15 individuals, who were predominantly Caucasian and male (Table 2). On average, participants had used METH 10 years, and the majority also currently used alcohol, marijuana, and nicotine. Participants did not meet DSM criteria for abuse or dependence on any substances except METH and nicotine.
Table 2.
Demographics and Drug Use
| Data reflect Mean±S.E.M. | (N=15) |
|---|---|
|
| |
| Gender | |
| Male | 13 |
| Female | 2 |
|
| |
| Ethnicity | |
| Caucasian | 12 |
| Hispanic | 2 |
| African American | 1 |
|
| |
| Age | 35.0±2.2 |
|
| |
| Education (years) | 13.3±0.6 |
|
| |
| IQ (WAIS) | 112.7±2.4 |
|
| |
| Methamphetamine Use | |
| Years of Use | 10.5±1.7 |
| Recent Use (#days last 30 days) | 17.5±2.4 |
|
| |
| Nicotine Use (N=12) | |
| Years of Use | 12.1±2.4 |
| Cigarettes/day | 17.0±3.0 |
|
| |
| Alcohol Use (N=11) | |
| Years of Use | 15.3±3.1 |
| Recent Use | 2.7±1.2 |
|
| |
| Marijuana Use (N=9) | |
| Years of Use | 15.0±2.9 |
| Recent Use | 9.6±3.3 |
Subjective Effects (time-course data)
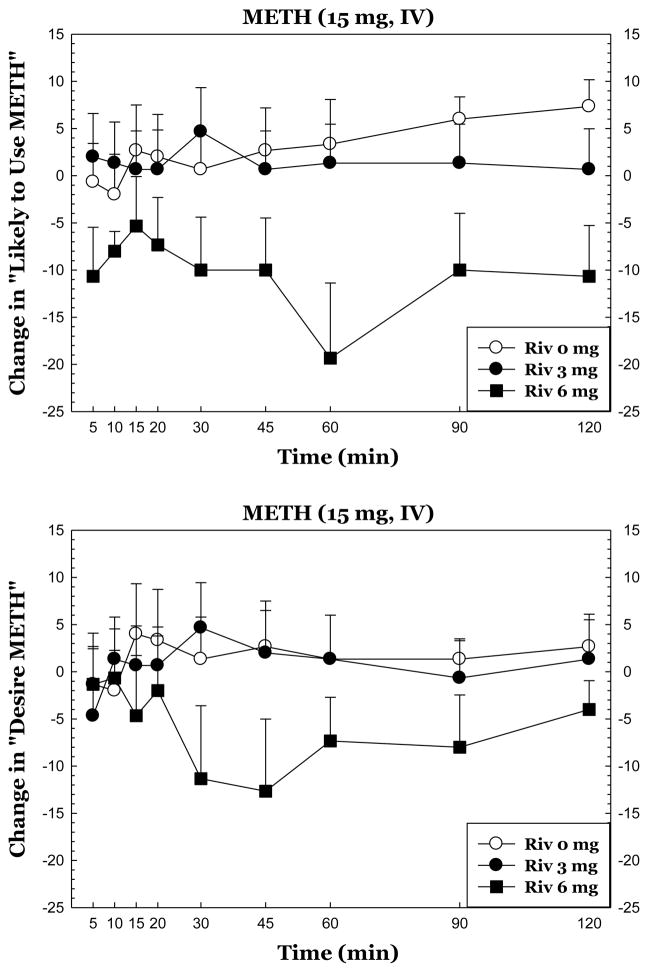
Statistical outputs for all time-course data are shown in Table 3 (left). Of these, the most intriguing outcomes are presented in Figure 1.
Table 3.
| Subjective Effect Adjective | Full Time Course | Peak Effects | |||
|---|---|---|---|---|---|
| METH Dose | 15 mg | 30 mg | 15 mg | 30 mg | |
| Desire | Rivastigmine | F2,42 = 0.87 p = 0.43 |
F2,42 = 0.3 p = 0.73 |
F2,42 = 1.3 p = 0.27 |
F2,42 = 0.5 p = 0.60 |
| Time | F8,336 = 0.6 p = 0.78 |
F8,336 = 1.2 p = 0.27 |
|||
| Rivastigmine*Time | F16,336 = 1.5 p = 0.09 |
F16,336 = 0.5 p = 0.96 |
|||
| Likely To Use | Rivastigmine | F2,42 = 2.7 p = 0.08 |
F2,42 = 0.4 p = 0.70 |
F2,42 =5.1 p = 0.01 |
F2,42 = 0.3 p = 0.72 |
| Time | F8,336 = 1.2 p = 0.33 |
F8,336 = 0.7 p = 0.69 |
|||
| Rivastigmine*Time | F16,336 = 2.0 p = 0.01 |
F16,336 = 0.6 p = 0.85 |
|||
| Any Drug Effect | Rivastigmine | F2,42 = 1.6 p = 0.22 |
F2,42 = 0.1 p = 0.90 |
F2,42 = 1.3 p = 0.27 |
F2,42 = 0.1 p = 0.86 |
| Time | F8,336 = 18.1 p = <0.0001 |
F8,336 = 16.1 p = <0.0001 |
|||
| Rivastigmine*Time | F16,336 = 1.4 p = 0.15 |
F16,336 = 1.3 p = 0.22 |
|||
| High | Rivastigmine | F2,42 = 1.1 p = 0.34 |
F2,42 = 0.0 p = 0.95 |
F2,42 = .7 p = 0.49 |
F2,42 = 0.1 p = 0.93 |
| Time | F8,336 = 17.0 p = <0.0001 |
F8,336 = 16.7 p = <0.0001 |
|||
| Rivastigmine*Time | F16,336 = 1.4 p = 0.15 |
F16,336 = 1.2 p = 0.23 |
|||
| Stimulated | Rivastigmine | F2,42 = 0.4 p = 0.69 |
F2,42 = 0.1 p = 0.92 |
F2,42 = 0.3 p = 0.76 |
F2,42 = 0.2 p = 0.80 |
| Time | F8,336 = 13.6 p = <0.0001 |
F8,336 = 13.6 p = <0.0001 |
|||
| Rivastigmine*Time | F16,336 = 1.4 p = 0.16 |
F16,336 = 1.3 p = 0.20 |
|||
Figure 1.
Subjective responses to METH during treatment with rivastigmine. The data reflect full time courses for METH 15 mg for “Desire METH” (top) and “Likely to Use METH” (bottom). All data reflect change from baseline (given value at a time point minus value at T=−15 min).
Subjective Effects (peak effects data)
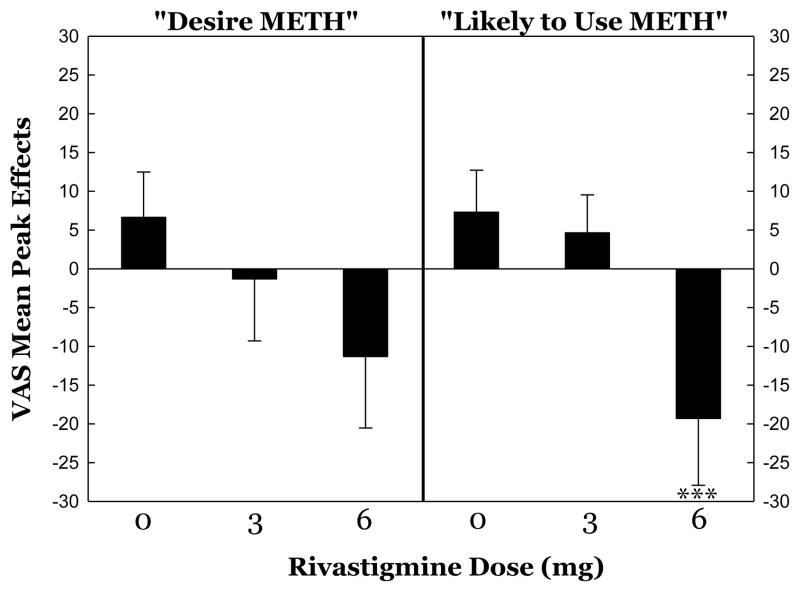
Statistical outputs for all peak effects data are shown in Table 3 (right). Of these, and following observations from full time-course analyses, the most intriguing outcomes are presented in Figure 2.
Figure 2.
Peak subjective effects responses to METH 15 mg during treatment with rivastigmine. All data reflect maximum change from baseline (given value at a time point minus value at T=−15 min). ***p<0.001 Rivastigmine 6 mg vs. 0 mg.
For “Likely to Use”, post-hoc tests confirmed significant differences between 0 mg vs. 6 mg (p=0.0058), as well as 3 mg vs. 6 mg dosages (p=0.0124), but not between 0 mg vs. 3 mg dosages (p=0.773).
Reinforcing Effects
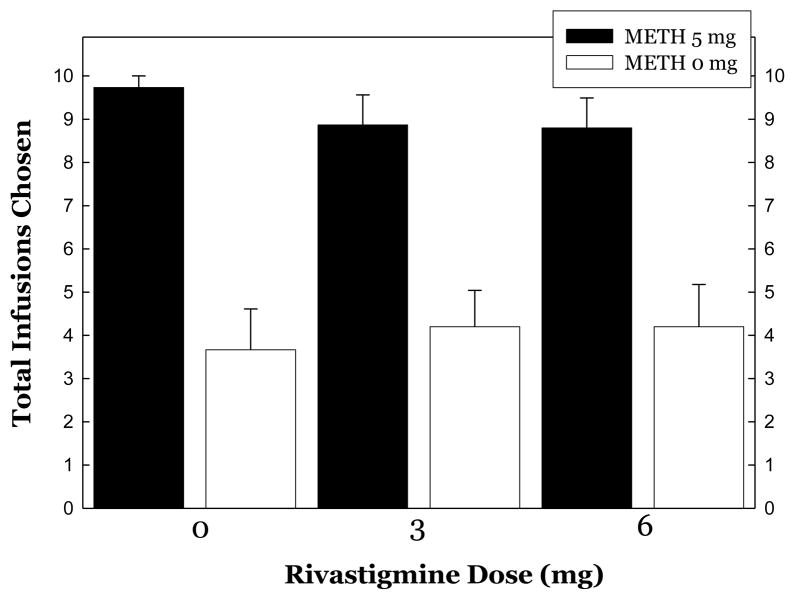
METH was more reinforcing than saline evidenced as more infusion choices (9.13±0.34 vs. 4.02±0.52, respectively) and revealed by ANOVA (F1,88 =587.8, p<0.0001). When considering choices as a function of rivastigmine dose, ANOVA did not reveal a significant effect of Rivastigmine (F2,84 =0.04, p=0.96), a significant effect was detected for METH (F1,84=587.8, p<0.0001), though a significant interaction was not detected for Rivastigmine × METH (F2,84=0.58, p=0.57)(Figure 3).
Figure 3.
Choices for METH (5 mg, IV/choice) or saline during treatment with rivastigmine.
Discussion
As shown before, acute METH exposure increased several positive subjective effects in METH-dependent volunteers. There was a trend for rivastigmine 6 mg to reduce METH-induced “Desire METH” and rivastigmine significantly attenuated “Likely to Use METH”. These effects were apparent across the time course, as well evaluation of peak effects. There were also non-significant trends for rivastigmine treatment to reduce “Desire METH” and “Likely to Use METH” at the 30 mg dosage of METH, though these did not reach statistical significance. These data serve as an extension, but not replication, of our previous findings (De La Garza, Shoptaw, 2008b). Also, as reported previously (De La Garza, Mahoney, 2008a), METH proved to be significantly more reinforcing than saline in a self-administration paradigm, though rivastigmine treatment did not reduce total choices for METH.
Overall, the results indicate some efficacy for rivastigmine in attenuating key subjective effects produced by METH, though the majority of measures were unaffected by rivastigmine treatment. The failure to see effects at higher doses of METH may simply be a function of the short duration of treatment with rivastigmine. The study design utilized is one set forth primarily for safety testing of drug interactions as required by the FDA, so answers to questions of efficacy can never be considered complete until outpatient clinical trials are conducted. In fact, the standard dose of rivastigmine used in the long-term treatment of Alzheimer’s disease is approximately 9 mg, so it is possible that even stronger reductions in “Desire” and “Likely to Use”, as well as other subjective effects, may have been detected if a dose higher than 6 mg and a longer treatment regimen was used. The modest effects observed thus far are not necessarily related to dose-limiting side effects since we did not detect increased adverse events in participants treated with rivastigmine as compared to placebo (to be reported elsewhere).
The lack of effect of rivastigmine on choices for METH is disappointing given the importance assigned to self-administration outcomes in other inpatient laboratory studies (Haney and Spealman, 2008). The disconnect between reductions in subjective effects, but not reinforcing effects, is also worth noting. It is possible, though cannot be proven, that short term rivastigmine treatment begins to change an individual’s perception of the drug, but that choice behavior remains intact and is much more difficult to alter until long-term treatment (accompanied by behavioral therapy) has been undertaken. Also of interest in this study was the unexpectedly high number of choices for saline (~4 of 10). The most likely cause was the study design utilized which involved the simple choice of an infusion or no infusion, while in other studies monetary alternatives are often utilized. In our previous report (De La Garza, Mahoney, 2008a), some reviewers commented that total choices for METH were quite low (~3 of 10) and therefore questioned whether 3 mg doses of METH were truly reinforcing for those participants. In an effort to improve upon our study design, we not only used a higher dose of METH for each choice (5 mg), but we eliminated the monetary alternative. In our design we were specifically interested in METH taking in the lab in the absence of a competing non-drug reinforcer. In so doing, we essentially removed any negative aspects of making choices even when participants clearly knew that saline was in the syringe. In previous study designs, making saline choices would have been accompanied by giving up some amount of money. In the current design participants could make a few saline choices and not be penalized for doing so. As expected, the study design change also resulted in an increase in METH infusions that was near the maximum possible (~9 of 10). These new data should assuage concerns about whether low doses of intravenous METH provided in the laboratory are reinforcing or not. Having a self-administration procedure that results in near maximum choices for METH being made during placebo treatment is considered an advantage since it is more likely that we will be able to detect medication effects in the future. Notwithstanding it is important to note that while subjects were exposed to 30–50 mg of METH on infusion days, this is considerably less than their reported daily use of METH (~800 mg per day). As a result, we do not known what effects rivastigmine would have on the much larger doses of METH that participants typically use in their natural environment.
Lastly, it is interesting to note our accompanying experiment evaluating the effects of rivastigmine on smoking behavior in these same participants. In that report, a trend toward reduced urges to smoke (p<0.09) was detected during treatment with rivastigmine at 3 mg (De La Garza and Yoon, 2011). Taken together, these new data are important since they highlight that cholinesterase inhibitors, and other drugs that target ACh systems, warrant continued consideration as treatments for METH-, and perhaps also cocaine-, and nicotine-dependence (Adinoff et al., Grasing et al., Williams and Adinoff, 2008). Notwithstanding, the general ability for cholinesterase inhibitors to increase ACh in brain very likely means that several populations of ACh receptors are being affected and this knowledge supports exploration of selective nicotinic and, possibly, muscarinic cholinergic medications. In fact, several of these are being evaluated including, for example, varenicline (a partial agonist of α4β2 nACh receptors) as a potential treatment for methamphetamine- (Zorick et al., 2009) and cocaine- (Plebani et al., 2011) dependence.
Research Highlights
Methamphetamine is a highly addictive stimulant and much work remains in identifying an effective pharmacotherapy.
Compounds that increase acetylcholine may attenuate subjective effects produced by methamphetamine in humans.
We characterized the effects of rivastigmine treatment on the subjective and reinforcing effects produced by administration of methamphetamine in human volunteers.
There was a trend for rivastigmine to reduce “Desire METH”, and rivastigmine significantly attenuated “Likely to Use METH”.
These data indicate that cholinesterase inhibitors warrant continued consideration as treatments for methamphetamine dependence.
Acknowledgments
This work was conducted at, and supported by the Michael E. DeBakey VA Medical Center, Houston, TX. Funding for this study was derived from a grant to R. De La Garza from the National Institutes on Drug Abuse (DA 023964) and included use of NIH-funded GCRC resources at BCM (RR 00188).
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ANOVA
analysis of variance
- BCM
Baylor College of Medicine
- BID
twice daily dosing
- DA
dopamine
- DSM
Diagnostic and Statistical Manual
- IV
intravenous
- MEDVAMC
Michael E. DeBakey Veteran Affairs Medical Center
- METH
methamphetamine
- NIDA
National Institute on Drug Abuse
- PO
oral administration
- S.E.M
standard error of the mean
- VAS
visual analog scale
Footnotes
Statement of Interest: None of the authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, et al. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology. 35:1485–99. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, et al. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1485–99. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Mahoney JJ, 3rd, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacology, biochemistry, and behavior. 2008a;89:200–8. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Yoon JH. Evaluation of the effects of rivastigmine on cigarette smoking by methamphetamine-dependent volunteers. Progress in neuro-psychopharmacology & biological psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008b:1–13. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. The Journal of pharmacology and experimental therapeutics. 2010;335:807–16. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug and alcohol dependence. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasing K, Mathur D, Newton TF, DeSouza C. Donepezil treatment and the subjective effects of intravenous cocaine in dependent individuals. Drug and alcohol dependence. 107:69–75. doi: 10.1016/j.drugalcdep.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. The American journal of drug and alcohol abuse. 2009;35:161–77. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob G, Ungerstedt U. The influence of cocaine self-administration on in vivo dopamine and acetylcholine neurotransmission in rat caudate-putamen. Neuroscience letters. 1990;109:227–33. doi: 10.1016/0304-3940(90)90568-t. [DOI] [PubMed] [Google Scholar]
- Kampman KM. The search for medications to treat stimulant dependence. Addiction science & clinical practice. 2008;4:28–35. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. British journal of clinical pharmacology. 2010;69:578–92. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Kalasinsky KS, Furukawa Y, Guttman M, Ang L, Li L, et al. Brain choline acetyltransferase activity in chronic, human users of cocaine, methamphetamine, and heroin. Molecular psychiatry. 1999;4:26–32. doi: 10.1038/sj.mp.4000462. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology. 1999a;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Keys AS. Involvement of acetylcholine in the nucleus accumbens in cocaine reinforcement. Annals of the New York Academy of Sciences. 1999b;877:792–5. doi: 10.1111/j.1749-6632.1999.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Herin D, Kjome KL. Use of stimulants to treat cocaine and methamphetamine abuse. Current psychiatry reports. 2008;10:385–91. doi: 10.1007/s11920-008-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug and alcohol dependence. 2010;106:72–8. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Yu Q, Pettinati HM, O’Brien CP, Kampman KM. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug and alcohol dependence. 2011 doi: 10.1016/j.drugalcdep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists. 2008;20:145–55. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clinical pharmacology and therapeutics. 2012;91:60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal D, Erickson J, Varoqui H, Ang L, Kalasinsky KS, Peretti FJ, et al. Brain vesicular acetylcholine transporter in human users of drugs of abuse. Synapse. 2004;52:223–32. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102 (Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–81. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, et al. Pilot Safety Evaluation of Varenicline for the Treatment of Methamphetamine Dependence. Journal of experimental pharmacology. 2009;2010:13–8. [PMC free article] [PubMed] [Google Scholar]