Abstract
Vibrio fischeri isolates from Euprymna scolopes are dim in culture but bright in the host. We found the luminescence of V. fischeri to be correlated with external osmolarity both in culture and in this symbiosis. Luminescence enhancement by osmolarity was independent of the lux promoter and unaffected by autoinducers or the level of lux expression, but the addition of an aldehyde substrate for luciferase raised the luminescence of cells grown at high and low osmolarities to the same high level. V. fischeri culture media have lower osmolarities than are typical in seawater or in cephalopods, partially accounting for the bacterium's low light output in culture.
The light organ symbiosis between the bioluminescent bacterium Vibrio fischeri and the Hawaiian bobtailed squid Euprymna scolopes has been developed as a model for studying mutualistic animal-bacterium interactions (15, 21). In establishing this system in our laboratory, we discovered that E. scolopes juveniles infected with V. fischeri in artificial seawater (Instant Ocean; Aquarium Systems, Mentor, Ohio) lost luminescence after 3 days, but remained colonized, if they were kept in diluted seawater (700 to 850 mosM) rather than seawater mixed to marine concentrations (975 to 1,025 mosM). Figure 1 illustrates this phenomenon with the results of one representative experiment. Squid were infected as previously described (16), and their luminescence was measured with a model LS 6500 counter (Beckman Coulter, Fullerton, Calif.). Although marine organisms are sometimes maintained at relatively low osmolarities, our data highlight the fact that this can perturb their natural biology in important ways.
FIG. 1.
The osmolarity of water affects the symbiotic luminescence of V. fischeri. Hatchling squid (n = 14) were exposed to V. fischeri strain ES114 and maintained in artificial seawater at 700 or 990 mosM, indicated by open bars or shaded bars, respectively. At 1, 2, and 3 days postinoculation, the animals were homogenized and plated to determine V. fischeri CFU counts (A) after animal luminescence (B) had been determined. Error bars indicate standard errors.
These data were intriguing because luminescence contributes to colonization persistence (20), and V. fischeri isolates from E. scolopes are unusual in that they are dim in culture (even dense culture) and bright only in the host (1, 10). Our data show wild-type cells exhibiting a dimness like that seen in culture in fully colonized squid, suggesting that we had mimicked culture conditions by placing the animals in dilute seawater. A simple explanation for our observations of symbiotic luminescence was that the squid, which maintain hyperosmotic tissues, lost the ability to osmoregulate against an unnaturally steep gradient and that the luminescence of the V. fischeri symbionts was dependent on the osmolarity of their surroundings (e.g., the light organ crypts). Consistent with the latter part of this model, in 1950 Farghaly reported a correlation between osmolarity and luminescence in culture for a bacterium that was probably a planktonic V. fischeri isolate (7).
We therefore tested the relationship between osmolarity and luminescence in a wild-type E. scolopes isolate, V. fischeri strain ES114 (1). Medium osmolarity was assessed using a freezing-point depression-automated osmometer (Osmette A; Precision Systems Inc., Natick, Mass.). The optical density at 595 nm (OD595) was determined with a BioPhotometer (Brinkmann Instruments Inc., Westbury, N.Y.) by measuring the culture density through 4-mm-wide cuvettes and dividing by 0.4 so that the reported values approximated the more commonly used OD595 for a 1-cm path. We measured luminescence with a TD20/20 luminometer (Turner Designs, Sunnyvale, Calif.), and we report these data in arbitrary, relative units that are comparable within a given experiment. Except as noted below, V. fischeri was cultured as described previously (8) in seawater tryptone (SWT) medium (1) made with differing amounts of Instant Ocean to vary the osmolarity.
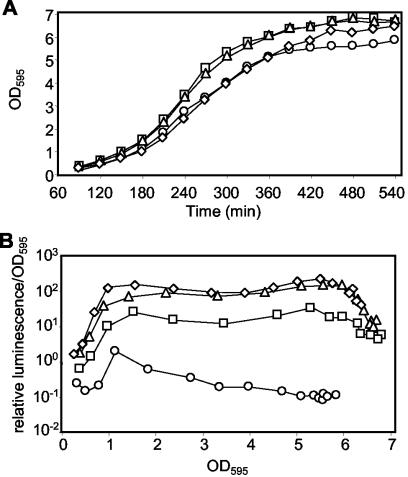
The luminescence of V. fischeri ES114 correlated with osmolarity (Fig. 2). The influence of osmolarity on luminescence was not due to effects on growth rate, as the conditions bracketed the optimal osmolarity for growth (Fig. 2A). Increased osmolarity also correlated with luminescence when the osmolarity of the SWT medium was adjusted from 700 to approximately 1,130 mosM with 240 mM NaCl or 390 mM MgSO4. However, the use of a nonmetabolizable sugar, xylose, inhibited growth at the required concentration and could not be used for comparison (data not shown). Previously, low light output by ES114 has been attributed to the underproduction of a homoserine-lactone (N-β-ketocaproyl-l-homoserine) autoinducer (AI) that stimulates lux gene transcription in concert with the regulator LuxR (1) and to the underproduction of the aliphatic aldehyde substrate of luciferase (11). We therefore tested whether the effect of osmolarity on luminescence was related to these phenomena.
FIG. 2.
Effects of medium osmolarity on V. fischeri growth and luminescence. V. fischeri strain ES114 was grown in SWT medium mixed with Instant Ocean to achieve different final osmolarities. Growth, measured as the OD595, is plotted versus time (A), and relative luminescence per OD595 is plotted versus the OD595 (B) for cultures grown in SWT at 570 mosM (circles), 810 mosM (squares), 1,050 mosM (triangles), or 1,300 mosM (diamonds).
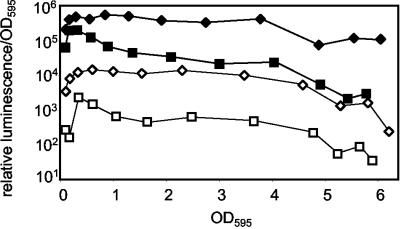
The effect of osmolarity on luminescence was independent of regulation by LuxR-AI and was not mediated by transcriptional lux promoters. The addition of AI (Sigma-Aldrich Co., St. Louis, Mo.) to achieve a final concentration of 200 ng/ml enhanced luminescence but did not alter the relative inhibitory effect of low osmolarity on luminescence (data not shown), suggesting that these were independent effects. Next, we used conjugation and allelic-exchange procedures (19) to generate a mutant of ES114, EVS101 (luxR::ermR-luxI::lacIq Ptac-luxCDABE [20]), in which the native lux promoters do not control luminescence. In this strain, luminescence is controlled by Ptac and LacIq and is induced by isopropyl-β-d-thiogalactopyranoside (IPTG). We found that the luminescence of EVS101 was enhanced at high osmolarities in the presence and in the absence of IPTG (Fig. 3). These data indicate that luminescence enhancement by osmolarity is not mediated by lux operon regulation, AI, or LuxR.
FIG. 3.
Luminescence enhancement at high osmolarity is independent of the lux promoter or expression level. Strain EVS101 (luxR::ermR-luxI::lacIq Ptac-luxCDABE) was grown with (filled symbols) and without (open symbols) IPTG (500 μg/ml−1) in SWT medium mixed with Instant Ocean to achieve final osmolarities of 810 mosM (squares) and 1,300 mosM (diamonds).
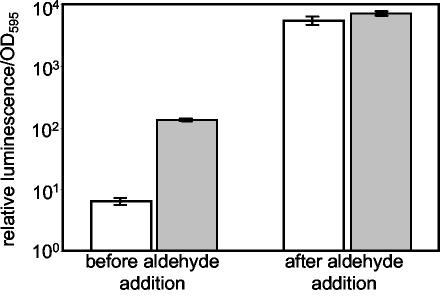
When aliphatic aldehyde (decanal; Sigma-Aldrich Co.), a substrate for luciferase, was added to ES114, the luminescence of cells grown at high and low osmolarities increased to indistinguishable high levels (Fig. 4). Thus, the luminescence of cells grown under either condition was aldehyde limited, but the limitation was less severe at high osmolarities. Boylan et al. (3) found that LuxE (aldehyde synthase) was stabilized in vitro upon the addition of 200 mM NaCl and 10% glycerol to the buffer, conditions of increased salt concentration and osmolarity. V. fischeri probably responds to high external osmolarity in part by increasing cytosolic osmolarity, as does Escherichia coli (4), and this might stabilize LuxE in vivo, resulting in a greater pool of aldehyde substrate and enhanced luminescence.
FIG. 4.
High osmolarity partially compensates for aldehyde limitation. V. fischeri strain ES114 was grown in SWT medium mixed with Instant Ocean to achieve final osmolarities of 810 mosM (open bars) or 1,300 mosM (shaded bars). Relative luminescence per OD595 was determined when the OD595 of the culture was between 2.0 and 4.0, before and immediately after the addition of a 1% aqueous decanal solution to a final concentration of 0.01%. Error bars indicate standard errors (three replicates).
Luminescence enhancement at high osmolarities was not specific to strain ES114. V. fischeri strains MJ1, ES12, and ES213 (2, 17) were brighter in SWT medium at 1,130 than at 700 mosM (data not shown). Strain ET101 (12), an isolate from Euprymna tasmanica and the brightest strain we examined, did not show enhanced luminescence in higher-osmolarity SWT medium. However, the addition of aldehyde also did not enhance ET101 luminescence, indicating that luminescence is not aldehyde limited (data not shown). Thus, our experiments with ET101 support our hypothesis that luminescence enhancement at high osmolarities is mediated by relieving aldehyde limitation.
The correlation between osmolarity and luminescence in ES114 may account in part for the strain's relative dimness in culture and its brightness in E. scolopes (1). We found that the osmolarity of SWT medium, which is often used in luminescence experiments (1, 8, 11), was 810 mosM and that the osmolarity of LBS (18), another commonly used growth medium, was 790 mosM. Each of these concentrations is below the typical concentration of seawater, 1,000 mosM. Furthermore, cephalopods, including squid and cuttlefish, maintain an osmolarity somewhat greater than that of the surrounding seawater (13, 14, 22). The small size of E. scolopes juveniles precluded measurement of the osmolarity of their blood; however, it seems likely that the osmolarity of SWT is lower than what the bacteria experience in the light organ. Based on the data in Fig. 2 and 4, this lower osmolarity may account for 10-fold of the 1,000-fold difference in luminescence between cultured and symbiotic cells (1).
Interestingly, Dunlap previously demonstrated a similar, but inverse, relationship between luminescence and osmolarity in the ponyfish symbiont Photobacterium leiognathi (5). P. leiognathi's luminescence is enhanced at low osmolarities, and because its host is a teleost fish, it presumably maintains a blood osmolarity below that of seawater (6, 9, 13). In both the P. leiognathi-ponyfish and V. fischeri-squid symbioses, the symbionts were initially described as brighter in the host than in culture; however, culture conditions were set at osmotic conditions closer to the bacterial growth optima than to the osmolarities in the respective hosts. In the future it may be useful to mimic the osmolarity of the host environment when studying the bioluminescence of symbiotic V. fischeri strains in culture.
Acknowledgments
We thank Claudia Lupp, Margaret McFall-Ngai, Ellen Neidle, and Edward Ruby for insightful comments, Michael Goodson and Kimberly Hutcheson for technical assistance, and Mark Rieger for use of the osmometer.
This work was supported by grants from the National Institutes of Health (R01 AI 50661) and the University of Georgia Research Foundation. D.M.A. was supported by a University of Georgia Graduate Research Fellowship.
REFERENCES
- 1.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boettcher, K. J., and E. G. Ruby. 1994. Occurrence of plasmid DNA in the sepiolid squid symbiont Vibrio fischeri. Curr. Microbiol. 29:279-286. [Google Scholar]
- 3.Boylan, M., C. Miyamoto, L. Wall, A. Grahm, and E. Meighen. 1989. Lux C, D, and E genes of the Vibrio fischeri luminescence operon code for the reductase, transferase, and synthetase enzymes involved in aldehyde biosyntheses. Photochem. Photobiol. 49:681-688. [DOI] [PubMed] [Google Scholar]
- 4.Csonka, L. N., and W. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 5.Dunlap, P. V. 1985. Osmotic control of luminescence and growth in Photobacterium leiognathi from ponyfish light organs. Arch. Microbiol. 141:44-50. [DOI] [PubMed] [Google Scholar]
- 6.Fange, R., U. Lidman, and A. Larsson. 1976. Comparative studies of inorganic substances in the blood of fishes from the Scagerac Sea. J. Fish Biol. 8:441-448. [Google Scholar]
- 7.Farghaly, A.-H. 1950. Factors influencing the growth and light production of luminous bacteria. J. Cell. Comp. Physiol. 36:165-183. [DOI] [PubMed] [Google Scholar]
- 8.Fidopiastis, P. M., C. Miyamoto, M. G. Jobling, E. A. Meighen, and E. G. Ruby. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131-143. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, M. S. 1982. Animal physiology: principles and adaptations. Macmillan Publishing Co., Inc., New York, N.Y.
- 10.Lee, K.-H., and E. G. Ruby. 1994. Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J. Bacteriol. 176:1985-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupp, C., M. Urbanowski, E. P. Greenberg, and E. G. Ruby. 2003. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 50:319-331. [DOI] [PubMed] [Google Scholar]
- 12.Nishiguchi, M. K., E. G. Ruby, and M. J. McFall-Ngai. 1998. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-vibrio symbioses. Appl. Environ. Microbiol. 64:3209-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosser, C. L. 1973. Comparative animal physiology, 3rd ed. W. B. Saunders and Company, Philadelphia, Pa.
- 14.Robertson, J. D. 1965. Studies on the chemical composition of muscle tissue. III. The mantle muscle of cephalopod molluscs. J. Exp. Biol. 42:153-175. [DOI] [PubMed] [Google Scholar]
- 15.Ruby, E. G. 1996. Lessons from a cooperative bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50:591-624. [DOI] [PubMed] [Google Scholar]
- 16.Ruby, E. G., and L. M. Asato. 1993. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159:160-167. [DOI] [PubMed] [Google Scholar]
- 17.Ruby, E. G., and K. H. Nealson. 1976. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica: a model of symbiosis based on bacterial studies. Biol. Bull. 151:574-586. [DOI] [PubMed] [Google Scholar]
- 18.Stabb, E. V., K. A. Reich, and E. G. Ruby. 2001. Vibrio fischeri genes hvnA and hvnB encode secreted NAD+-glycohydrolases. J. Bacteriol. 183:309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stabb, E. V., and E. G. Ruby. 2002. New RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 358:413-426. [DOI] [PubMed] [Google Scholar]
- 20.Visick, K. L., J. Foster, J. Doino, M. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]