Abstract
Compared to smokers alone, smokers with co-morbid substance use disorders are at greater risk of suffering from smoking-related death. Despite this, relatively few studies have examined smoking cessation treatments for those with stimulant dependence. In the current study, we sought to evaluate the effects of produced by short-term exposure to the cholinesterase inhibitor rivastigmine (0, 3 or 6 mg) on cigarette smoking in non-treatment-seeking, methamphetamine-dependent volunteers. This was a double-blind, placebo-controlled, crossover study that took place over 9 days. The data indicate that rivastigmine treatment did not alter Fagerström Test for Nicotine Dependence scores, carbon monoxide readings, or cigarettes smoked per day, but a trend toward reduced urges to smoke (p<0.09) was detected during treatment with rivastigmine 3 mg. These data, while preliminary, indicate that cholinesterase inhibitors warrant further consideration as treatments for nicotine dependence, including use in stimulant-dependent individuals who exhibit significantly higher rates of smoking than the general population.
Keywords: smoking, nicotine-dependence, methamphetamine, treatment, addiction, rivastigmine, acetylcholine
Introduction
Cigarette smoking is the leading cause of death and morbidity in the US (Centers for Disease Control and Prevention 2008). Among those with substance use disorders, rates of smoking are almost 70%, approximately 3- to 4- fold greater than the general population (Kalman et al. 2005). Even higher rates of smoking, 87–92%, are reported among those with methamphetamine-dependence (Weinberger and Sofuoglu 2009). In our own research, we have routinely shown similarly high rates of smoking among methamphetamine-dependent individuals (De La Garza et al. 2009; De La Garza et al.; De La Garza et al. 2008; Newton et al.; Zorick et al. 2009). Compared to smokers alone, smokers with co-morbid substance use disorders are at greater risk of suffering from smoking-related death (Hser et al. 1994; Hurt et al. 1996). While the cause of increased negative health outcomes are unknown, likely reasons are that co-morbid smokers are a more treatment-resistant population (using more drug more frequently, earlier initiation, etc) and there are likely additive effects of combining to drugs with known negative health effects (Weinberger & Sofuoglu, 2009). The U.S. Department of Health and Human Services Clinical Practice Guidelines explicitly recommends addressing cigarette smoking in treatments for substance dependence (Fiore et al. 2008). However, relatively few studies have examined smoking cessation treatments for those with stimulant dependence (Morisano et al. 2009; Weinberger and Sofuoglu 2009). In many addiction treatment settings, smoking is actually endorsed in the form of regular smoke breaks (Knapp et al. 1993); a strategy apparently adopted by treatment providers concerned that reducing smoking may impede successful treatment outcomes. However, a meta-analysis of smoking interventions in addiction treatment settings suggests smoking cessation treatments may actually enhance longer-term addiction outcomes (Prochaska et al. 2004).
Among the general population of smokers, millions attempt to quit smoking each year, but the majority of those who attempt to quit on their own fail within 8 days of the initial quit attempt (Hughes et al. 2004). Currently, first-line pharmacotherapies for smoking cessation include nicotine replacement therapy, bupropion, and varenicline. These medications consistently improve smoking cessation rates relative to placebo in randomized control trials, however rates of abstinence are only 20–33% at 6-months after the initial quit attempt (Fiore et al. 2008). Therefore, it is important to continue the search for new pharmacotherapies to mitigate withdrawal symptoms and prevent relapse during smoking cessation.
Substantial evidence points to nicotine as the primary agent in tobacco leading to abuse and dependence (Dwoskin et al. 2009; Govind et al. 2009; Livingstone and Wonnacott 2009). Nicotine binds to nicotinic acetylcholine receptors (nAChRs) in brain and activates the mesocorticolimbic dopamine system (Grenhoff et al. 1986; Zhang et al. 2009) in the same manner as other drugs of abuse (Pierce and Kumaresan 2006) and rewarding stimuli (Hyland et al. 2002). Chronic nicotine administration results in upregulation of high affinity nicotine binding sites in neuronal nAChRs, which has been suggested as the neurobiological basis of nicotine dependence (Govind et al. 2009). Based on nicotine’s effects on the brain, compounds that inhibit the breakdown of ACh warrant consideration as possible smoking cessation pharmacotherapies. In fact, the acetylcholinesterase (AChE) inhibitor galantamine reduced smoking and craving for cigarettes in alcohol-dependent participants (Diehl et al. 2006), although similar effects were not observed in smokers with schizophrenia (Kelly et al. 2008). More recently, 4 weeks of the AChE and butyrylcholinesterase (BuChE) inhibitor rivastigmine (6 mg/day) produced significant decreases in both smoking and craving relative to placebo in alcohol-dependent participants (Diehl et al. 2009). To our knowledge there are no data on the effects of AChE inhibitors on smoking behaviors in healthy, non-drug-dependent individuals.
The purpose of the current study was to evaluate the effects produced by short-term exposure to rivastigmine on smoking behavior in non-treatment seeking methamphetamine (METH)-dependent volunteers.
Methods
This double-blind, placebo-controlled, crossover study was funded by the National Institute on Drug Abuse, and approved by the Baylor College of Medicine and Michael E. DeBakey VA Medical Center Institutional Review Boards. All volunteers provided written informed consent after being apprised of potential risks of study participation.
Sample
Participants were English-speaking volunteers who were not seeking abstinence-focused treatment for cigarette smoking or METH use at the time of the study, between 18–55 years of age, met DSM-IV-TR (DSM-IV-TR 2000) criteria for METH dependence, had a breathalyzer test indicating an undetectable blood alcohol level upon admission, had a medical history and brief physical examination demonstrating no clinically significant contraindications for study participation, and had a negative urine drug screen, with the exception of METH or marijuana. Exclusion criteria included having neurological or psychiatric disorders, as assessed by MINI (Sheehan et al. 1998), such as episode of major depression within the past 2 years, lifetime history of schizophrenia, other psychotic illness, or bipolar illness, current organic brain disease or dementia assessed by clinical interview, history of or any current psychiatric disorder which would require ongoing treatment or which would make study compliance difficult, history of suicide attempts within the past three months and/or current suicidal ideation/plan, or history of psychosis occurring in the absence of current METH use, or meet current or past DSM criteria for dependence on alcohol or other drugs, except for nicotine.
Procedure
Participants resided for 9 days on the Research Commons of the Michael E. DeBakey VA Medical Center. During the 9-day stay, participants were exposed to low doses of methamphetamine (study days 2, 6, 7, and 8) to measure physiological, subjective and reinforcing effects (those data and outcomes have been submitted for publication elsewhere). In this manuscript, we focus on changes in smoking behavior that occurred during the protocol.
The participants were not only non-treatment seeking methamphetamine users, but they were also non-treatment seeking nicotine-dependent individuals. Participants were randomized to one of 3 rivastigmine dosing conditions (0, 3 and 6 mg) from days 1–8, and on day 9 they were discharged from the study and returned for enrollment and randomization to alternate rivastigmine dosing conditions. The period between study enrollments was >7 days but < 28 days).
During screening, all participants tested METH-positive (via urine toxicology) indicating that they were active METH users. Upon enrollment, however, all participants were required to report to the hospital METH-negative, which necessitated cessation of use for at least 4–6 days. No participants exhibited signs of withdrawal at enrollment or during the study as assessed by daily mood questionnaires or adverse events reporting (data not shown).
Assessment of Nicotine Dependence
The Fagerström Test for Nicotine Dependence (FTND) is a 5-item questionnaire that has been previously demonstrated to be reliable and valid and is widely used to characterize nicotine dependence (Heatherton et al., 1991). The FTND score was assessed once daily every morning at 7 AM.
Self-Reported Cravings for Cigarettes
The Urge to Smoke Questionnaire (UTS) has been previously demonstrated to be positively associated with nicotine withdrawal (Jarvik et al., 2000). The UTS consists of 10 items, with each item scored on a 7-pt scale. Participants were administered the UTS once daily every morning at 7 AM. The mean score was calculated for all 10 questions for each day and this value was used in the final data analyses.
Assessment of Cigarette Smoking
Cigarette smoking was assessed via daily breath carbon monoxide (CO; Vitalograph® Inc, Lenexa, KS) and the Self-Report of Nicotine Use (SRNU) questionnaire. The key question from the SRNU was “in the past 24 h how many cigarettes have you smoked?” Available answers were 1) 1–3 cigarettes, 2) 3–10 cigarettes, 3) 10–20 cigarettes, and 4) greater than 1 pack (i.e., 20 cigarettes).
Rivastigmine
Rivastigmine inhibits both AChE and BuChE with equal potency and has selectivity for central activity. Following oral dosing, the plasma half-lives of rivastigmine and its primary metabolite are roughly 1 h and 2 h, respectively; however, cholinesterase inhibition lasts much longer (~10 h) than the plasma half-life indicates. Commercially available rivastigmine tablets (3 mg) were encapsulated in gelatin capsules by the VA Medical Center research pharmacy, and placebo was prepared in a similar manner. Two rivastigmine or matched placebo pills were administered orally at 7:00 AM and 7:00 PM (i.e., 0+0 mg, 3+0 mg, or 3+3 mg) beginning on day 1 (in the PM) and continuing through day 8 (in the AM) of the protocol.
Data Analyses
Data were analyzed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were compiled for demographic and drug use variables. All primary measures, including FTND scores, breath CO, UTS scores, and SRNU scores were analyzed individually using repeated measures analysis of variance (ANOVA) as a function of rivastigmine dose (0, 3 and 6 mg) and Time (days 1–8). All data, except time, were analyzed as between-subjects factors. For all measures, statistical significance was set at p<0.05. All data are presented as mean standard error of the mean (S.E.M.).
Results
Of the original 17 participants who completed all three portions of the main protocol, 13 were cigarette smokers (76%) and were therefore included in this report. Most participants were Caucasian and male (Table 1). On average, participants had used methamphetamine for 10 years and had used the drug every other day of the month, and they had smoked cigarettes for 13 years and smoked on average one pack per day. Participants did not meet DSM criteria for abuse or dependence on other substances except methamphetamine and nicotine.
Table 1. Demographic and Drug Use.
Data reflect Mean ± S.E.M. Recent use indicates use of the specified drug in the 30 days prior to study enrollment.
| Demographics and Drug Use | (N=13) |
|---|---|
|
| |
| Gender | |
| Male | 10 |
| Female | 3 |
|
| |
| Race/Ethnicity | |
| Caucasian | 11 |
| Hispanic | 2 |
|
| |
| Age (years) | 33.7±2.4 |
|
| |
| Education (years) | 13.2±0.6 |
|
| |
| Methamphetamine | |
| Years of Use | 9.8±1.9 |
| Recent Use (last 30 days) | 16.1±2.7 |
|
| |
| Nicotine | |
| Years of Use | 13.4±2.0 |
| Cigarettes/day | 17.8±2.5 |
|
| |
| Alcohol Use (N=12) | |
| Years of Use | 13.5±2.9 |
| Recent (last 30 days) | 2.6±1.1 |
|
| |
| Marijuana Use (N=10) | |
| Years of Use | 12.7±3.2 |
| Recent (last 30 days) | 8.8±3.1 |
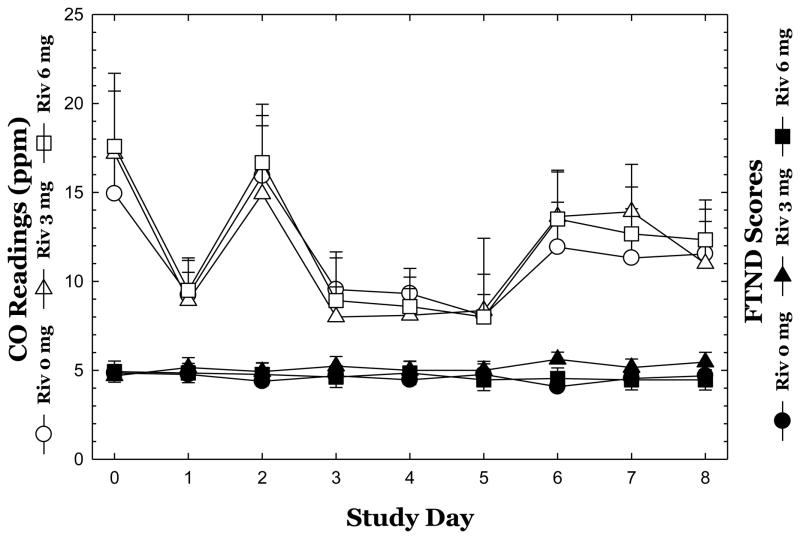
For FTND scores (Figure 1), repeated-measures ANOVA did not reveal a significant effect of Rivastigmine (F2,36 =0.34, p=0.72) nor Time (F8,288=0.43, p=0.90), and no significant interaction of Rivastigmine x Time (F8,288=0.98, p=0.45).
Figure 1.
Mean carbon monoxide (CO) readings and Fagerström Test for Nicotine Dependence (FTND) scores are shown across study days for each dose of Rivastigmine. Data reflect Mean ± S.E.M.
For CO readings (Figure 1), repeated-measures ANOVA did not reveal a significant effect of Rivastigmine (F2,33 =0.03, p=0.97) though one was detected for Time (F8,264=10.64, p<0.0001), and no significant interaction of Rivastigmine x Time (F8,264=0.27, p=0.99).
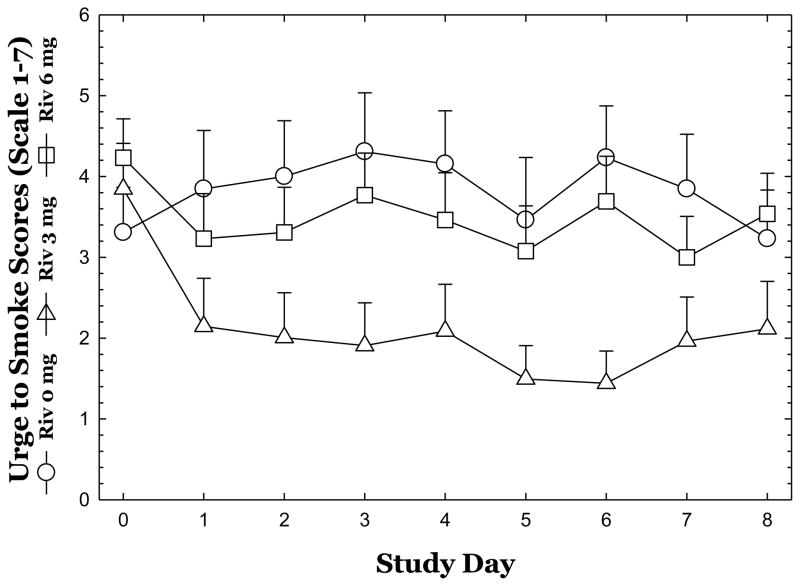
For UTS scores (Figure 2), repeated-measures ANOVA did not reveal a significant effect of Rivastigmine (F2,36 =0.21, p=0.81) nor Time (F8,288=0.81, p=0.59), and no significant interaction of Rivastigmine x Time (F8,288=1.50, p=0.09); though a trend for rivastigmine 3 mg to reduce UTS scores was apparent.
Figure 2.
Mean Urge to Smoke (UTS) scores are shown across study days for each dose of Rivastigmine. Data reflect Mean ± S.E.M.
For SRNU scores (data not shown), repeated-measures ANOVA did not reveal a significant effect of Rivastigmine (F2,36 =0.06, p=0.94) though one was detected for Time (F8,288=2.43, p=0.015), and no significant interaction of Rivastigmine x Time (F8,288=1.06, p=0.39).
Discussion
In the current study, we sought to characterize the effects produced by short-term exposure to rivastigmine on cigarette smoking in non-treatment seeking, methamphetamine-dependent research volunteers. The role of rivastigmine and related medications can be described in terms of negative reinforcement. During withdrawal, the receptor binding of exogenous nicotine is diminished, leading to several aversive symptoms. As relapse occurs in untreated patients, symptoms are relieved as exogenous nicotine is made available. Rivastigmine treatment increases the availability and binding of endogenous ACh at nicotinic receptors, and may therefore prevent withdrawal symptoms and decrease the potential for negative reinforcement.
In this report, and as shown by several other groups, the majority of methamphetamine-dependent participants were also current cigarette smokers. In general, the data did not reveal an effect for rivastigmine in reducing smoking behaviors, though a trend was observed for rivastigmine 3 mg to reduce daily urge to smoke scores. The explanation for the effect of rivastigmine at 3 mg, but not 6 mg, could be a function of nAChR desensitization at higher rivastigmine doses, though future studies would have to be conducted to determine if this is indeed the case.
Of interest, apparent increases in CO readings were detected on Days 2 and 6–8, which coincided with methamphetamine exposure that occurred on those days. This finding is worth commentary since many have speculated that methamphetamine use increases desire for smoking and vice versa (Weinberger and Sofuoglu 2009). In fact, animal data show that nicotine and nicotine agonists fully substitute for methamphetamine in a drug discrimination task (Desai and Bergman 2010; Gatch et al. 2008). Notwithstanding, our group recently conducted a large survey of methamphetamine-dependent cigarette smokers (N>100), and the data surprisingly indicate that these individuals self-report that smoking does not affect the high or desire experienced during concurrent methamphetamine use. Instead, participants self-report smoking cigarettes during methamphetamine use mostly out of habit or boredom (De La Garza et al., unpublished findings).
The dissociation between subjective effects data indicating reduced urge to smoke versus the actual choice to smoke cigarettes (evidenced by all other metrics) is difficult to reconcile. We postulate (though we have no definitive proof) that short-term treatment with rivastigmine may alter the way a smoker thinks about cigarettes, but that it is not sufficient to change behavior. This is not be surprising since these individuals had been using nicotine on average for greater than 13 years and smoking about one pack of cigarettes per day. More importantly, these were non-treatment seeking cigarette smokers. It is likely that a longer duration of treatment is needed to change behavior. For example, while self-report measures of smoking and withdrawal returned to baseline levels following 7-days of smoking abstinence in non-treatment seeking cigarette smokers, decreased likelihood to smoke in a laboratory model of smoking relapse was observed only after 14-days of abstinence, despite no additional changes in self-report measures (Lussier et al. 2005). A definitive answer to this question can be obtained from a clinical trial in which treatment-seeking individuals are exposed to several weeks of treatment with rivastigmine in combination with cognitive behavioral therapy and other supportive methods to discourage cigarette smoking. Further, given the ability for rivastigmine to increase ACh, it may be preferable (in future studies) to examine its effects during abstinence, as increasing ACh may have limited effect under satiated conditions (given that these individuals were allowed to smoke throughout enrollment) where nicotinic receptors are bound to by nicotine and may also be desensitized to further stimulation.
As specified in the Methods, the primary thrust of the main protocol was to evaluate the effects of rivastigmine on methamphetamine-induced physiological, subjective and reinforcing effects. In general, the same profile has emerged with the most prominent effects occurring at the 3 mg, but not 6 mg dose. It is possible, though not certain, that the effects of rivastigmine on nicotine smoking and methamphetamine are related, since the most prominent effects observed in the main study included reductions in methamphetamine-induced “desire” at 3 mg, which we have shown previously (De La Garza et al. 2008).
A number of limitations should be conceded, including the lack of smoking-related inclusion criteria (i.e., cigarettes per day, FTND scores), the lack of more precise biochemical measures of smoking (i.e., salivary or urinary cotinine) for data outputs, and the lack of more sensitive instruments of smoking behavior (e.g., topographic assessment). In addition, given that these were non-treatment seeking smokers, who had no knowledge that the treatment might change cigarette smoking behaviors, the placebo/expectation effects of a regular treatment trial were essentially eliminated; allowing the analyses of the effects of the pharmacological agent in the absence of any “psychological contamination” (Benedetti; Benedetti and Amanzio). The sample size is a key limitation, which was considerably smaller than that used in conventional trials for smoking cessation treatments. Another important important limitation was that smokers were not allowed to smoke ad libitum. Smoking was allowed only at specified times during the day, which is standard for inpatient medications studies which necessitate that participants be accompanied outdoors by a staff member. As such, these restrictions very likely affected regular smoking patterns and perhaps skewed data outcomes. Lastly, the data are unlikely relevant to the majority of outpatients in rehabilitation clinics (i.e., individuals motivated to quit) who may have very distinct profile of response to rivastigmine treatment.
Taken together, these findings, while preliminary, indicate that cholinesterase inhibitors warrant consideration as treatments for nicotine dependence, including evaluation in stimulant-dependent individuals.
Research Highlights.
Stimulant-dependent individuals exhibit significantly higher rates of smoking than the general population.
We evaluated the effects produced by short-term exposure to the cholinesterase inhibitor rivastigmine on cigarette smoking in non-treatment-seeking, methamphetamine-dependent volunteers.
A clear trend toward reduced urges to smoke was detected during treatment with rivastigmine 3 mg.
These data indicate that cholinesterase inhibitors warrant further consideration as treatments for nicotine dependence.
Acknowledgments
Funding for this study was derived from a grant to R. De La Garza from the National Institutes on Drug Abuse (DA 023964).
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ANOVA
analysis of variance
- BuChE
butyrylcholinesterase
- CO
carbon monoxide
- DSM
diagnostic and statistical manual
- FTND
Fagerström Test for Nicotine Dependence
- METH
methamphetamine
- nAChRs
nicotinic acetylcholine receptors
- SRNU
Self-Report of Nicotine Use
- UTS
Urge to Smoke
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Benedetti F. No prefrontal control, no placebo response. Pain. 148:357–8. doi: 10.1016/j.pain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M. The placebo response: How words and rituals change the patient’s brain. Patient Educ Couns. doi: 10.1016/j.pec.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–8. [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, Heinzerling KG, Nusinowitz S, London ED, Shoptaw S, Moody DE, Newton TF. The cardiovascular and subjective effects of methamphetamine combined with gamma-vinyl-gamma-aminobutyric acid (GVG) in non-treatment seeking methamphetamine-dependent volunteers. Pharmacol Biochem Behav. 2009;94:186–93. doi: 10.1016/j.pbb.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 106:173–80. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008;11:729–41. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, Croissant B, Smolka MN, Batra A, Mann K. Galantamine reduces smoking in alcohol-dependent patients: a randomized, placebo-controlled trial. Int J Clin Pharmacol Ther. 2006;44:614–22. doi: 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, Mutschler J, Hermann D, Kiefer F. Rivastigmine reduces tobacco craving in alcohol-dependent smokers. Pharmacopsychiatry. 2009;42:89–94. doi: 10.1055/s-0028-1103295. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks PA, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem Pharmacol. 2009;78:732–43. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Bailey WC, Benowitz NL, et al. Treating Tobacco Use and Dependency: 2008 Update Clinical Practice Guideline. U. S. Department of Health and Humans Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–65. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–8. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev Med. 1994;23:61–9. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–23. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Weiner E, Boggs DL, Dickinson D, Conley RR, Buchanan RW. Lack of beneficial galantamine effect for smoking behavior: a double-blind randomized trial in people with schizophrenia. Schizophr Res. 2008;103:161–8. doi: 10.1016/j.schres.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp JM, Rosheim CL, Meister EA, Kottke TE. Managing tobacco dependence in chemical dependency treatment facilities: a survey of current attitudes and policies. J Addict Dis. 1993;12:89–104. doi: 10.1300/J069v12n04_07. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78:744–55. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology (Berl) 2005;181:486–95. doi: 10.1007/s00213-005-0008-5. [DOI] [PubMed] [Google Scholar]
- Morisano D, Bacher I, Audrain-McGovern J, George TP. Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Can J Psychiatry. 2009;54:356–67. doi: 10.1177/070674370905400603. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Grasing K. The angiotensin-converting enzyme inhibitor perindopril treatment alters cardiovascular and subjective effects of methamphetamine in humans. Psychiatry Res. 179:96–100. doi: 10.1016/j.psychres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72:1144–56. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–7. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, De La Garza R, Newton TF, London ED. Pilot Safety Evaluation of Varenicline for the Treatment of Methamphetamine Dependence. J Exp Pharmacol. 2009;2010:13–18. [PMC free article] [PubMed] [Google Scholar]