Abstract
Although stroke is one of the leading causes of death and disability worldwide, preventive or therapeutic options are still limited. Therefore, a better understanding of the pathophysiological characteristics of this life-threatening disease is urgently needed. The incidence and prevalence of ischemic stroke are increased by exposure to certain types of xenobiotics, including heavy metals, suggesting the possible toxicological contribution of these compounds to the onset or aggravation of stroke. Among the potential targets, we have focused on alterations to cerebral endothelial cells (CECs), which play important roles in maintaining the functional integrity of brain tissue.
Keywords: Ischemic stroke, Blood-brain barrier, Tight junction, Neurovascular unit, Heavy metals
INTRODUCTION
Several environmental factors including heavy metals are reported to be associated to ischemic stroke, which is the leading cause of death and disability (1). Although thousands of neuroprotective therapeutic drug candidates were developed, all of them failed during clinical trials raising the urgent need to understand the dynamics of pathophysiological alterations during ischemic stroke (2). Recent studies suggest that each components of the integrated neurovascular unit plays key roles in ischemic damage. Here we will discuss the importance of alteration of blood brain barrier (BBB), which is essential in functional integrity of the brain and the possible contribution of BBB impairment to heavy metal-associated ischemic brain damage.
ISCHEMIC STROKE
Ischemic brain damage. The brain shows a high requirement of energy to maintain the heavy synaptic interactions in neuronal network, and almost 15% of the total cardiac output is used for cerebral blood flow (CBF). Since the brain is not capable to reserve energy supply, which is derived from oxygen and glucose in CBF, it is highly susceptible to interruption of blood flow in the brain, which is called stroke. Ischemic stroke is usually caused by blockade of blood circulation by an embolus or by in situ thrombus (3), and it represents 87% of the total stroke (4). During ischemic brain injury, the neuronal damage is immediately initiated at the site of ischemic core. According to the ischemic duration, the necrotic lesions of neuronal death expand. Even with a successful reperfusion within few minutes after ischemic onset, the neuronal death can occur after several days representing delayed neuronal death. The major cell types affected by ischemic insults are neurons, but also astrocytes and CECs can be also damaged by sustained duration of ischemia.
Molecular mechanisms of ischemic damage. Ischemic insult in brain induces a complicated array of pathological mechanisms, i.e. ischemic cascade, which ultimately results in irreversible neuronal injury and brain infarction (5). The most typical phenomenon of ischemic neuronal death is an excessive excitation (6,7). There are several types of ion channels to regulate the membrane potential for neuronal excitation, and ATP is critical to maintain the function of these ion channels. Upon ischemic insult, ATP depletion immediately results in depolarization of neuronal membrane and excessive influx of calcium and sodium into neuronal cytosol. Also, dysregulated release of neurotransmitters such as glutamate occurs by reversed operation of transporters, and the excessive activation of glutamate receptors further increase calcium influx into neuronal cytosol. Following calcium ion elevation, cytoskeletal degradation and enzymatic activation occurs, resulting in further activation of cell death pathways including mitochondrial perturbation (8). Along with the exciting stimulation, radical generation and suicidal apoptotic pathways are also involved in neuronal cell death (9).
NEUROVASCULAR UNIT AS AN INTEGRATED SYSTEM
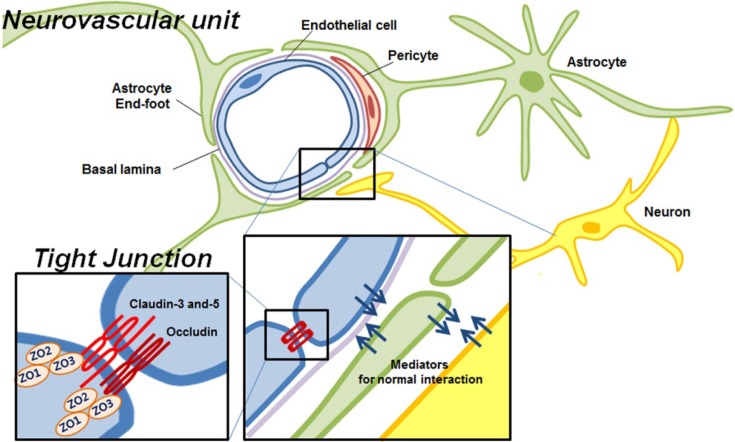
Neurovascular unit. Traditional approach for stroke treatment has primarily focused on neuroprotection, preserving the neurons to lessen the cerebral brain damage following ischemic stroke. However, all of neuroprotective experimental drugs have failed during clinical trials, showing side effects or no efficacy (10). To overcome the weakness and limitation of neuroprotectants, the idea regarding multi-functional protective drugs has been raised, based on the complicated integrated brain physiology. Especially, to understand the complexity of post-ischemic brain damage, the importance of ‘neurovascular unit’ has been revisited (Fig. 1). The fundamental concept of neurovascular unit is that emphasizing the interaction in between cells themselves, and between cells and matrix for the tissue outcome in neurological disorders (9). In this context, ischemic brain damage is not the matters of neurons alone but that of the complex of neurons, vascular cells and the supportive system such as astrocytes. Ischemic stroke is basically a vascular disorder affecting neuronal function (11), and the alterations of local environment influence surrounding neurons, allowing them to survive or die.
Fig. 1. Integrated neurovascular unit.
Blood-brain barriers. The blood-brain barrier (BBB) is a theoretical functional concept that describes unique characteristics of intracranial blood vessels. BBB is a physical and metabolic barrier essential for the normal function and the stability of the central nervous system, limiting the paracelluar flux of hydrophilic molecules from systemic blood circulation (12). The structural and biochemical features of BBB are maintained by the specific contribution of the CECs, pericytes and glial end-foot (Fig. 1). Among them, endothelial tight junctions (TJs) are the most important components of the BBB integrity, determining alterations in BBB vascular permeability. The TJs consist of a combination of trans-membrane proteins such as claudin, occludin, and junctional adhesion molecules, and cytosolic proteins like zonulaoccluden (ZO)-1, ZO-2 and ZO-3. The cytoplasmic proteins interact with transmembrane proteins in multi-protein complexes linked to the actin-cytoskeleton (13,14). Multiple cellular signaling pathways including calcium, cAMP, and phospholipase C are associated with TJ assembly and regulation of BBB permeability (15). Under normal physiological conditions, the BBB is tightly regulated to be impermeable; however, a variety of mediators such as glutamate, endothelin-1, serotonin, and interleukin-beta, which increase BBB permeability, are released in pathological conditions (12) like ischemic stroke and traumatic brain injury (16). While the physiological and structural properties of BBB have been studied over the past few decades, the disruption of BBB under pathological condition still remains largely unknown.
Role of vascular system in ischemic injury. Like neurons, the energy requirement of BBB is very high to maintain the dynamic transport system for its proper function (17). Therefore the BBB is very susceptible to the ischemic damage even by a short period of ischemic insult. A number of studies using in vitro or in vivo models demonstrated that cerebral ischemia was associated with increased micro vascular permeability (18,19), and TJ proteins were affected by ischemic/hypoxic stimuli resulting in BBB breakdown (20). Impairment of BBB during ischemic damage consequently results in infiltration of local inflammatory cells and post-ischemic edema and swelling (21). The BBB opening is likely to be differently regulated during an early acute phase within several hours (22,23), and in extended period up to 4~5 weeks (24,25) showing ‘biphasic’ pattern following ischemia (26). Understanding of BBB permeability transition is becoming a critical point to prevent and treat ischemic stroke, and also evaluation of toxicological susceptibility of BBB may be important to predict aggravation of ischemic damage.
BBB IMPAIRMENT DURING ISCHEMIC STROKE
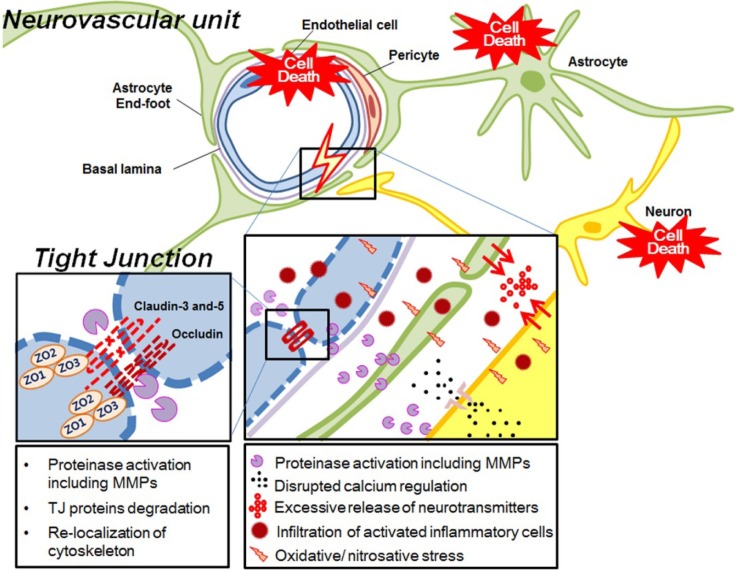
Mechanism of BBB impairment. The exact molecular pathways of BBB disruption has not been fully understood, however, the morphological BBB disruption is closely linked to the degradation/redistribution of TJ proteins and re-organization of cytoskeleton. The most important mediator for TJ alteration is the activation of matrix-metalloproteinase (MMPs) (27,28). MMP-2 and MMP-9 are the main factors inducing BBB disruption by degradation of TJ proteins and basal lamina proteins thereby leading to BBB leakage, although these two enzymes have their unique function in terms of the activation time frame, specific substrates, and the representative contribution to the BBB opening (28). It is well established that the activation level of MMPs are correlated to the infarction volume, stroke severity, and the functional outcome (29). Beside the role of MMPs, the ischemic damage on TJ proteins was also observed by protein expression and re-localization (13,30). Caveolin-1 is found to regulate expression of TJ proteins (31), and redistribute TJ proteins from outer membrane to cytosol (32). Mediators released to neurovascular unit during ischemic periods also involve in the regulation of TJ proteins as found in ZO-1 expression by VEGF (33) and TJ protein change by glutamate (34). Other factors with the onset of ischemia also participate in cerebral EC impairment, contributing to BBB opening. Depletion of ATP, imbalance of ionic homeostasis including calcium, loss of metabolic function with increased acidosis, oxidative/ nitrosative stress signaling and the associated intracellular signaling such as phosphorylation of TJ accessory proteins can subsequently result in BBB disruption (16,26,35,36). Suggested mechanisms underlying ischemic damage in neurovascular units were summarized in Fig. 2.
Fig. 2. Ischemic damage in neurovascular unit. MMP, matrix-metalloproteinase; TJ, tight junction; ZO, zonular occluden.
Implication of BBB disruption in ischemic damage. Along with the concept of ischemic BBB disruption, the importance of this phenomenon has recently revisited. The most direct effect of BBB opening is increased paracelluar permeability and resultant vasogenic edema, contributing to increased mortality (37,38). Besides the edema, early disruption of BBB is suggested as an important risk factor for hemorrhagic damage associated with tPA therapy (39), which is currently the only approved treatment option for acute ischemic stroke (2). Another interesting finding is that alteration of BBB permeability is not spatially limited to the peri-lesional tissue which is around ischemic core, but also expanded to remote functionally connected brain areas (40). Since focal ischemia leads to changes in the overall CBF, the brain metabolism and excitability in remote area can also be affected, ultimately leading to metabolic failure and inflammatory responses (6,41). Consistently, disruption of BBB during brain damage including ischemia is reported to contribute to development of epilepsy (42,43), i.e. epileptogenesis. Based on these observations, maintaining of BBB integrity is currently suggested to be critical in clinical functional outcome following ischemic damage.
ROLE OF BBB IN HEAVY METAL-ASSOCIATED ISCHEMIC BRAIN INJURY
Heavy metal-associated ischemic stroke. Traditional risk factors for cardiovascular diseases (CVD), such as diabetes, hypertension, and the advanced age are associated to stroke (1). Beside these CVD risk factors, several environmental factors for stroke are identified, including high risk behavior such as smoking, high fat diet and poor-nutrition (44). Notably, exposure to certain environmental factors has been suggested to be an emerging risk factor for stroke, and a recent paper reported a significant association of air pollution and stroke risk (45). Accumulating evidences indicate that exposure to toxicological heavy metals such as lead, cadmium, mercury, and arsenic is linked to the cardiovascular risk as well as stroke. Epidemiological cohort studies demonstrated that the incidence of stroke showed significant association with higher lead levels (46-48). These observations are further supported by other studies reporting lead exposure as one of the contributing factors to the incidence of CVDs including stroke (49-52). There was an interesting study on blood or urinary cadmium level was correlated with prevalence of stroke, which was found to be increased by 35% and 9% when 50% of cadmium concentration was increased in blood and urine, respectively (53). In terms of mercury, it is known that occupational exposure to mercury is associated to 1.17 fold increased risk of stroke (54). Consumption of inorganic arsenic through drinking water is also found to be related with increased prevalence of cerebral infarction (55,56). The mechanism of heavy metal-associated CVD risk has been intensively studied. Disturbed cardiovascular homeostasis such as increased thrombosis, higher blood tension, and impaired fibrinolysis can potentially contribute to the onset of ischemic stroke. Nevertheless, the effect of heavy metals on BBB integrity, which is a critical factor determining the outcome of ischemic damage, should be also noted to understand heavy-metal associated ischemic stroke.
BBB injury by heavy metals. Since BBB permeability plays a crucial role in determination of the extent of ischemic brain damage, factors that modify the susceptibility of BBB integrity can alter the overall outcome of ischemic stroke. Hyperglycemia significantly worsened BBB breakdown during ischemic condition, contributing to the aggravation of the consequences of stroke (57), and exogenous excitatory amino acid neurotransmitters disrupted BBB integrity during focal cerebral ischemia (58). Although the studies investigating the effect of heavy metals on BBB integrity are limited, several reports have given insights for heavy metalinduced BBB disruption (59). Acute lead poisoning results in brain swelling with micro vascular damage with TJ opening (60,61), suggesting that BBB is one of the main targets of lead-associated toxicity. Notably, lead showed a higher affinity to CECs, so that it accumulated in higher concentration in CECs than in other brain cells (62), and irreversibly potentiated cytokine- and glutamate-mediated decrease in BBB resistance (63). In vivo exposure to cadmium in drinking water increased the BBB permeability and the concentration of malondialdehyde in brain micro vessels in rats, whereas the activities of anti-oxidant enzymes were significantly decreased (64), suggesting that BBB may be an important target for cadmium toxicity. In CECs, cadmium induced activation of apoptotic signaling (65), and stimulated adhesion molecule expression which is an indicator for BBB injury (66). The CNS damage following mercuryexposure has been largely focused on BBB transport of methyl-mercury (MeHg). While MeHg easily crosses BBB due to its high lipophilicity (67), resulting in encephalopathy, inorganic mercury has been considered less neurotoxic, but BBB breakdown was induced by inorganic mercury (68). Chronic arsenic exposure is also associated with the increased prevalence of micro vascular diseases including neurological diseases (69). Alteration of vascular permeability of arsenic was reported in arsenic-exposed rats (70). Previous observations regarding heavy metal-induced alteration of permeability, especially in BBB, were listed in Table 1. Moreover, recent studies demonstrated that TJ proteins such as ZO-1, 2, 3, occluding and claudins in kidney (71) or in blood-testes barrier (72) were impaired by heavy metal exposure, explaining TJ protein damage as a key factor of heavy metal toxicity. These findings may give a clue for the dysregulation of TJ proteins by heavy metals in BBB permeability.
Table 1.
Impairment of permeability by heavy metals
| Heavy metal | System | Concentration | Exposure duration | Toxicity | Ref | |
|---|---|---|---|---|---|---|
|
| ||||||
| Pb | in vivo | Rats of 5 days age | highest non-lethal dosage (1 mg Pb/g body weight/day) | 2 days | Hemorrhagic encephalopathy, abnormal morphology in capillaries and microvessels, swollen and vacuolated ECs | 62 |
| in vivo | Mice between 10~40 days of age | 2.5 and 5 μg/g (subcutaneous injection) | five injections between 2 and 10 days after birth | Potentiating BBB disruption by lipopolysaccharide, interleukin-6 or glutamate measured by transendothelial electrical resistance | 63 | |
| Cd | in vivo | Rats of 21 days age | 10 ppm | 90 days | Decrease in microvessel antioxidant potential | 64 |
| in vitro | Cerebral endothelial cells (bEnd.3) | 10, 30 and 50 μM | upto 24 hr | Remarkable decrease in cell viability | 65 | |
| in vitro | Cerebral endothelial cells (bEnd.3) | 1, 3 and 10 μM | upto 24 hr | Stimulation of the expression of ICAM-1 | 66 | |
| Hg | in vivo | Adult cats | 6 × 10−5 M solution of mercuric chloride (intracarotid injection) | 30 min | BBB damage examined by Evans blue | 68 |
| As | in vivo | Adult rats | 1.92, 3.85 and 7.70 μM of sodium arsenite (intradermal injection) | upto 60 min | Vascular dysfunction (vascular leakage) determined by Evans blue | 70 |
DISCUSSION
There is increasing evidence that BBB permeability may be an important mediator for brain damage determining the clinical outcome following ischemic stroke, such as edema, inflammation, and epileptogenesis. The loss of BBB integrity is becoming a critical factor that must be considered for accurate evaluation of ischemic stroke. Although the processes governing BBB TJ permeability during ischemic damage are not simple, BBB alteration by heavy metal exposure needs to be further re-visited to understand the mechanism of heavy metal-associated ischemic stroke.
Acknowledgments
This work was supported by the Korea Foundation for the Advancement of Science & Creativity (KOFAC) 2012 URP grant funded by the Korean Government (MEST).
References
- 1.O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., Rangarajan S., Islam S., Pais P., Mcqueen M.J., Mondo C., Damasceno A., Lopez-Jaramillo P., Hankey G.J., Dans A.L., Yusoff K., Truelsen T., Diener H.C., Sacco R.L., Ryglewicz D., Czlonkowska A., Weimar C., Wang X., Yusuf S., Investigators I. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010);376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M. New approaches to neuroprotective drug development. Stroke. (2011);42:S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 3.Savitz S.I., Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann. Neurol. (2007);61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Soliman E.Z., Sorlie P.D., Sotoodehnia N., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. (2012);125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. (2003);4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. (1999);22:391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald J.F., Xiong Z.G., Jackson M.F. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. (2006);29:75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Graham S.H., Chen J. Programmed cell death in cerebral ischemia. J. Cereb. Blood Flow Metab. (2001);21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lo E.H., Moskowitz M.A., Jacobs T.P. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. (2005);36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 10.Green A.R. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br. J. Pharmacol. (2008);153 Suppl 1:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Zoppo G.J. Stroke and neurovascular protection. N. Engl. J. Med. (2006);354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 12.Ballabh P., Braun A., Nedergaard M. The bloodbrain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. (2004);16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Lai C.H., Kuo K.H., Leo J.M. Critical role of actin in modulating BBB permeability. Brain Res. Brain Res. Rev. (2005);50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Wolburg H., Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vasc. Pharmacol. (2002);38:323–337. doi: 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Matter K., Balda M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. (2003);4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins B.T., Davis T.P. The blood-brain barrier/ neurovascular unit in health and disease. Pharmacol. Rev. (2005);57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 17.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. (1977);1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 18.del Zoppo G.J., Mabuchi T. Cerebral microvessel responses to focal ischemia. J. Cereb. Blood Flow Metab. (2003);23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 19.Petty M.A., Wettstein J.G. Elements of cerebral microvascular ischaemia. Brain Res. Brain Res. Rev. (2001);36:23–34. doi: 10.1016/S0165-0173(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 20.Mark K.S., Davis T.P. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. (2002);282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dénes A., Ferenczi S., Kovács K.J. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, bloodbrain barrier damage and brain oedema independently of infarct size. J. Neuroinflammation. (2011);8:164. doi: 10.1186/1742-2094-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahles T., Luedike P., Endres M., Galla H.J., Steinmetz H., Busse R., Neumann-Haefelin T., Brandes R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. (2007);38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 23.Belayev L., Busto R., Zhao W., Ginsberg M.D. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. (1996);739:88–96. doi: 10.1016/S0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 24.Strbian D., Durukan A., Pitkonen M., Marinkovic I., Tatlisumak E., Pedrono E., Abo-Ramadan U., Tatlisumak T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. (2008);153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Abo-Ramadan U., Durukan A., Pitkonen M., Marinkovic I., Tatlisumak E., Pedrono E., Soinne L., Strbian D., Tatlisumak T. Post-ischemic leakiness of the bloodbrain barrier: a quantitative and systematic assessment by Patlak plots. Exp. Neurol. (2009);219:328–333. doi: 10.1016/j.expneurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. (2008);32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg G.A., Estrada E.Y., Dencoff J.E. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. (1998);29:2189–2195. doi: 10.1161/01.STR.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Fernandez M., Bellolio M.F., Stead L.G. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J. Stroke Cerebrovasc. Dis. (2011);20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham L.A., Wetzel M., Rosenberg G.A. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glial. (2005);50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 30.Koto T., Takubo K., Ishida S., Shinoda H., Inoue M., Tsubota K., Okada Y., Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am. J. Pathol. (2007);170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L., Ge S., Pachter J.S. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. (2007);109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Jin X., Liu K.J., Liu W. Matrix metalloproteinase- 2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. (2012);32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer S., Wobben M., Marti H.H., Renz D., Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc. Res. (2002);63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 34.András I.E., Deli M.A., Veszelka S., Hayashi K., Hennig B., Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J. Cereb. Blood Flow Metab. (2007);27:1431–1443. doi: 10.1038/sj.jcbfm.9600445. [DOI] [PubMed] [Google Scholar]
- 35.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. (2008);57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Gasche Y., Copin J.C., Sugawara T., Fujimura M., Chan P.H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. (2001);21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Heo J.H., Han S.W., Lee S.K. Free radicals as triggers of brain edema formation after stroke. Free Radical Biol. Med. (2005);39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg G.A., Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus. (2007);22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 39.Kastrup A., Gröschel K., Ringer T.M., Redecker C., Cordesmeyer R., Witte O.W., Terborg C. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. (2008);39:2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- 40.Krysl D., Deykun K., Lambert L., Pokorny J., Mares J. Perifocal and remote blood-brain barrier disruption in cortical photothrombotic ischemic lesion and its modulation by the choice of anesthesia. J. Physiol. Pharmacol. (2012);63:127–132. [PubMed] [Google Scholar]
- 41.Fagan S.C., Hess D.C., Hohnadel D.C., Pollock D.M., Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. (2004);35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 42.Ivens S., Kaufer D., Flores L.P., Bechmann I., Zumsteg D., Tomkins O., Seiffert E., Heinemann U., Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. (2007);130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 43.Seiffert E., Dreier J.P., Ivens S., Bechmann I., Tomkins O., Heinemann U., Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. (2004);24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernal-Pacheco O., Roman G.C. Environmental vascular risk factors: new perspectives for stroke prevention. J. Neurol. Sci. (2007);262:60–70. doi: 10.1016/j.jns.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 45.Mateen F.J., Brook R.D. Air pollution as an emerging global risk factor for stroke. JAMA. (2011);305:1240–1241. doi: 10.1001/jama.2011.352. [DOI] [PubMed] [Google Scholar]
- 46.Kromhout D. Blood lead and coronary heart disease risk among elderly men in Zutphen, The Netherlands. Environ. Health Perspect. (1988);78:43–46. doi: 10.1289/ehp.887843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott P., Arnold R., Cockings S., Eaton N., Järup L., Jones J., Quinn M., Rosato M., Thornton I., Toledano M., Tristan E., Wakefield J. Risk of mortality, cancer incidence, and stroke in a population potentially exposed to cadmium. Occup. Environ. Med. (2000);57:94–97. doi: 10.1136/oem.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moller L., Kristensen T.S. Blood lead as a cardiovascular risk factor. Am. J. Epidemiol. (1992);136:1091–1100. doi: 10.1093/oxfordjournals.aje.a116574. [DOI] [PubMed] [Google Scholar]
- 49.Schober S.E., Mirel L.B., Graubard B.I., Brody D.J., Flegal K.M. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ. Health Perspect. (2006);114:1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navas-Acien A., Selvin E., Sharrett A.R., Calderon-Aranda E., Silbergeld E., Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. (2004);109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 51.Menke A., Muntner P., Batuman V., Silbergeld E.K., Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. (2006);114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 52.Lustberg M., Silbergeld E. Blood lead levels and mortality. Arch. Intern. Med. (2002);162:2443–2449. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 53.Peters J.L., Perlstein T.S., Perry M.J., Mcneely E., Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ. Res. (2010);110:199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García Gómez M., Boffetta P., Caballero Klink J.D., Español S., Gómez Quintana J. [Cardiovascular mortality in mercury miners]. Med. Clin. (Barcelona) (2007);128:766–771. doi: 10.1157/13106327. [DOI] [PubMed] [Google Scholar]
- 55.Lisabeth L.D., Ahn H.J., Chen J.J., Sealy-Jefferson S., Burke J.F., Meliker J.R. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke. (2010);41:2499–2504. doi: 10.1161/STROKEAHA.110.585281. [DOI] [PubMed] [Google Scholar]
- 56.Chiou H.Y., Huang W.I., Su C.L., Chang S.F., Hsu Y.H., Chen C.J. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. (1997);28:1717–1723. doi: 10.1161/01.STR.28.9.1717. [DOI] [PubMed] [Google Scholar]
- 57.Dietrich W.D., Alonso O., Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke. (1993);24:111–116. doi: 10.1161/01.STR.24.1.111. [DOI] [PubMed] [Google Scholar]
- 58.Chi O.Z., Hunter C., Liu X., Weiss H.R. Effects of exogenous excitatory amino acid neurotransmitters on blood-brain barrier disruption in focal cerebral ischemia. Neurochem. Res. (2009);34:1249–1254. doi: 10.1007/s11064-008-9902-7. [DOI] [PubMed] [Google Scholar]
- 59.Zheng W., Aschner M., Ghersi-Egea J.F. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. (2003);192:1–11. doi: 10.1016/S0041-008X(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldstein G.W., Asbury A.K., Diamond I. Pathogenesis of lead encephalopathy. Uptake of lead and reaction of brain capillaries. Arch. Neurol. (1974);31:382–389. doi: 10.1001/archneur.1974.00490420048005. [DOI] [PubMed] [Google Scholar]
- 61.Struzyñska L., Walski M., Gadamski R., Dabrowska-Bouta B., Rafalowska U. Lead-induced abnormalities in blood-brain barrier permeability in experimental chronic toxicity. Mol. Chem. Neuropathol. (1997);31:207–224. doi: 10.1007/BF02815125. [DOI] [PubMed] [Google Scholar]
- 62.Toews A.D., Kolber A., Hayward J., Krigman M.R., Morell P. Experimental lead encephalopathy in the suckling rat: concentration of lead in cellular fractions enriched in brain capillaries. Brain Res. (1978);147:131–138. doi: 10.1016/0006-8993(78)90777-1. [DOI] [PubMed] [Google Scholar]
- 63.Dyatlov V.A., Platoshin A.V., Lawrence D.A., Carpenter D.O. Lead potentiates cytokine- and glutamatemediated increases in permeability of the blood-brain barrier. Neurotoxicology. (1998);19:283–291. [PubMed] [Google Scholar]
- 64.Shukla A., Shukla G.S., Srimal R.C. Cadmiuminduced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum. Exp. Toxicol. (1996);15:400–405. doi: 10.1177/096032719601500507. [DOI] [PubMed] [Google Scholar]
- 65.Jung Y.S., Jeong E.M., Park E.K., Kim Y.M., Sohn S., Lee S.H., Baik E.J., Moon C.H. Cadmium induces apoptotic cell death through p38 MAPK in brain microvessel endothelial cells. Eur. J. Pharmacol. (2008);578:11–18. doi: 10.1016/j.ejphar.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 66.Jeong E.M., Moon C.H., Kim C.S., Lee S.H., Baik E.J., Moon C.K., Jung Y.S. Cadmium stimulates the expression of ICAM-1 via NF-kappaB activation in cerebrovascular endothelial cells. Biochem. Biophys. Res. Commun. (2004);320:887–892. doi: 10.1016/j.bbrc.2004.05.218. [DOI] [PubMed] [Google Scholar]
- 67.Clarkson T.W. Mercury: major issues in environmental health. Environ. Health Perspect. (1993);100:31–38. doi: 10.1289/ehp.9310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson E.W., Cardoso E.R. The blood-brain barrier following experimental subarachnoid hemorrhage. Part 2: Response to mercuric chloride infusion. J. Neurosurg. (1983);58:345–351. doi: 10.3171/jns.1983.58.3.0345. [DOI] [PubMed] [Google Scholar]
- 69.Chiou J.M., Wang S.L., Chen C.J., Deng C.R., Lin W., Tai T.Y. Arsenic ingestion and increased microvascular disease risk: observations from the south-western arseniasis-endemic area in Taiwan. Int. J. Epidemiol. (2005);34:936–943. doi: 10.1093/ije/dyi108. [DOI] [PubMed] [Google Scholar]
- 70.Chen S.C., Tsai M.H., Wang H.J., Yu H.S., Chang L.W. Vascular permeability alterations induced by arsenic. Hum. Exp. Toxicol. (2004);23:1–7. doi: 10.1191/0960327104ht407oa. [DOI] [PubMed] [Google Scholar]
- 71.Reyes J.L., Molina-Jijón E., Rodríguez-Muñoz R., Bautista-García P., Debray-García Y., Namorado Mdel C. Tight junction proteins and oxidative stress in heavy metalsinduced nephrotoxicity. Biomed. Res. Int. (2013);2013:730789. doi: 10.1155/2013/730789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong E.W., Cheng C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. (2011);32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]