Abstract
In-utero exposure to valproic acid (VPA) has been known as a potent inducer of autism spectrum disorder (ASD), not only in humans, but also in animals. In addition to the defects in communication and social interaction as well as repetitive behaviors, ASD patients usually suffer from gastrointestinal (GI) problems. However, the exact mechanism underlying these disorders is not known. In this study, we examined the gross GI tract structure and GI motility in a VPA animal model of ASD. On embryonic day 12 (E12), 4 pregnant Sprague-Dawley (SD) rats were subcutaneously injected with VPA (400 mg/kg) in the treatment group, and with phosphate buffered saline (PBS) in the control group; the resulting male offspring were analyzed at 4 weeks of age. VPA exposure decreased the thickness of tunica mucosa and tunica muscularis in the stomach and ileum. Other regions such as duodenum, jejunum, and colon did not show a significant difference. In high-resolution microscopic observation, atrophy of the parietal and chief cells in the stomach and absorptive cells in the ileum was observed. In addition, decreased staining of the epithelial cells was observed in the hematoxylin and eosin (H&E)-stained ileum section. Furthermore, decreased motility in GI tract was also observed in rat offspring prenatally exposed to VPA. However, the mechanism underlying GI tract defects in VPA animal model as well as the association between abnormal GI structure and function with ASD is yet to be clearly understood. Nevertheless, the results from the present study suggest that this VPA ASD model undergoes abnormal changes in the GI structure and function, which in turn could provide beneficial clues pertaining to the pathophysiological relevance of GI complications and ASD phenotypes.
Keywords: Valproic acid, ASD, GI motility, GI thickness, Epithelial cells
INTRODUCTION
Valproic acid (VPA) is an anticonvulsant and mood stabilizer widely used in clinical situation. However, the use of VPA during pregnancy has raised many concerns due to the possibility of teratogenic outcomes. Prenatal exposure of VPA has been reported as a potent teratogen, which causes abnormal brain development. The adverse consequences of prenatal exposure to VPA is collectively called fetal valproate syndrome. Recently, the link between prenatal VPA exposure and manifestation of autism spectrum disorder (ASD) has been acknowledged by many researchers (1,2). In addition to human, animals prenatally exposed to VPA showed autistic behaviors, which made it one of the versatile animal models of ASD (3,4).
Although ASD is characterized by three core symptoms including impairment in social interaction, communication, and restricted/repetitive behavior, gastrointestinal problem has also commonly reported in ASD patients as a comorbid condition (5). The affected individuals show diverse symptoms including diarrhea, constipation, bloating, gas, and history of reflux (6,7). In ASD patients, gastrointestinal (GI) complications such as gastrointestinal inflammation, lymphoid- nodular hyperplasia (LNH), increased intestinal permeability, inactive disaccharidase enzyme, dysbiosis, and food intolerance are relatively common. Furthermore, GI problems can also be misrecognized as non-GI problems such as disturbed sleep disorder (8,9).
Although, hypotheses on the linkage between ASD and GI problem have been presented in many different aspects (10-12), the mechanism leading to the dysregulated GI function in the substantial proportion of ASD patients is not clear yet. Mainly due to the difficulties in studying GI tract problems in ASD patients and lack of alternative study model, in-depth study to find out the relevance between ASD and GI tract problem is not an easy task. Therefore, extrapolating study from ASD animal models can be useful to understand the mechanistic link between ASD and GI disturbance in human. These approaches may also provide opportunities to figure out the common mechanism regulating gut and brain development as well as targeting the biological effects of environmental, medical and chemical toxicants.
VPA animal model of ASD suffice face and construct validity in that it shows all three core symptoms of ASD both in human and rodents. Mechanistically, VPA animal model shows dysregulated differentiation of cortical neurons especially, glutamatergic and GABAergic neuron. The altered glutamatergic and GABAergic differentiation is one of the common underlying mechanism observed human as well as both genetic and environmental animal model of ASD. In addition to the core symptoms, we reported increased seizure susceptibility of VPA animal model similar to human ASD patients (3). ASD showed gender differences in prevalence rate, which skewed high to male over female (13). We also reported that VPA model of ASD show male gender preference (14). These results suggest that VPA animal model of ASD is an ideal model system to investigate the possible GI complications in ASD.
In this study, we examined whether prenatal VPA exposure induces abnormal anatomical structure of GI tract in rat offspring and compromises general GI function as measured by the GI motility.
MATERIALS AND METHODS
Materials. Methylcellulose (Cat No. M0262), paraformaldehyde (PFA, Cat No. 158127), VPA sodium salt (Cat No. P4543), metoclopramide (Cat No. M0763), were purchased from Sigma Aldrich (MO, U.S.). RevertAid First Strand cDNA Syntheis Kit was purchased from Thermo Scientific (MA, U.S.). SolGent™ Taq DNA Polymerase was purchased from Solgent (Daejeon, Korea). All other reagents were purchased from Sigma and were the reagent grade or above.
Animals. Timed pregnant Sprague-Dawley (SD) rats were purchased from OrientBio (Kyungkido, Korea). And rats were maintained on a standard light-dark cycle, at ambient temperature (22 ± 2℃) and humidity (55 ± 5%) with free access to chow pellets and water. Prenatal exposure of VPA was performed as previously reported (3). VPA (400 mg/kg) was injected to pregnant rats subcutaneously at embryonic day 12 (E12). PBS was injected for control group. Each group contained four pregnant rats and those were housed individually or with respective offspring till the end of experiment. For the experiment, only male offspring were used from each dam and the remaining female offspring was euthanized by ether anesthesia. Animal treatments including anesthesia, euthanasia and administration were carried out in accordance with the Principle of Laboratory Animal Care (NIH publication No. 85-23, revised 1985) and were approved by Animal Care and Use Committee of Konkuk University, Korea (KU12115). All efforts were made to minimize the number of animals as well as their suffering.
Tissue preparation. Animals were anesthetized using ether and perfused and fixed with 4% PFA. Stomach, small intestine and colon were extracted and containments were removed by washing with saline. Extracted intestine were tied and filled with 4% PFA and immersed in it for 18 hrs at 4℃. Fixed samples were washed and dissected into five parts; upper body portion of stomach, duodenum, jejunum, ileum and colon. Those parts were treated in common procedure and embed in paraffins. Each embedded sample were sliced (5 μm) and stained by hematoxylin and eosin (H&E) staining method.
Thickness measurement. Coronal section of stomach (×40), small intestine and large intestine (×100) were taken by 2048 × 1546-pixel digital CCD camera (DP70, Olympus, Tokyo, Japan). The thickness of tunica mucosa and tunica muscularis was measured using analysis TS AUTO software. Perpendicular distance was measured from luminal surface to muscularis mucosa for the determination of tunica mucosa thickness and from inner muscle layer to external muscle layer for tunica muscularis thickness. In each field, thickness of tunica mucosa and tunica muscularis was measured in 6 part at regular intervals.
RT-PCR. Colon, stomach and ileum were homogenized with PBS. Total RNA was isolated using Trizol reagent (Invitrogen, CA, USA) and 1 mg of total RNA was converted to cDNA, according to the manufacturer’s instructions. Specific DNA bands were amplified by PCR. The amplified DNA products were resolved by 1.0% agarose gel electrophoresis and visualized by staining with ethidium bromide and exposed to Bio-Rad (CA, U.S.) electrophoresis image analyzer. The primers used in this analysis are:
ACTA2, 5' TGACTGAGCGTGGCTATTCC (forward)
5' TCAGCAATGCCTGGGTACAT (reverse)
KRT7, 5' GCGTGCCAAGTTAGAGTCCA (forward)
5' CCCCTGCGGGTAGTAGATGT (reverse)
GAPDH, 5' TCCCTCAAGATTGTCAGCAA (forward)
5' AGATCCACAACGGATACATT (reverse).
GI tract motility test. This test was performed using a method previously reported (15). Briefly, rats were fasted for 6 hrs and 1 ml of a semiliquid nonnutrient dye (Evans blue 50 mg/ml dissolved in 0.5% methylcellulose) was administrated via oral gavage. After 30 minutes, rats were euthanized by ether anesthesia. The abdomen was cut off and the small intestine was carefully removed. Total length of GI tract was measured form pyloric sphincter to the ileocecal junction and the distance travelled by the dye was recorded. The GI transit index is defined as (distance the marker traveled/total length of intestine) × 100 and is reported as %. To validate our study, metoclopramide (3 mg/kg) was used as a positive control to increase GI motility.
Statistical analysis. Data were expressed as the mean ± standard error of mean (S.E.M) and analyzed for statistical significance using one-way analysis of variance followed by Newman-Keuls test as a post-hoc test. Differences were considered statistically significant when the P value was less than 0.05 (p < 0.05). All statistical analyses were conducted using GraphPad Prism 5 (CA, U.S.).
RESULTS
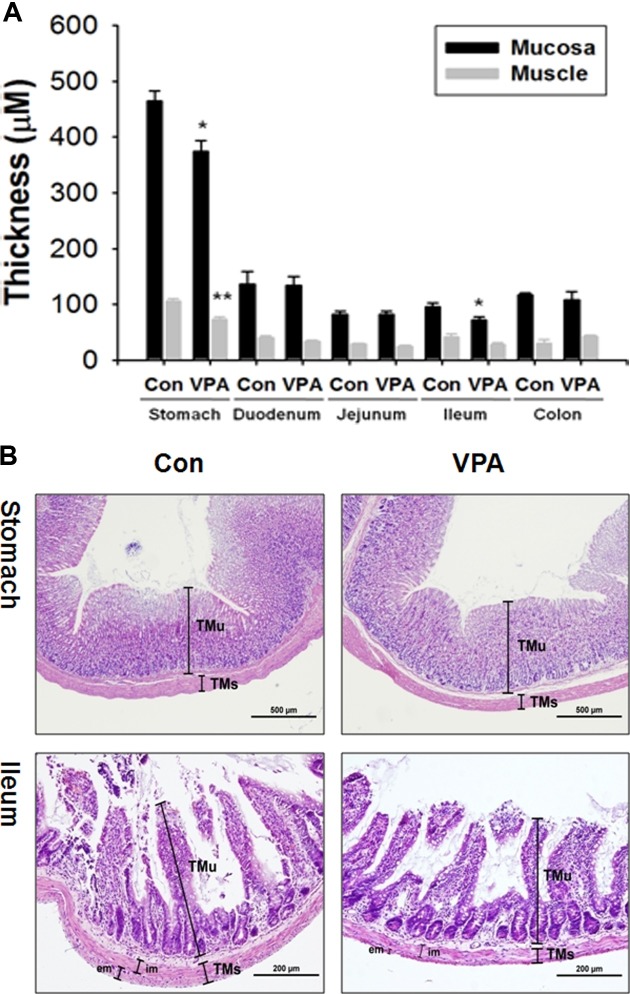
Gastrointestinal mucosa and muscle layer shrinkage in VPA-induced autistic animal model. To identify gastrointestinal impairment in VPA-induced autistic animal model, stomach, duodenum, jejunum, ileum, and colon thickness of 4 weeks old male rat offspring was measured after H &E staining. Tunica mucosa (TMu) and tunica muscularis (TMs) thickness is significantly decreased in the stomach and ileum of VPA animal model (Fig. 1A). In the H&E staining picture (Fig. 1B), thin mucosa and muscle was evident both in stomach and ileum. Especially, the thickness of external part of muscle is markedly decreased in ileum. The epithelial thickness of colon in VPA exposed rat offspring was slightly decreased but there was no statistical significance.
Fig. 1. Thickness changes in gastrointestinal mucosa and muscle layers in VPA animal model of ASD. A. Thickness of mucosa and muscle was measured in the stomach, duodenum, jejunum, ileum and colon by analySIS TS AUTO software. Thickness of mucosa and muscle were measured from luminal surface to muscularis mucosa and from luminal surface to external muscle layer each. Thickness was measured in total six areas per sample with fixed interval (*p<0.05, ** p< 0.01. N = 4) B. Stomach and ileum section was stained by H&E. Picture of stomach and ileum was taken with 40x and 100x resolution, respecitively using 2048x1536-pixel digital CCD camera (DP70, Olympus, Tokyo, Japan) (TMu: Tunica Mucosa, TMs: Tunica Muscularis, im: Tunica Musccularis interna, em: Tunica Muscularis externa).
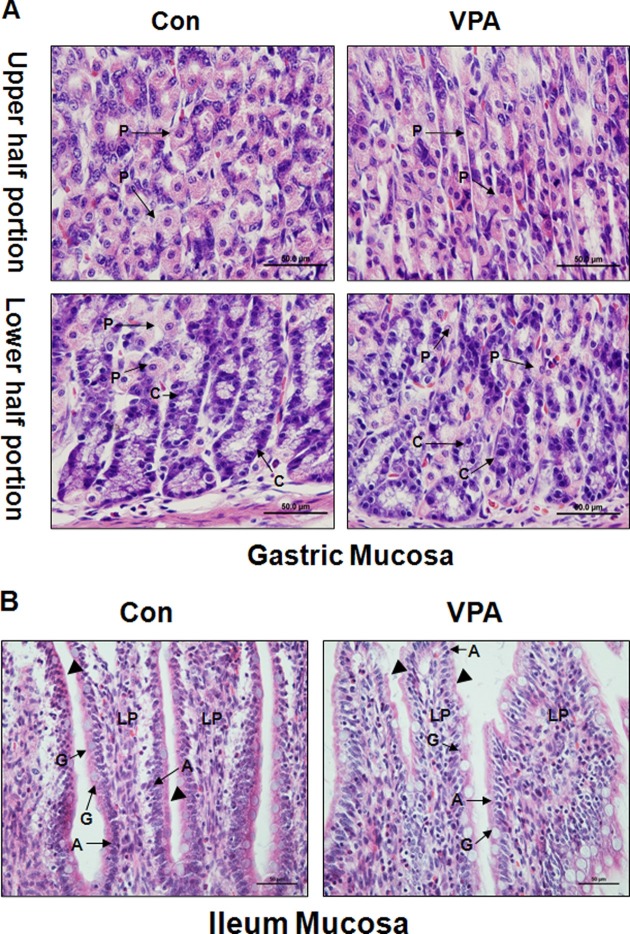
Abnormal morphologies in the gastrointestinal epithelial cell in VPA-induced autistic animal model. To specify gastrointestinal impairment in prenatally VPAexposed rat offspring, epithelial cells of stomach and ileum was observed in high resolution (400×). In the stomach, parietal cell (P) (upper and lower half portion) and chief cell (C) (lower half portion) showed atrophy and weak cytoplasm staining in VPA animal model. Especially, arrangement of chief cell was distracted compared to control animal (lower half portion) (Fig. 2A).
Fig. 2. Morphological changes in gastrointestinal mucosa of VPA model of ASD. H&E stained gastric and ileac mucosa was taken with 400× resolution to observe epithelial cell. A. Gastric epithelial cell was taken in the upper portion and lower portion of stomach. Morphological changes of parietal cell(P) and chief cell(C) were observed. B. Ileac epithelial cells including absorptive cell(A), goblet cell(G) and lamina propria were observed. Striated border line was indicated by arrowheads.
In the ileum mucosa, goblet cell(G), absorptive cell(A), and lamina propria were observed. Cellular atrophy and decreased stainability for H&E was observed in columar absorptive cell of VPA animal model. Striated border line was not clear or thinner than control group (Arrowhead) (Fig. 2B).
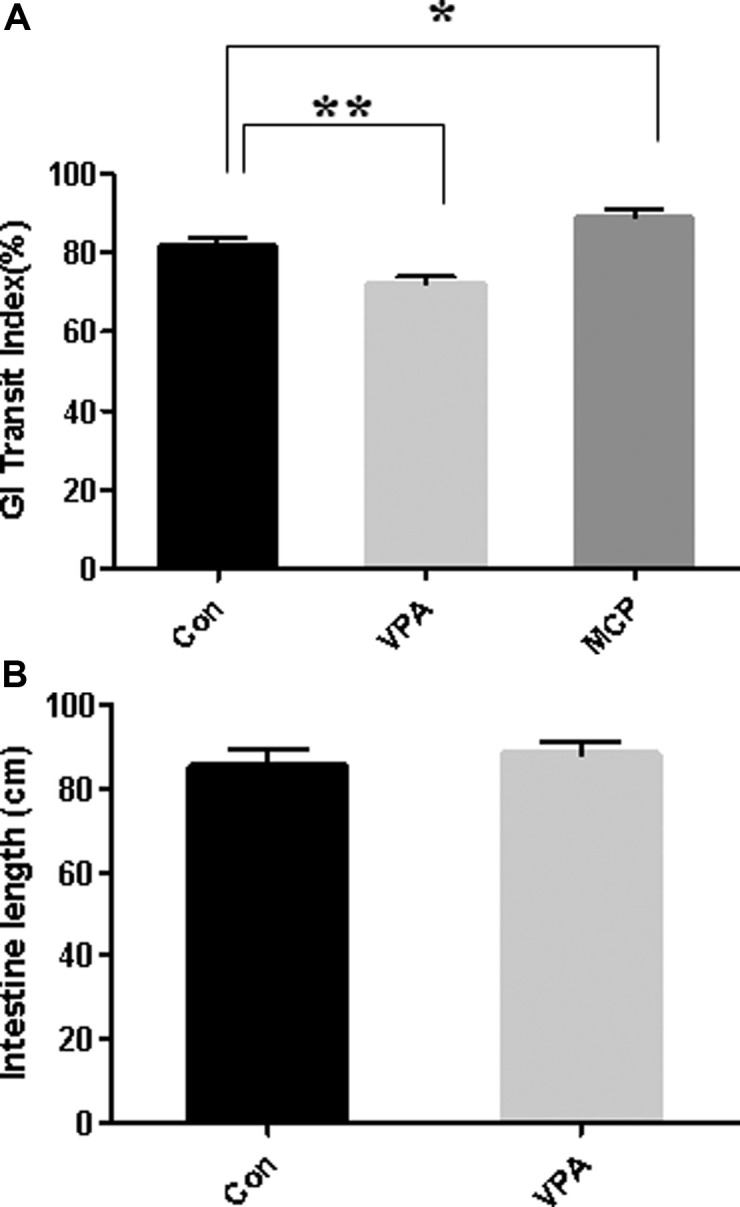
Impairment of gastrointestinal motility of rat offspring prenatally exposed to VPA. To investigate whether the anatomical changes in thickness of stomach and ileum is linked to their functional deficit, we investigated GI tract motility. GI tract motility was monitored by measuring the movement of Evans Blue dye along the entire GI tract as described in methods. Metoclopramide (MCP) was used as a positive control to increase GI motily (16). While metoclopramide treatment to control rats significantly increased GI transit index (MCP:88.8% ± 7.0, Con:81.7% ± 7.7, P < 0.05), the GI transit index in VPA treated group was significantly decreased (VPA:72.0% ± 7.0, Con:81.7% ± 7.7) as compared with control suggesting the impaired GI tract function (Fig. 3A). But total length of intestine was not significantly different in the control and VPA animal (Fig. 3B).
Fig. 3. Gastrointestinal motility test. After fasted for 6 hrs, rats were administered with Evans Blue via oral gavage (50mg/ml suspended in 0.5% methyl cellulose). Dye traveled distance was measured (cm) from the pyloric sphincter to the ileocecal junction. GI transit index is defined as (dye moved distance/total length of intestine) × 100(%). Metoclopramide (MCP) was used as positive control for the study (*p<0.05, **p<0.01. N = 12~15).
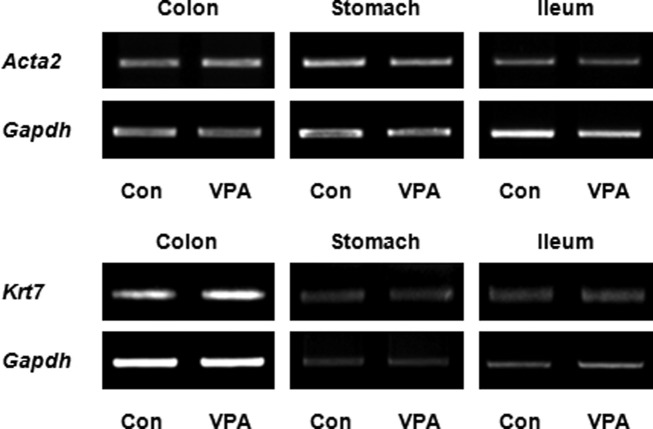
ACTA2 and KRT7 mRNA were not significantly changed in colon, stomach, and ileum. As an effort to identify the molecular changes associated with altered gastrointestinal thickness, we investigated the expression of ACTA2, a smooth muscle marker gene encoding alpha-smooth muscle actin, and KRT7, an epithelium marker encoding cytokeratin-7, by RT-PCR. In all the tissue examined, i.e. stomach, ileum and colon, the mRNA expressions of ACTA2 and KRT7 normalized to GAPDH expression were not significantly changed by prenatal exposure to VPA (Fig. 4) These result suggested that cytoskeletal protein expression of ACTA2 and KRT7 was not related to the decreased thickness of gastrointestinal muscle and mucosa layer.
Fig. 4. ACTA2 and KRT7 mRNA expression in colon, stomach and ileum. ACTA2 and KRT7 mRNA expression was identified by RT-PCR in the colon, stomach and ileum. Each tissue was homogenized and RNA was isolated using trizol. The data are representative of three independent experiments.
DISCUSSION
Autism patient usually suffer from GI disturbance including gastroesophageal reflux disease (GERD), diarrhea, constipation, bloating, abdominal pain, discomfort, gas and so on (6). However, the causal relationship and relevance between GI tract disturbance and ASD is under debate. In this study, we found abnormal GI tract motility and structural impairment in prenatal VPA-exposure model of ASD.
In the stomach and ileum, the thickness of tunica muscosa and tunica muscularis was significantly decreased. The decreased thickness may result in increased permeability and vulnerability to external attacks. Mucous layer is primary immunological barrier against external invasion. Reduced thickness of the gastric mucous layer and decreased hexosamine contents in the mucus gel layer were observed in the toxicant induced gastritis animal model. Consequently, the decreased thickness of mucosa increased vulnerability to external attacks (17). Increased gut permeability has been associated as one of the pathophysiological factors underlying ASD. Intestinal permeability was measured in 21 ASD children and nine of them showed altered permeability (18). These results were hypothesized as the leaky gut theory. In this theory, intestinal mucosa is abnormally permeable in ASD. Food intake containing gluten or casein causes influx of neuroactive metabolites such as casomorphins and gliadomorphins, known as potent psychosis-inducing factors, into brain via permeable intestine and cerebrospinal fluid in the ASD (19). However, linkage between permeability change and prognosis of ASD is not clear yet. Further studies should be performed to derive the conclusion whether the decreased thickness and permeability changes has causal relationship with ASD behaviors. In this regard, GI function and abnormalities of VPA animal model should be examined not only at 4 weeks of age but also at earlier time points of development.
We also found decreased thickness of tunica muscularis and GI motility in the VPA- induced animal model of ASD. Tunica muscularis is composed of smooth muscle cells and muscle fibers which plays a major role in autonomously regulating contractility of intestine. Decreased thickness might arise from the reduced cell number of smooth muscle cell or smooth muscle fibers and this might underlie decreased motility of GI tract in VPA animal model. Although we did not find the reason for the reduced thickness, growth factor mediated proliferation and differentiation of enterocyte might be responsible for the effects. Hepatocyte growth factor (HGF) which is expressed broadly in GI tract mediates gastric and intestinal epithelial cell proliferation and migration (20). HGF usually induced not only in an experimental model of colitis but also in human gastric ulcer, suggesting that up-regulated HGF played a role in healing responses in inflammatory condition of GI tract (20,21). HGF ameliorates gastroenteritis via interaction with its receptor, c-MET. HGF/c-MET signaling has been suggested as a potential therapeutic target for gastroenteritis (22). Interestingly, linkage between HGF/c-MET and ASD was reported in the previous study. The reduced HGF expression in the serum was observed in ASD patients who have severe GI tract problems (23). Furthermore, a significant polymorphism in the receptor tyrosine kinase MET promoter region was observed in ASD patient group having GI problem in a genetic analysis study (24). MET signaling activate N-mehtyl-D-aspartate (NMDA) mediated receptor function and increase glutamatergic synapse formation and post synaptic protein clustering in mature neuron (25-27). In addition, MET signaling played an important role in epithelial cell proliferation in GI tract system and activated MET signaling by exogenous HGF reduced intestinal inflammation and abnormal intestinal function (28). Whether HGF/MET signaling pathways modulate CNS and GI tract development in common in VPA animal model should be investigated further in the future.
Not much study about gastrointestinal histological abnormalities in ASD has been performed. Horvath et al. reported that reflux esophagitis, chronic gastritis and paneth’s cell hyperplasia was observed in ASD children in a study using endoscopy (6). Another endoscopic study also showed lymphonodular hyperplasia in ASD children, especially in ileum (29). These reports suggest that hyperplasia is commonly observed both in paneth’s cell and lymphonodule that are connected with immune function, which is consistent with the observed inflammation in GI tract although it did not have linkage with helicobacter pylori infection (30). In our histological study, parietal cell and chief cell in stomach and absorptive cell in intestine showed atrophy and decreased stainability in cytoplasm. These results suggest that cell activity was decreased compared to control and it may induce decreased digestive reaction. Parietal cell secretes gastric acid and chief cell release zymogen pepsinogen to digest food in the stomach. Intestinal absorptive cell uptakes nutrient and water from food and secretes immunoglobulin to protect from immunological attack. These cells are very important in digestive process and immune function. Therefore, malfunction or decreased number of these cells may results in decreased GI motility and digestive problem.
In this study, we reported structural defect of mucosa and muscle in ileum and stomach of VPA animal model of ASD, which may be linked to the impaired GI motility in VPA animal model. Although the exact mechanism and relevance between ASD and GI motility could not be identified here, VPA animal model may provide a versatile model for an in depth study of GI tract problem in ASD. For example, we and others reported abnormal glutamatergic neuronal differentiation from neural progenitors cells by exposure to VPA during developmental periods both in vivo and in vitro. Whether prenatal VPA exposure modulates enteric nervous system differentiation from enteric stem cell would be one such interesting topic to be investigated in the future.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of health & welfare, Republic of Korea (No. A120029).
References
- 1.Lindhout D., Schmidt D. In-utero exposure to valproate and neural tube defects. Lancet. (1986);1:1392–1393. doi: 10.1016/S0140-6736(86)91711-3. [DOI] [PubMed] [Google Scholar]
- 2.Williams G., King J., Cunningham M., Stephan M., Kerr B., Hersh J.H. Fetal valproate syndrome and autism: additional evidence of an association. Dev. Med. Child. Neurol. (2001);43:202–206. [PubMed] [Google Scholar]
- 3.Kim K.C., Kim P., Go H.S., Choi C.S., Yang S.I., Cheong J.H., Shin C.Y., Ko K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol. Lett. (2011);201:137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Schneider T., Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. (2005);30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 5.Valicenti-McDermott M., McVicar K., Rapin I., Wershil B.K., Cohen H., Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J. Dev. Behav. Pediatr. (2006);27:S128–S136. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- 6.Horvath K., Papadimitriou J.C., Rabsztyn A., Drachenberg C., Tildon J.T. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. (1999);135:559–563. doi: 10.1016/S0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 7.Wasilewska J., Jarocka-Cyrta E., Kaczmarski M. [Gastrointestinal abnormalities in children with autism]. Pol. Merkuriusz Lek. (2009);27:40–43. [PubMed] [Google Scholar]
- 8.Wakefield A.J., Murch S.H., Anthony A., Linnell J., Casson D.M., Malik M., Berelowitz M., Dhillon A.P., Thomson M.A., Harvey P., Valentine A., Davies S.E., Walker-Smith J.A. Ileal-lymphoid-nodular hyperplasia, nonspecific colitis, and pervasive developmental disorder in children. Lancet. (1998);351:637–641. doi: 10.1016/S0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 9.Horvath K., Perman J.A. Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr. (2002);14:583–587. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Sousa I., Clark T.G., Toma C., Kobayashi K., Choma M., Holt R., Sykes N.H., Lamb J.A., Bailey A.J., Battaglia A., Maestrini E., Monaco A.P. MET and autism susceptibility: family and case-control studies. Eur. J. Hum. Genet. (2009);17:749–758. doi: 10.1038/ejhg.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwood P., Anthony A., Pellicer A.A., Torrente F., Walker-Smith J.A., Wakefield A.J. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J. Clin. Immunol. (2003);23:504–517. doi: 10.1023/B:JOCI.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- 12.Kang D.W., Park J.G., Ilhan Z.E., Wallstrom G., Labaer J., Adams J.B., Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. (2013);8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron-Cohen S. The extreme male brain theory of autism. Trends Cognit. Sci. (2002);6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.C., Kim P., Go H.S., Choi C.S., Park J.H., Kim H.J., Jeon S.J., Dela Pena I.C., Han S.H., Cheong J.H., Ryu J.H., Shin C.Y. Male-specific alteration in excitatory post-synaptic development and social interaction in prenatal valproic acid exposure model of autism spectrum disorder. J. Neurochem. (2013);124:832–843. doi: 10.1111/jnc.12147. [DOI] [PubMed] [Google Scholar]
- 15.Mittelstadt S.W., Hemenway C.L., Spruell R.D. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J. Pharmacol. Toxicol. Methods. (2005);52:154–158. doi: 10.1016/j.vascn.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Marathe P.H., Wen Y., Norton J., Greene D.S., Barbhaiya R.H., Wilding I.R. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br. J. Clin. Pharmacol. (2000);50:325–332. doi: 10.1046/j.1365-2125.2000.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi A., Kumar M.M., Bhawani G., Chaturvedi H., Kumar M., Goel R.K. Effect of ethanolic extract of Eugenia jambolana seeds on gastric ulceration and secretion in rats. Indian J. Physiol. Pharmacol. (2007);51:131–140. [PubMed] [Google Scholar]
- 18.D’Eufemia P., Celli M., Finocchiaro R., Pacifico L., Viozzi L., Zaccagnini M., Cardi E., Giardini O. Abnormal intestinal permeability in children with autism. Acta Paediatr. (1996);85:1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindstrom L.H., Nyberg F., Terenius L., Bauer K., Besev G., Gunne L.M., Lyrens S., Willdeck-Lund G., Lindberg B. CSF and plasma beta-casomorphin-like opioid peptides in postpartum psychosis. Am. J. Psychiatry. (1984);141:1059–1066. doi: 10.1176/ajp.141.9.1059. [DOI] [PubMed] [Google Scholar]
- 20.Dignass A.U., Lynch-Devaney K., Podolsky D.K. Hepatocyte growth factor/scatter factor modulates intestinal epithelial cell proliferation and migration. Biochem. Biophys. Res. Commun. (1994);202:701–709. doi: 10.1006/bbrc.1994.1987. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno M., Shiota G., Umeki K., Kawasaki H., Kojo H., Miura K. Induction of plasma hepatocyte growth factor in acute colitis of mice. Inflammation Res. (1997);46:166–167. doi: 10.1007/s000110050161. [DOI] [PubMed] [Google Scholar]
- 22.Ido A., Numata M., Kodama M., Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J. Gastroenterol. (2005);40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- 23.Russo A.J., Krigsman A., Jepson B., Wakefield A. Decreased Serum Hepatocyte Growth Factor (HGF) in Autistic Children with Severe Gastrointestinal Disease. Biomarker Insights. (2009);4:181–190. doi: 10.4137/bmi.s3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell D.B., Buie T.M., Winter H., Bauman M., Sutcliffe J.S., Perrin J.M., Levitt P. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. (2009);123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- 25.Akimoto M., Baba A., Ikeda-Matsuo Y., Yamada M.K., Itamura R., Nishiyama N., Ikegaya Y., Matsuki N. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. (2004);128:155–162. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Tyndall S.J., Walikonis R.S. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle. (2006);5:1560–1568. doi: 10.4161/cc.5.14.2918. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez H., Dolcet X., Tolcos M., Davies A. HGF regulates the development of cortical pyramidal dendrites. Development. (2004);131:3717–3726. doi: 10.1242/dev.01209. [DOI] [PubMed] [Google Scholar]
- 28.Ido A., Numata M., Kodama M., Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J. Gastroenterol. (2005);40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield A.J., Anthony A., Murch S.H., Thomson M., Montgomery S.M., Davies S., O’Leary J.J., Berelowitz M., Walker-Smith J.A. Enterocolitis in children with developmental disorders. Am. J. Gastroenterol. (2000);95:2285–2295. doi: 10.1111/j.1572-0241.2000.03248.x. [DOI] [PubMed] [Google Scholar]
- 30.Torrente F., Anthony A., Heuschkel R.B., Thomson M.A., Ashwood P., Murch S.H. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. Am. J. Gastroenterol. (2004);99:598–605. doi: 10.1111/j.1572-0241.2004.04142.x. [DOI] [PubMed] [Google Scholar]