Abstract
The anti-inflammatory effects of glycosaminoglycan (GAG) derived from Isaria sinclairii (IS) and of IS extracts were investigated in a complete Freund’s adjuvant (CFA)-treated chronic arthritis rat model. Groups of rats were treated orally with 30 mg/kg one of the following: [1] saline control, extracts of [2] water-IS, [3] methanol-IS, [4] butanol-IS, [5] ethyl acetate-IS, or [6] Indomethacin® as the positive control for a period of two weeks. The anti-paw edema effects of the individual extracts were in the following order: water-IS ex. > methanol ex. > butanol ex. > ethyl acetate ex. The water/methanol extract from I. sinclairii remarkably inhibited UV-mediated upregulation of NF-κB activity in transfected HaCaT cells. GAG as a water-soluble alcohol precipitated fraction also produced a noticeable anti-edema effect. This GAG also inhibited the pro-inflammatory cytokine levels of prostaglandin E2-stimulated lipopolysaccharide in LAW 264.7 cells, cytokine TNF-α production in splenocytes, and atherogenesis cytokine levels of vascular endothelial growth factor (VEGF) production in HUVEC cells in a dose-dependent manner. In the histological analysis, the LV dorsal root ganglion, including the articular cartilage, and linked to the paw-treated IS GAG, was repaired against CFA-induced cartilage destruction. Combined treatment with Indomethacin® (5 mg/kg) and IS GAG (10 mg/kg) also more effectively inhibited CFA-induced paw edema at 3 hr, 24 hr, and 48 hr to levels comparable to the anti-inflammatory drug, indomethacin. Thus, the IS GAG described here holds great promise as an anti-inflammatory drug in the future.
Keywords: Isaria sinclairii, Glycosaminoglycan, Inflammation
INTRODUCTION
As an Oriental medicine, one of Dongchunghacho (Tochukaso), Cicada Dongchunghacho (Isaria sinclairii) has been developed as a new potential Korean crude drug. It contains the fruiting bodies of I. sinclairii (IS) from its parasitic host larva of silkworm. Previous studies have investigated the antiobesity activity of IS (1) in four strains of rat including SHR, WKY, Zücker-fa/fa rats and Sprague- Dawley (SD) rats, anti-hypertensive effect (2) and its antidiabetic effect in db/db mice (3). In addition, the molecular mechanism behind IS biological activity has been investigated by purifying and examining the second metabolites and macromolecules produced by IS. The anti-oxidant potential of the individual fractions was determined to be in the following order: ethylacetate > n-BuOH > chloroform > n-hexan. N-(2-Hydroxyethyl) adenosine (HEA) was a one of the active compounds in the n-BuOH layer (4). Following n-BuOH extraction, the remaining aqueous fraction or water-soluble fraction could be used as potential nutraceutical agents.
As a purified IS substance, FTY720, semi-synthetic derivatives of Myriocin (ISP-1) from IS could act as a safe and potent immunomodulator by decreasing the number of peripheral lymphocytes (5). Fingolimod (FTY720), which is derived from the lipid fraction, spingosine, of IS has also been shown to be a potent immunosuppressant (6) and approved as multiple sclerosis drug based on a fugal secondary metabolite (ISP-1) (7). However, the immunosuppressant FTY720 has been shown to result in glycosaminoglycan (GAG) depletion in articular cartilage; therefore, clinical trials using FTY720 for the treatment of rheumatic diseases have not yet been performed (8). On the other hand, ISGAG also possesses anti-hypertensive effect (9) against one of the causes of coronary artery disease.
In this study, we identified an IS-GAG that was water soluble, found in the aqueous fraction and displayed anti-inflammatory properties as a potential articular cartilage repairing agent in the form of combined drug.
MATERIALS AND METHODS
Materials. I. sinclairii was collected in Mountain Halla located in South Korea, cultured in a potato dextrose agar (PDA) medium, sprayed (inoculated) on silkworm for infection, proliferated inside of the silkworm body, and cultivated with forming fruiting bodies in the Department of Agricultural Biology, National Academy of Agricultural Science, Korea.
Preparation of I. sinclairii extract. As an aqueous extract, the dried I. sinclairii (IS, 50 g) was homogenized and soaked with deionized water or methanol for the extract. The samples were filtered through Whatman filter paper, and concentrated by evaporation and freezing drying. The dried powder (water/methanol extract) was dissolved in saline prior to use as a test solution. As an organic solvent extract, the dried IS (1 kg) was soaked and extracted thrice with MeOH by ultrasonification for 30 min. The extracts obtained were dried on a rotary evaporation; the residue was suspended in water and sequentially partitioned with hexane, chloroform, ethylacetate, n-butanol and H2O. The residues separated from solvent extracts were used for glycosaminoglycan purification steps. Each sample (5 mg) was dissolved in 500 μl of phosphate buffered saline (PBS) (final concentration 0.5% ethanol or 0.5% DMSO) as a test solution.
Preparation of IS glycosaminoglycans. The dried IS (1 kg) was soaked and extracted trice with ethanol by ultrasonification for 30 min. The residues separated from the alcohol extracts were defatted twice with 2 volumes of acetone. Approximately 200 g of dried, defatted and pulverized powder was suspended in 2 L of 0.05 M sodium bicarbonate buffer (pH 9). The suspension was incubated for 48 hr at 60℃ after adding 28 ml (1.4%) of Alcalase (2.4 Anson units/g Sigma Co., USA). The digestion mixture was cooled to 4℃, and trichloroacetic acid was added to a final concentration of 5%. The sample was mixed, allowed to stand for 1 hr, and then centrifuged for 30 min at 8000 × g. Three volumes of 5% potassium acetate in ethanol were added to one volume of supernatant. After mixing, the suspension was stored overnight at 4℃ and then centrifuged. The precipitate (20 g) was dissolved in 40 ml of 0.2 M NaCl and centrifuged, cetylpyridinium chloride (5%) was added to 0.2 times the volume of the supernatant, and the precipitate was collected by centrifugation. The precipitate was dissolved in 20 ml of 2.5 M NaCl, 5 volumes of ethanol were added, and the precipitate was centrifuged. The precipitate was dissolved in water and dialyzed against 100 volumes of water (10), and the dialyzate was freeze-dried to obtain about 0.78 g of GAG as a white powder. Crude ISGAG was loaded onto a DEAE Sephadex A-25 gel chromatography column (40 × 1.2 cm) equilibrated with 50 mM phosphate buffer (pH 7.4). The fractions were eluted using a linear sodium chloride gradient from 0 to 2.5 M NaCl in phosphate buffer at a flow rate of 20 ml/h, and the dialyzate was freeze-dried to pure GAG.
Animal model. Specific pathogen-free SD rats (weighing 220 ± 20 g, male) were purchased from Samtako Co. Ltd. (Osan, Korea). Anti-inflammatory activity was measured using complete Freud ajuvant (CFA, sigma, 0.1 ml/rat) induced rat paw edema. CFA was injected into the subplantar tissue of the right hind paw.
Experimental design. Three animal experiments were conducted.
In Experiment 1, Anti-inflammatory effect of IS extract
Rats were divided into seven groups (n = 8 per group): [1] PBS-treated control rat, [2] CFA-induced arthritis rat, [3] water extract-treated arthritis rat, [4] methanol-treated arthritis rat, [5] ethylacetate-treated arthritis rat, [6] buthanol extract-treated arthritis rat, [7] indomethacin-treated arthritis rat. The right foot pad of each rat was injected subcutaneously with 0.12 ml of complete Freund’s adjuvant (CFA, Sigma co., USA), except the control group, to induce rat paw edema as a chronic arthritis experimental model at the first day. IS extract (water, ethylacetate, buthanol extract, respectively) and Indomethacin (on 1st day, 5 mg/kg, and then from 2nd day to 14th day, 1 mg/kg) as a positive control, were treated to rat intraperitoneally over a 2 week. Paw size was measured at 1 hr, 2 hr, 6 hr and everyday using a digimatic caliper (Mitutoyo, Co., Japan). After 2 weeks of treatment, blood was collected from the posterior vena cava under light CO2 inhalation and used for serum chemistry measurements (11).
In Experiment 2, Anti-inflammatory effect of IS-GAG
The right foot pad of each rat was injected subcutaneously with CFA above mentioned method. Rats were divided into 4 groups (n = 8 per group): [1] PBS-treated control rat, [2] CFA-induced arthritis rat, [3] IS-GAGtreated arthritis rat, [4] indomethacin-treated arthritis rat. ISGAG (2 mg/kg) and Indomethacin (5 → 1: 5 mg/kg on 1st day, from 2nd day to 8th day, 1 mg/kg) treated intraperitoneally over an 8-day period, respectively. Paw size detected at 1, 2, 6 hr and every day. After 14 days of treatment, blood was collected and used for serum chemistry measurements. At that time, the LV dorsal root ganglion including articular cartilage and near leg bones were dissected from the rats, fixed in fixative, decalcified, embedded in paraffin, stained hematoxylin and eosin, and analyzed with microscopy.
In Experiment 3, Anti-paw edema effect of Indomethacin combined IS-GAG
Ten SD rats were divided into 4 groups: Rats were divided into 4 groups: [1] PBS-treated control rat, [2] CFAinduced arthritis rat, [3] IS-GAG plus indomethacin-treated arthritis rat, [4] indomethacin-treated arthritis rat. The right sole of foot of each rat was injected subcutaneously with CFA according to upper mentioned method. IS-GAG (10 mg/kg), IS-GAG (10 mg/kg) with Indomethacin (5 mg/kg), and Indomethacin (5 mg/kg), was injected intraperitoneally over a week, respectively. Paw size detected at 1, 3, 5 hr and every day. After third, 7th and 14th day of treatment, blood was collected from posterior vena cava for serum biochemical analysis. The LV dorsal root ganglions including articular cartilage were extracted and histopathological study processed.
Measurement of Prostaglandin E (PGE2) assay. The concentration of PGE2 was determined by enzyme - linked immunoassay (EIA) according to the manufacturer’s manual (Cayman Chemicals, Ann Arbor, MI, USA) by using a monoclonal antibody/enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA). Concentrations of PGE2 were measured at 405 nm using ELISA (12).
Cytokine (TNF-α) production measurements in splenocytes. The splenocytes were separated from a C57BL/6 female mouse (SLC Co, Shizuoka, Japan). The supernatants from the splenocytes incubated with IS-GAG 0 M, 0.1 M, or 1 M from DEAE Sephadex A-25 at 37℃, and the TNF-α levels in these fractions were measured by ELISA using an ELISA kit for TNF-α purchased from QuantikineRM, R&D systems, Inc. (Minneapolis, MN, USA) according to the manufacturer’s instructions.
Secretary phospholipase A2 measurements in CPAE cells. Phospholipase A2 (sPLA2) cleaves membrane phospholipids to release arachidonic acid, the precursor to a large family of pro-inflammatory eicosanoids (13). The sPLA2 levels in these 24 hr-incubated IS-GAG 0 M, 0.1 M, or 1 M from DEAE Sephadex A-25 were measured by ELISA using a sPLA2 assay kit purchased from Cayman Chemicals, (Ann Arbor, MI, USA).
Endothelial VEGF assay. The level of VEGF production was also measured in human umbilical vein endothelial cells (HUVEC) (ATCC, Manassas, VA, USA) in an endothelial cell basal medium (EBM)-2 with EGM™-2 singlequots (Cambrex, Walkersville, USA) at 37℃ in an atmosphere containing 5% CO2. The levels of VEGF production were measured, using an ELISA kit for VEGF kit (hunan VEGF Immunoassay) purchased from QuantikineRM, R&D systems, Inc. (Minneapolis, MN, USA) according to the manufacturer’s.
Measurement of UV-upregulation NF-κB activity in transfectant HaCaT cells by IS extract and LPS-activation NF-κB activity in RAW 264.7 cells by IS-GAG. HaCaT keratinocytes (3 × 106 cells) that were pretreated with Sample, ISMC (1 mg/ml) for 3 hr were exposed to UV irradiation (40 mJ cm−2). After the UVB exposure, cells were incubated with ISMC (1 mg/ml) in fresh culture media containing 10% FCS for 24 hr. The NF-κB activity was measured as previously described (14).
As a another inflammatory assay of NFκB assay, RAW 264.7 (1 × 105 cells) cells were treated with Sample, ISGAG (10, 50, 100, 200 mg/ml) and incubated for 2 hr. After inactivation of enzymes, The NF-κB activity was measured as previously described [14]. For NF-κB-SEAP reporter gene assay of IS-GAG, samples were treated: control, LPS: lipopolysaccharide (1 mg/ml), IS-GAG 10, 50, 100 μg/ml or TPCK (20 μM) as a positive control.
Determination of molecular weight of IS-GAG using MALDI mass and EI mass spectrometry. Matrix-Assisted Laser Desorption Ionization (MALDI) Mass spectrometer (Voyager-DE STR, Applied Biosystems, Germany) MS analysis was performed using a High resolution Tandem Mass spectrometer (Micromass Autospec OA-TOF, Manchester, UK) using an EI (electron ionization)-mode, Electron 70 eV DIP (Direct Inlet Probe) mode in National instrumentation center for environmental management of Seoul National University.
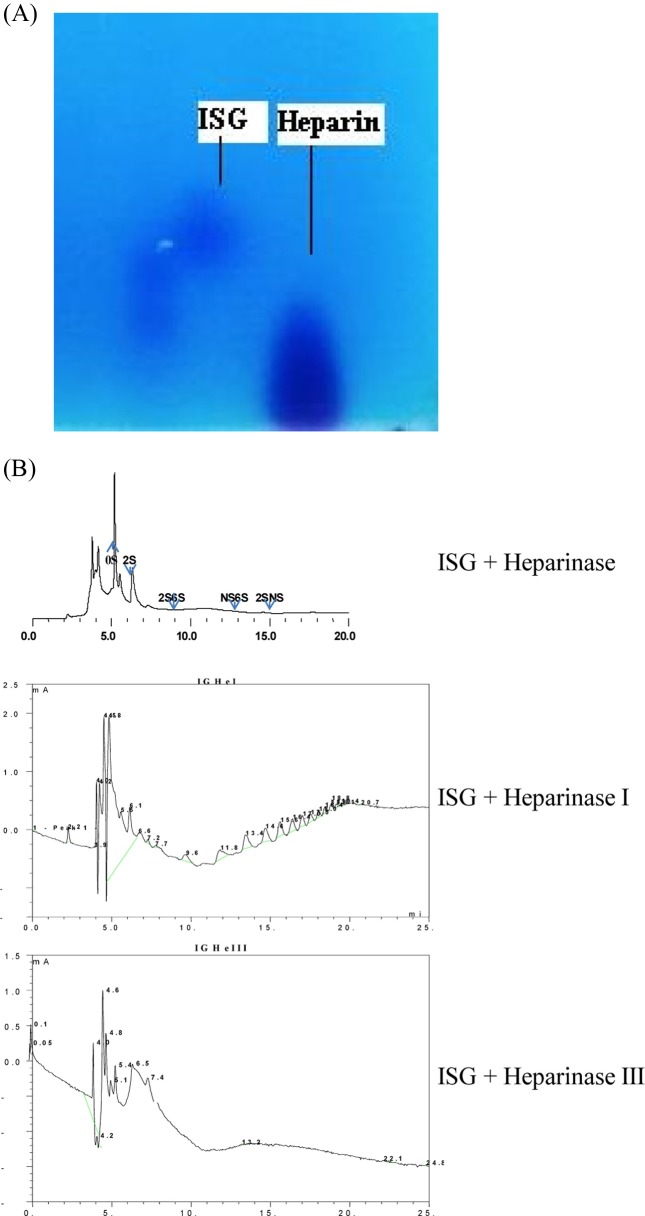
Identification of used IS-GAG. IS-GAG was electrophoresed, compared to heparin (Sigma Co., USA). Agarose (1%) gel electrophoresis was conducted in TAE buffer, stained with Alcian blue (Sigma Co., USA) and destained 8% acetic acid. The composition of disaccharides and oligosaccharides produced from IS GAG by Hj-15 GAG enzyme were analyzed using strong anion-exchange (SAX)- high performance liquid chromatography. The HPLC system equipped with UV detector was operated under a linear gradient system of NaCl (0.1 M~1.6 M, pH 3.5). The flow rate was adjusted at 1.0 ml/min and the detector was set at 232 nm (Dionex submmit HPLC, Sunnyvale, USA).
Statistical analysis. Mean and standard error values of all the studied parameters were determined for each group. Student’s t-test was conducted to establish significant differences between the final biochemical level in control and treated groups. p < 0.05 was considered statistically significant.
RESULTS
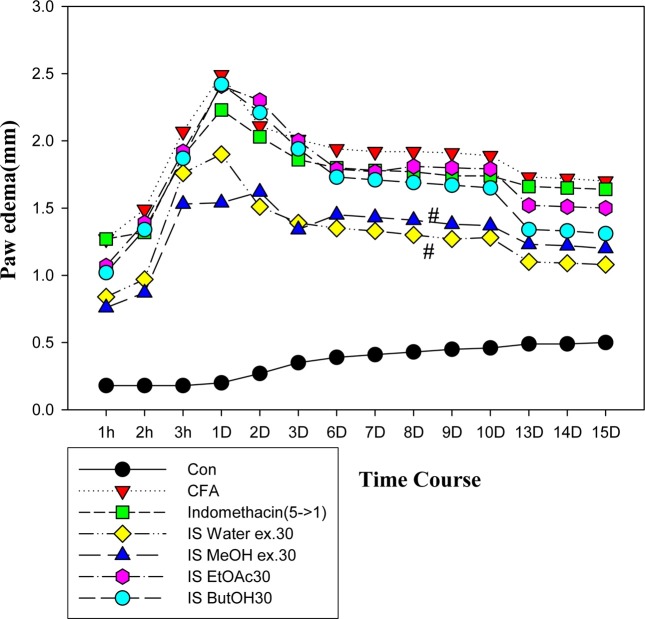
Anti-inflammatory effect of IS extract. The total MeOH extract of IS was partitioned into n- hexane, CHCl3, EtOAC, n-BuOH, and H2O fractions. To identify the active compounds in the extracts of IS, five groups were orally treated with each samples (30 mg/kg); saline as the control, the water, methanol, butanol and ethylacetate extracts, and Indomethacin® (5 → 1 mg/kg), which was used as the positive control. The rats received each samples every day for 2 weeks. The anti-paw edema effect of individual extract was in the following: H2O (water) fraction > methanol > n-BuOH > ethyl acetate. Interestingly, H2O fraction following n-BuOH extraction, remaining aqueous fraction (Fig. 1) and glycosaminoglycan, which was used as the water-soluble alcohol precipitation fraction, meaningful, further purified, also displayed anti-edema effects (Fig. 2). The mean change in the paw edema size (mm) from 1h to 14 day for each group were as follows: control (0.34 ± 0.03), CFA (1.87 ± 0.08), IND (1.74 ± 0.07), IS water (1.30 ± 0.08), IS methanol ex. (1.31 ± 0.07), IS ethylacetate ex. (1.76 ± 0.09), and IS buthanol ex. (1.66 ± 0.10).
Fig. 1. Anti-inflammatory activity of the ethanol extract and their fractions of IS on CFA-induced paw edema in rat arthritis model. Each fraction (30mg/kg) of water, methanol, ethylacetate, butanol and indomethacin (5 mg/kg, 1st day, extra day 1 mg/kg) was treated over half of a month. #p< 0.05 vs each vehicle (CFA) (p< 0.05).
Fig. 2. Anti-inflammatory evaluation of IS-GAG on CFA paw edema rat model. (A) Anti-inflammatory effect of IS-GAG on CFA-induced paw edema in rat arthritis mode (8th day). CFA: 100 μl, ISG (1M): IS-GAG throughout 1M NaCl salt isocratic fraction by a DEAE sephadex A-25 chromatography (2 mg/kg). Indomethacin (1st day: 5mg/kg, from 2nd day to 14th day: 1mg/kg). Values are means ± SE. (B) Anti-paw edema effect of Indomethacin (IND) combined IS-GAG. CFA: 120 μl, ISG + IND group: IS-GAG (10mg/kg) and Indomethacin (1st day: 5mg/kg, from 2nd day, 2.5mg/kg) after 1st day CFA treatment, and Indomethacin (5 → 2.5mg/kg) only. Values are means ± SD. (C) Histopathological finding in the LV dorsal root ganglion, including the articular cartilage linked to the paw treated IS-GAG for 14 days.
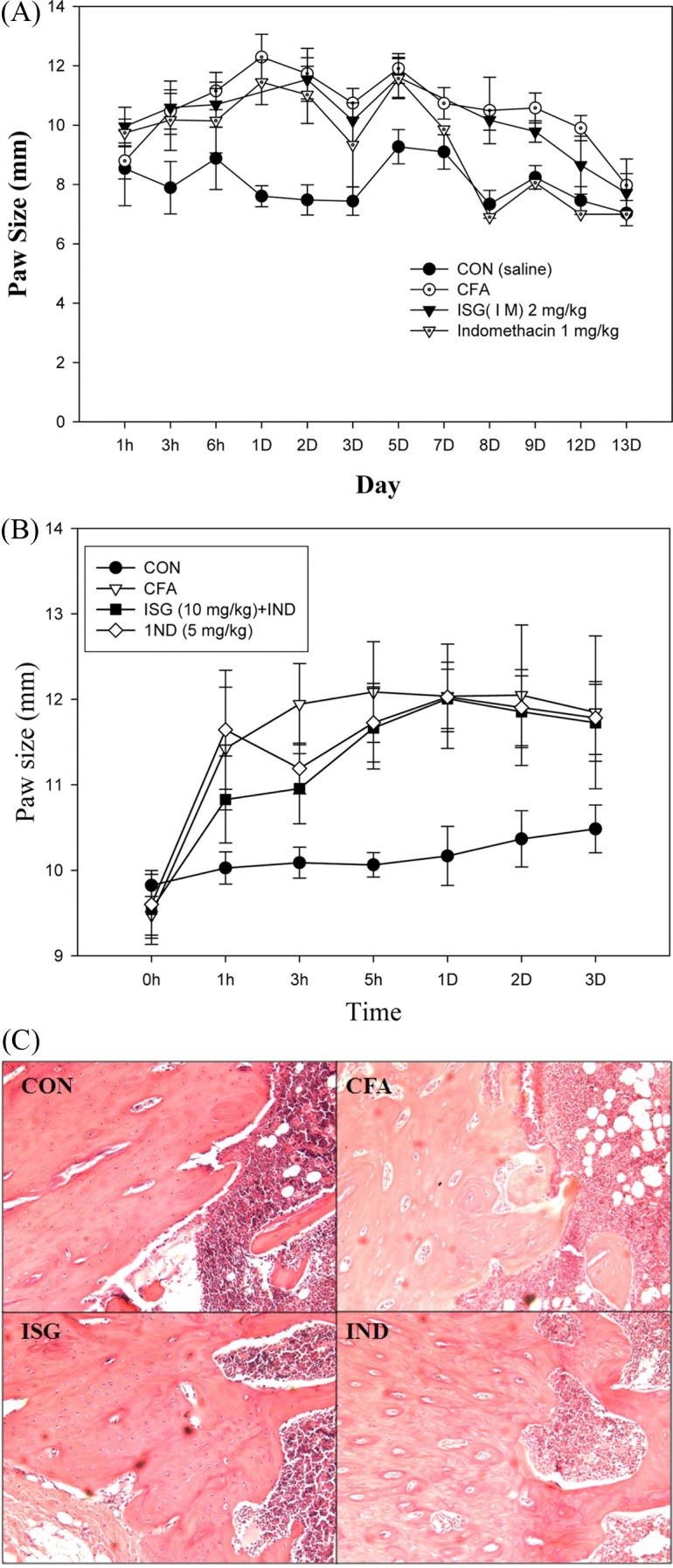
Anti-inflammatory effect of IS-GAG. IS-GAG (2 mg/kg) inhibited CFA-induced paw edema at 3 hr, 2nd, 3rd day to comparable levels to anti-inflammatory drug, indomethacin (Fig. 2A). The mean group values (m ± S.E.) of paw size (not subtracted initial paw size) for each group were as follows: control (8.02 ± 0.22 mm), CFA (10.56 ± 0.36 mm), indomethacin (9.35 ± 0.50 mm), IS GAG (10.09 ± 0.08 mm).
Anti-paw edema effect of Indomethacin combined IS-GAG. The combined Indomethacin (5 mg/kg)-IS GAG (10 mg/kg) inhibited CFA-induced paw edema at 3 hr, 2nd, 3rd day to levels comparable to anti-inflammatory drug, indomethacin (Fig. 2B). The mean group values (m ± S.D.) of paw size (not subtracted initial paw size) for each group were as follows: control (10.14 ± 0.22 mm), CFA (11.55 ± 0.94 mm), IS-GAG + indomethacin (11.23 ± 0.87 mm), indomethacin (11.41 ± 0.45 mm) (Fig. 2B).
Articular cartilage destruction repair by IS-GAG. In the histological analysis, the LV dorsal root ganglion, including the articular cartilage and linked to the paw treated ISGAG, was repaired against CFA induced cartilage destruction. This was in contrast to the effects observed in the CFA treated group, where the destructed with erosion of articular cartilage (Fig. 2C).
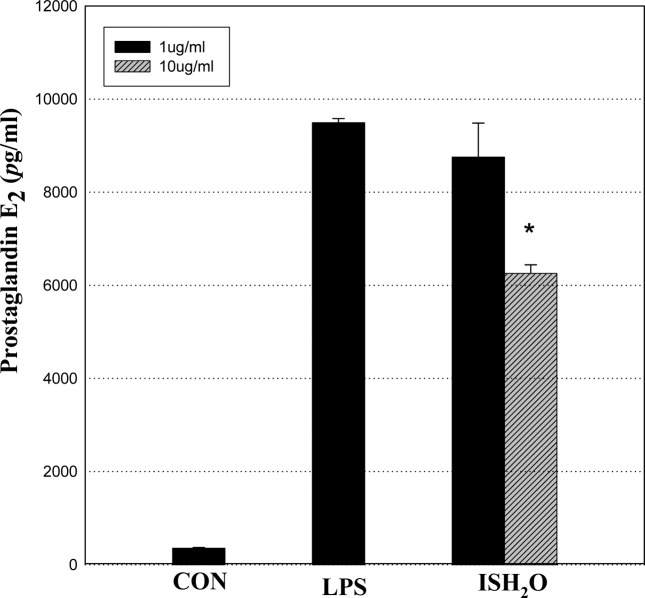
Cytokine (Prostaglandin E2) production measurements in inflammatory state (LPS-induced macrophage cells). As an other factors indicating anti-inflammation, prostaglandin E2 (PGE2) levels were decreased (34.11%) in a dosedependent manner, were observed in macrophage 264.7 cells in the ISH2O fraction (previous purified one of ISGAG), which suggests anti-inflammatory action (Fig. 3). There were also little sPLA2 activities in these 24 hr-incubated IS-GAG 0 M, 0.1 M, or 1 M from DEAE Sephadex A-25 (data not shown).
Fig. 3. Effect of IS H2O (aqueous) fraction on LPS-induced PGE2 productions in RAW 264.7 cells. *p<0.05 vs. vehicle (LPS). Values are means ± SD.
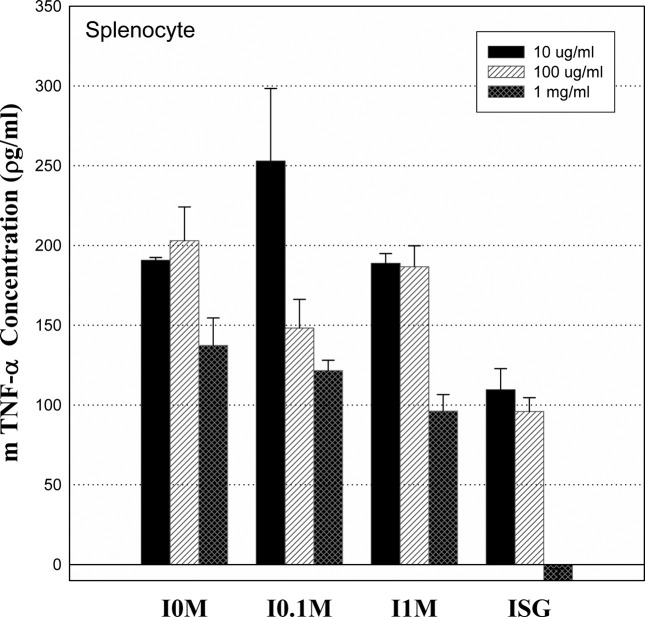
Tumor necrosis factor-α production measurements in normal splenocytes. IS-GAG did not increase the level of TNF-α, showing dose-dependent regulation to inflammation (Fig. 4).
Fig. 4. Production of TNF-alpha from normal splenocytes stimulated by IS glycosaminoglycan in 96 well plates for 24hr. I0M, I0.1M and I1M mean IS-GAG from DEAE Sephadex A-25 fraction that the bound fraction was eluted using a linear sodium chloride stepwise from 0M, 1M, 0.1M and 1M NaCl in phosphate buffer (pH 7.4). Values are means ± SD.
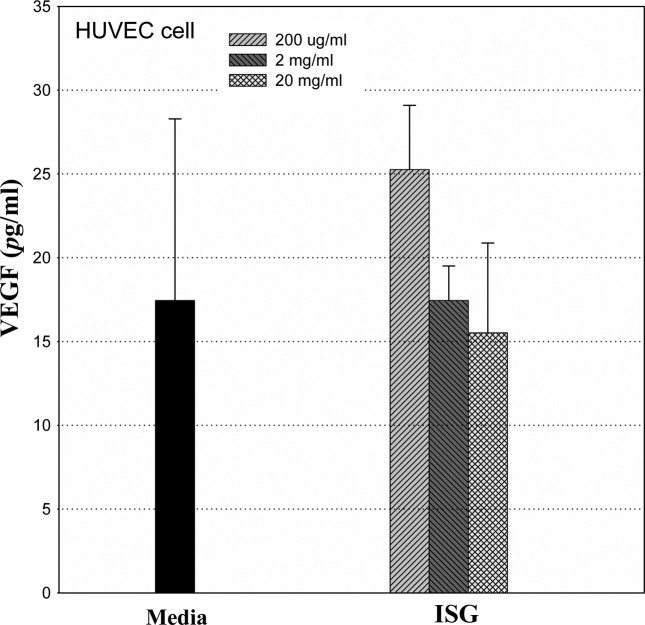
Vascular Endothelial Growth Factor (VEGF) related to anti-angiogenesis. In this study, there was a dose-dependent regulation of VEGF production in the HUVEC endothelial cells, which suggests that IS-GAG has virtually somewhat relationship with anti-angiogenesis associated with the cancer progenesis state (Fig. 5). That result shows that the level of VEGF production in HUVEC cells did not increase dose dependently but decrease after a 24 hr treatment.
Fig. 5. Effect of IS-GAG on VEGF cytokine production in HUVEC cells. *p<0.05 vs. vehicle. Values are means ± SD.
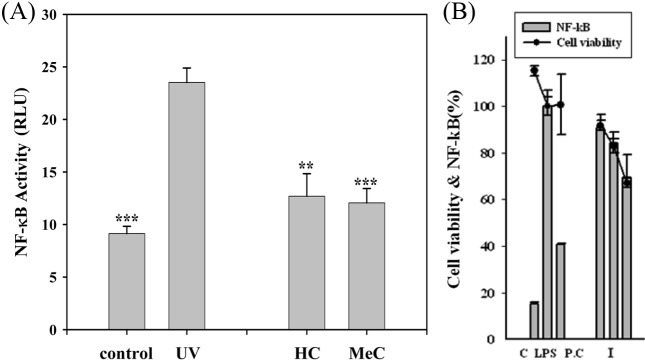
Suppression of UV-induced NF-κB activity in transfectant HaCaT cells by IS extract. Treatment of the transfectant HaCaT cells with IS for 24 h such as ISMC (methanol extract) and ISHC (water extract) significantly inhibited the level of UV-induced NF-κB activity by 75.3 and 75.7%, respectively, with respect o the unradiated control (Fig. 6). Additionally, Effect of cellular NF-κB activation by UV stimuli (60 mj/cm−2) after pre-incubation (1 hr) of samples (ethylacetate extract and butanol extract) in Transfectant Human HaCaT cells inhibited the level of UV-upregulating NF-κB activity by 72.1 and 84.9% (data not shown).
Fig. 6. (A) Inhibition effect of cellular NF-κB activation by UV stimuli (40mj/cm2) after pre-incubated (3 hr) of IS-extract (water extract and methanol extract) in Transfectant Human HaCaT cells. ***p< 0.001 and **p < 0.05 vs. UV-stimulated cells. (B) Inhibition effect of cellular NF-κB activation by lipopolysaccharide after pre-incubated (2 hr) of IS-GAG in transfectant RAW 264.7 cells. C: control, LPS: lipopolysaccharide (1 mg/ml), I: IS-GAG 10, 50, 100 μg/ml; P.C. (positive control): TPCK 20 mM.
Identification of used IS-GAG. The molecular weight of IS-GAG was estimated to be approximately 6000 dalton by MALDI-TOF mass data (9). IS-GAG was also identified by alcian blue stain compared to heparin standard (Fig. 7A). The digested pattern of IS-GAG co-incubated heparin-lyase (HJ-15) was matched to heparin disaccharides standards (Fig. 7B).
Fig. 7. (A) Alcian blue staining gel (1% agarose gel) of glycosaminoglycan derived from I. sinclairii. (B) SAX-HPLC chromatogram of digested ISG compared to standard heparin disaccharides. 0S: ΔUA (4-deoxy-L-threo-hex-4-enopyranosyluronic acid)-GlcN (D-glucosamine) Ac; 2S: ΔUA-GlcN; 2S6S: ΔUA-GlcN6S.
DISCUSSION
GAG, which contains a repeating disaccharide unit, is the most abundant group of heteropolysaccharides found in the body (15). The IS-GAG can also serve as lubricants in joints while at the same time providing structure rigidity to cells. In complete Freund’s adjuvant (CFA) induced paw edema, the histopathologic results of IS-GAG on rat arthritis model indicated that hyaline articular cartilage destruction was repaired. There was a different report that GAG was a potential cause of rheumatoid arthritis (RA) and inhibition of the abnormal growth or adhesion of immune cells reactive to GAGs might open new therapeutic avenues for treatment of RA (16), but the source of used GAG (mixture of commercial chondrotin sulfate, hyaluronic acid and heparin) in this journal, was different from insect source (I. sinclairii from silkworm).
As a novel immunosuppressive drug of IS, the spingosine derivative, FTY720 was investigated in clinical trial that examined its effects on organ including kidney transplantation; however, this drug was shown to enhance GAG depletion in articular cartilage (8). However, the IS-GAG, which corresponds to the carbohydrate fraction derived from I. sinclairii, also produced an immunosuppressive effect and did not cause glycosaminoglycan depletion in articular cartilage. Thus, this IS-GAG holds great promise as an anti-inflammatory drug in the future; waiting for good pharmaceutical large scale extraction and commercial combined preparation.
Acknowledgments
This study was supported by the Rural Development Administration, Basic research project 2009-2012.
References
- 1.Ahn M.Y., Jee S.D., Lee B.M. Antiobesity effects of Isaria sinclairii by repeated oral treatment in obese Zucker rats over a 4-month period. J. Toxicol. Environ. Health Part A. (2007A);70:1395–1401. doi: 10.1080/15287390701428556. [DOI] [PubMed] [Google Scholar]
- 2.Ahn M.Y., Jung Y.S., Jee S.D., Kim C.S., Lee S.H., Moon C.H., Cho S.I., Lee B.M., Ryu K.S. Anti-hypertensive effect of the Dongchunghacho, Isaria sinclairii, in the spontaneously hypertensive rats. Arch. Pharmacal Res. (2007B);30:493–501. doi: 10.1007/BF02980225. [DOI] [PubMed] [Google Scholar]
- 3.Ahn M.Y., Jee S.D., Lee B.M., Yeon J.H., Park K.K., Hwang J.S., Yun E.Y. Antidiabetic effects and gene expression profiling in obese mice treated with Isaria sinclairii over a 6-month period. J. Toxicol. Environ. Health Part A. (2010);73:1511–1520. doi: 10.1080/15287394.2010.511575. [DOI] [PubMed] [Google Scholar]
- 4.Ahn M.Y., Heo J.E., Ryu J.H., Jeong H., Ji S.D., Park H.C., Sim H.S. Antioxidant activity of N-hydroxyethyl adenosine from I. sinclairii. Int. J. Indust. Entomol. (2008);17:197–200. [Google Scholar]
- 5.Ueda H., Takahara S., Itoh S., Nomi H., Shibahara N., Katsuoka Y. Preoperative administration of FTY720 prolonged renal allograft survival. Transplant Immunol. (2005);14:1–8. doi: 10.1016/j.trim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Takano F., Yahagi N., Yahagi R., Takada S., Yamaguchi M., Shoda S., Murase T., Fushiya S., Ohta T. The liquid culture filtrates of Paecilomyces tenuipes (Peck) Samson (= Isaria japonica Yasuda) and Paecilomyces cicadae (Miquel) Samson (= Isaria sinclairii (Berk.) Llond) regulate Th1 and Th2 cytokine response in murine Peyer’s patch cells in vitro and ex vivo. Int. Immunopharmacol. (2005);5:903–916. doi: 10.1016/j.intimp.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Strader R.C., Pearce C.J., Oberlies N.H. Fingolimod (FTY720): A recently approved multiple sclerosis drug based on a fungal secondary metabolite. J. Nat. Prod. (2011);74:900–907. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 8.Stradner M.H., Angerer H., Ortner T., Fuerst F.C., Setznagl D., Kremser M.L., Hermann J., Graninger W.B. The Immunosupressant FTY720 (fingolimod) enhances glycosaminoglycan depletion in articular cartilage. BMC Musculoskeletal Disord. (2011);12:279. doi: 10.1186/1471-2474-12-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn M.Y., Jung Y.S., Jee S.D., Han J.W., Hwang J.S., Yun E.Y., Lee B.M. Blood pressure lowering effect of the glycosaminoglycan derived from Isaria sinclairii in SHR rats. J. Toxicol. Environ. Health Part A. (2013);76:391–399. doi: 10.1080/15287394.2013.765370. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.S., Jo Y.Y., Chang I.M., Toida T., Park Y., Linhardt R.J. A New glycosaminoglycan from the giant African snail Achatina fulica. J. Biol. Chem. (1996);271:11750–11755. doi: 10.1074/jbc.271.20.11750. [DOI] [PubMed] [Google Scholar]
- 11.Nackley A.G., Suplita R.L., Hohmann A.G. A peripheral cannabinoid mechanism suppresses spinal pos protein expression and pain behavior in a rat model of inflammation. Neuroscience. (2003);117:659–670. doi: 10.1016/S0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- 12.Kwon J.H., Kim J.H., Choi S.E., Park K.H., Lee M.W. Inhibitory effects of phenolic compounds from needles of Pinus densiflora on nitric oxide and PGE2 production. Arch. Pharm. Res. (2010);33:2011–2016. doi: 10.1007/s12272-010-1217-y. [DOI] [PubMed] [Google Scholar]
- 13.Reid R.C. Inhibitors of secretory phospholipase A2 group IIA. Curr. Med. Chem. (2005);12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 14.Ahn K.S., Moon K.Y., Lee J., Kim Y.S. Downregulation of NF-κB activation in human keratinocytes by melanogenic inhibitors. J. Dematol. Sci. (2003);31:193–201. doi: 10.1016/S0923-1811(03)00039-2. [DOI] [PubMed] [Google Scholar]
- 15.Lauver D.A., Lucchesi B.R. Sulodexide: A renewed interest in this glycosaminoglycan. Cardiovasc. Drug Rev. (2006);24:214–226. doi: 10.1111/j.1527-3466.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang J.Y., Roehrl M.H. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc. Natl. Acad. Sci. (2002);99:14362–14367. doi: 10.1073/pnas.222536599. [DOI] [PMC free article] [PubMed] [Google Scholar]