Abstract
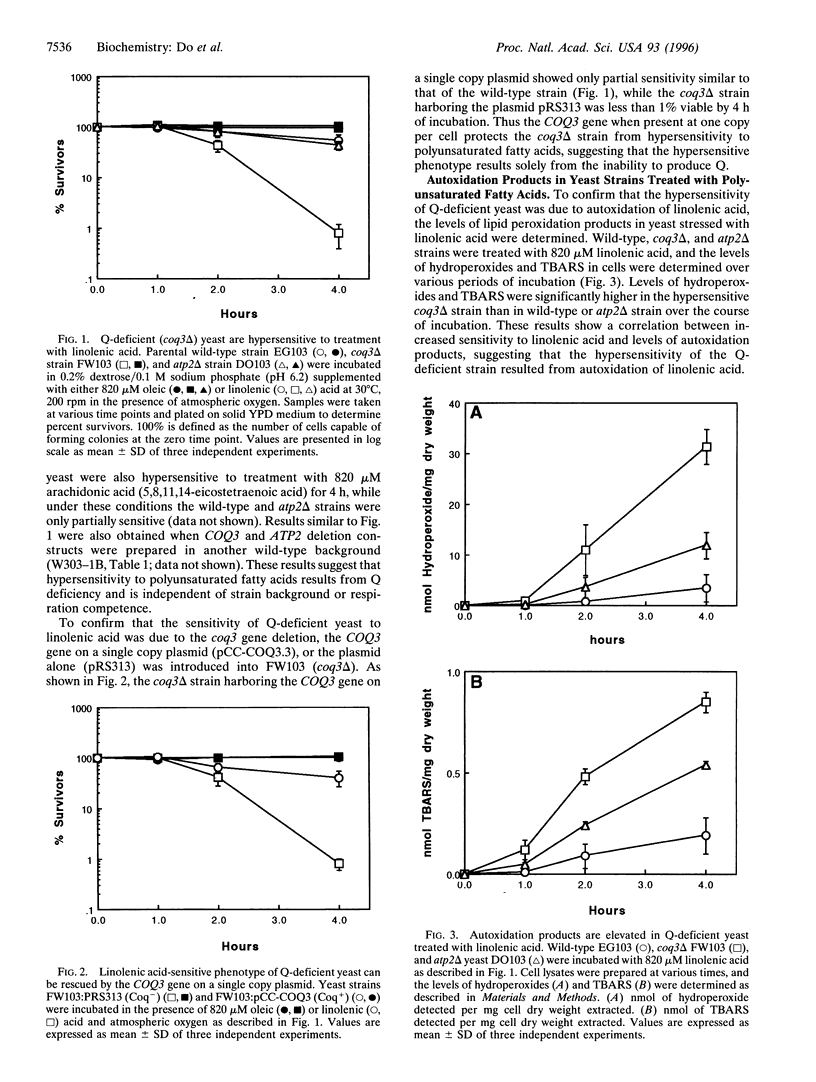
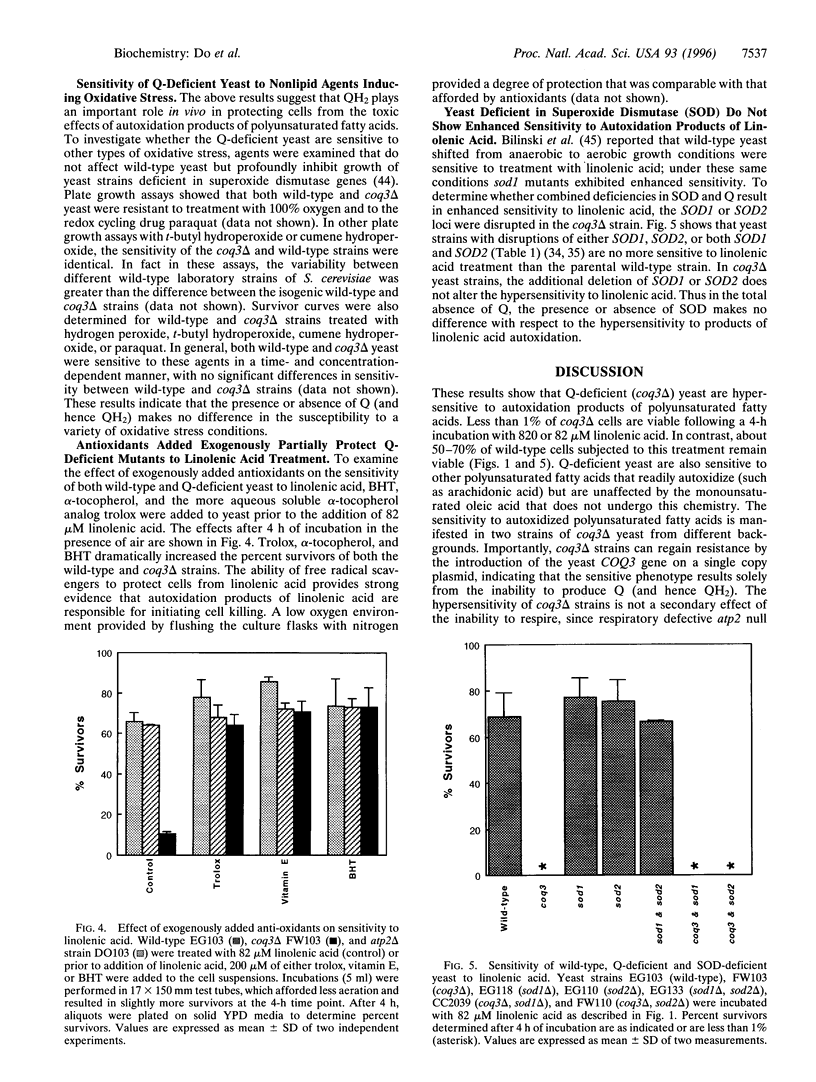
Coenzyme Q (ubiquinone or Q) plays a well known electron transport function in the respiratory chain, and recent evidence suggests that the reduced form of ubiquinone (QH2) may play a second role as a potent lipid-soluble antioxidant. To probe the function of QH2 as an antioxidant in vivo, we have made use of a Q-deficient strain of Saccharomyces cerevisiae harboring a deletion in the COQ3 gene [Clarke, C. F., Williams, W. & Teruya, J. H. (1991) J. Biol. Chem. 266, 16636-16644]. Q-deficient yeast and the wild-type parental strain were subjected to treatment with polyunsaturated fatty acids, which are prone to autoxidation and breakdown into toxic products. In this study we find that Q-deficient yeast are hypersensitive to the autoxidation products of linolenic acid and other polyunsaturated fatty acids. In contrast, the monounsaturated oleic acid, which is resistant to autoxidative breakdown, has no effect. The hypersensitivity of the coq3delta strains can be prevented by the presence of the COQ3 gene on a single copy plasmid, indicating that the sensitive phenotype results solely from the inability to produce Q. As a result of polyunsaturated fatty acid treatment, there is a marked elevation of lipid hydroperoxides in the coq3 mutant as compared with either wild-type or respiratory-deficient control strains. The hypersensitivity of the Q-deficient mutant can be rescued by the addition of butylated hydroxytoluene, alpha-tocopherol, or trolox, an aqueous soluble vitamin E analog. The results indicate that autoxidation products of polyunsaturated fatty acids mediate the cell killing and that QH2 plays an important role in vivo in protecting eukaryotic cells from these products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S., Willett W. C. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer R. E. An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem Cell Biol. 1992 Jun;70(6):390–403. doi: 10.1139/o92-061. [DOI] [PubMed] [Google Scholar]
- Biliński T., Litwińska J., Błaszczyński M., Bajus A. Superoxide dismutase deficiency and the toxicity of the products of autooxidation of polyunsaturated fatty acids in yeast. Biochim Biophys Acta. 1989 Jan 23;1001(1):102–106. doi: 10.1016/0005-2760(89)90312-3. [DOI] [PubMed] [Google Scholar]
- Bossie M. A., Martin C. E. Nutritional regulation of yeast delta-9 fatty acid desaturase activity. J Bacteriol. 1989 Dec;171(12):6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. F., Williams W., Teruya J. H. Ubiquinone biosynthesis in Saccharomyces cerevisiae. Isolation and sequence of COQ3, the 3,4-dihydroxy-5-hexaprenylbenzoate methyltransferase gene. J Biol Chem. 1991 Sep 5;266(25):16636–16644. [PubMed] [Google Scholar]
- Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995 May 24;1271(1):195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Ernster L., Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71(8 Suppl):S60–S65. doi: 10.1007/BF00226842. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Forsmark-Andrée P., Dallner G., Ernster L. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Radic Biol Med. 1995 Dec;19(6):749–757. doi: 10.1016/0891-5849(95)00076-a. [DOI] [PubMed] [Google Scholar]
- Forsmark-Andrée P., Ernster L. Evidence for a protective effect of endogenous ubiquinol against oxidative damage to mitochondrial protein and DNA during lipid peroxidation. Mol Aspects Med. 1994;15 (Suppl):s73–s81. doi: 10.1016/0098-2997(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Forsmark P., Aberg F., Norling B., Nordenbrand K., Dallner G., Ernster L. Inhibition of lipid peroxidation by ubiquinol in submitochondrial particles in the absence of vitamin E. FEBS Lett. 1991 Jul 8;285(1):39–43. doi: 10.1016/0014-5793(91)80720-n. [DOI] [PubMed] [Google Scholar]
- Frei B., Kim M. C., Ames B. N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R. Ubiquinone formation in wild-type and petite yeast: the effect of catabolite repression. Biochim Biophys Acta. 1969 Apr 1;177(2):358–360. doi: 10.1016/0304-4165(69)90150-0. [DOI] [PubMed] [Google Scholar]
- Gralla E. B., Kosman D. J. Molecular genetics of superoxide dismutases in yeasts and related fungi. Adv Genet. 1992;30:251–319. doi: 10.1016/s0065-2660(08)60322-3. [DOI] [PubMed] [Google Scholar]
- Gralla E. B., Valentine J. S. Null mutants of Saccharomyces cerevisiae Cu,Zn superoxide dismutase: characterization and spontaneous mutation rates. J Bacteriol. 1991 Sep;173(18):5918–5920. doi: 10.1128/jb.173.18.5918-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaki Y., Sugiyama S., Ozawa T., Ohno M. Ratio of low-density lipoprotein cholesterol to ubiquinone as a coronary risk factor. N Engl J Med. 1991 Sep 12;325(11):814–815. doi: 10.1056/nejm199109123251116. [DOI] [PubMed] [Google Scholar]
- Jain S. K. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim Biophys Acta. 1988 Jan 22;937(2):205–210. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- Janda S., Gille G., Sigler K., Höfer M. Effect of hydrogen peroxide on sugar transport in Schizosaccharomyces pombe. Absence of membrane lipid peroxidation. Folia Microbiol (Praha) 1993;38(2):135–140. doi: 10.1007/BF02891695. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Hunt J. V., Wolff S. P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992 May 1;202(2):384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Woollard A. C., Wolff S. P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991 Oct;26(10):853–856. doi: 10.1007/BF02536169. [DOI] [PubMed] [Google Scholar]
- Kagan V., Serbinova E., Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990 Jun 29;169(3):851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- Kalén A., Norling B., Appelkvist E. L., Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta. 1987 Oct 8;926(1):70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- Kohlwein S. D., Paltauf F. Uptake of fatty acids by the yeasts, Saccharomyces uvarum and Saccharomycopsis lipolytica. Biochim Biophys Acta. 1984 Mar 7;792(3):310–317. doi: 10.1016/0005-2760(84)90198-x. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- Liu X. F., Elashvili I., Gralla E. B., Valentine J. S., Lapinskas P., Culotta V. C. Yeast lacking superoxide dismutase. Isolation of genetic suppressors. J Biol Chem. 1992 Sep 15;267(26):18298–18302. [PubMed] [Google Scholar]
- Marbois B. N., Hsu A., Pillai R., Colicelli J., Clarke C. F. Cloning of a rat cDNA encoding dihydroxypolyprenylbenzoate methyltransferase by functional complementation of a Saccharomyces cerevisiae mutant deficient in ubiquinone biosynthesis. Gene. 1994 Jan 28;138(1-2):213–217. doi: 10.1016/0378-1119(94)90810-9. [DOI] [PubMed] [Google Scholar]
- Marbois B. N., Xia Y. R., Lusis A. J., Clarke C. F. Ubiquinone biosynthesis in eukaryotic cells: tissue distribution of mRNA encoding 3,4-dihydroxy-5-polyprenylbenzoate methyltransferase in the rat and mapping of the COQ3 gene to mouse chromosome 4. Arch Biochem Biophys. 1994 Aug 15;313(1):83–88. doi: 10.1006/abbi.1994.1362. [DOI] [PubMed] [Google Scholar]
- Matsura T., Yamada K., Kawasaki T. Antioxidant role of cellular reduced coenzyme Q homologs and alpha-tocopherol in free radical-induced injury of hepatocytes isolated from rats fed diets with different vitamin E contents. Biochim Biophys Acta. 1992 Aug 19;1127(3):277–283. doi: 10.1016/0005-2760(92)90232-k. [DOI] [PubMed] [Google Scholar]
- McDonough V. M., Stukey J. E., Martin C. E. Specificity of unsaturated fatty acid-regulated expression of the Saccharomyces cerevisiae OLE1 gene. J Biol Chem. 1992 Mar 25;267(9):5931–5936. [PubMed] [Google Scholar]
- Mlakar A., Spiteller G. Reinvestigation of lipid peroxidation of linolenic acid. Biochim Biophys Acta. 1994 Sep 15;1214(2):209–220. doi: 10.1016/0005-2760(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Mohr D., Bowry V. W., Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992 Jun 26;1126(3):247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- Mordente A., Martorana G. E., Santini S. A., Miggiano G. A., Petitti T., Giardina B., Battino M., Littarru G. P. Antioxidant effect of coenzyme Q on hydrogen peroxide-activated myoglobin. Clin Investig. 1993;71(8 Suppl):S92–S96. doi: 10.1007/BF00226847. [DOI] [PubMed] [Google Scholar]
- Mueller D. M. Arginine 328 of the beta-subunit of the mitochondrial ATPase in yeast is essential for protein stability. J Biol Chem. 1988 Apr 25;263(12):5634–5639. [PubMed] [Google Scholar]
- Mukai K., Kikuchi S., Urano S. Stopped-flow kinetic study of the regeneration reaction of tocopheroxyl radical by reduced ubiquinone-10 in solution. Biochim Biophys Acta. 1990 Jul 20;1035(1):77–82. doi: 10.1016/0304-4165(90)90176-w. [DOI] [PubMed] [Google Scholar]
- Navab M., Fogelman A. M., Berliner J. A., Territo M. C., Demer L. L., Frank J. S., Watson A. D., Edwards P. A., Lusis A. J. Pathogenesis of atherosclerosis. Am J Cardiol. 1995 Sep 28;76(9):18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- Noack H., Kube U., Augustin W. Relations between tocopherol depletion and coenzyme Q during lipid peroxidation in rat liver mitochondria. Free Radic Res. 1994 Jun;20(6):375–386. doi: 10.3109/10715769409145637. [DOI] [PubMed] [Google Scholar]
- Repetto B., Tzagoloff A. Structure and regulation of KGD1, the structural gene for yeast alpha-ketoglutarate dehydrogenase. Mol Cell Biol. 1989 Jun;9(6):2695–2705. doi: 10.1128/mcb.9.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schultz J. R., Ellerby L. M., Gralla E. B., Valentine J. S., Clarke C. F. Autoxidation of ubiquinol-6 is independent of superoxide dismutase. Biochemistry. 1996 May 28;35(21):6595–6603. doi: 10.1021/bi960245h. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky D. A., Osipov A. N., Quinn P. J., Kagan V. E. Ubiquinone-dependent recycling of vitamin E radicals by superoxide. Arch Biochem Biophys. 1995 Nov 10;323(2):343–351. doi: 10.1006/abbi.1995.9955. [DOI] [PubMed] [Google Scholar]
- Takada M., Ikenoya S., Yuzuriha T., Katayama K. Studies on reduced and oxidized coenzyme Q (ubiquinones). II. The determination of oxidation-reduction levels of coenzyme Q in mitochondria, microsomes and plasma by high-performance liquid chromatography. Biochim Biophys Acta. 1982 Feb 17;679(2):308–314. doi: 10.1016/0005-2728(82)90301-2. [DOI] [PubMed] [Google Scholar]
- Tribble D. L., van den Berg J. J., Motchnik P. A., Ames B. N., Lewis D. M., Chait A., Krauss R. M. Oxidative susceptibility of low density lipoprotein subfractions is related to their ubiquinol-10 and alpha-tocopherol content. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1183–1187. doi: 10.1073/pnas.91.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpower B. L. New concepts on the role of ubiquinone in the mitochondrial respiratory chain. J Bioenerg Biomembr. 1981 Apr;13(1-2):1–24. doi: 10.1007/BF00744743. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990 Jul 15;265(20):11409–11412. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system. Characterization of nuclear mutants of Saccharomyces cerevisiae with defects in mitochondrial ATPase and respiratory enzymes. J Biol Chem. 1975 Oct 25;250(20):8228–8235. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system: isolation of nuclear and cytoplasmic mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. J Bacteriol. 1975 Jun;122(3):826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



