Abstract
Up to 60% of cases of equine colitis have no known cause. To improve understanding of the causes of acute colitis in horses, we hypothesized that Clostridium perfringens producing enterotoxin (CPE) and/or beta2 toxin (CPB2) are common and important causes of severe colitis in horses and/or that C. perfringens producing an as-yet-undescribed cytotoxin may also cause colitis in horses. Fecal samples from 55 horses (43 adults, 12 foals) with clinical evidence of colitis were evaluated by culture for the presence of Clostridium difficile, C. perfringens, and Salmonella. Feces were also examined by enzyme-linked immunosorbent assay (ELISA) for C. difficile A/B toxins and C. perfringens alpha toxin (CPA), beta2 toxin (CPB2), and enterotoxin (CPE). Five C. perfringens isolates per sample were genotyped for the following genes: cpa, cpb, cpb2 consensus, cpb2 atypical, cpe (enterotoxin), etx (epsilon toxin), itx (iota toxin), netB (necrotic enteritis toxin B), and tpeL (large C. perfringens cytotoxin). The supernatants of these isolates were also evaluated for toxicity for an equine cell line. All fecal samples were negative for Salmonella. Clostridium perfringens and C. difficile were isolated from 40% and 5.4% of samples, respectively. All fecal samples were negative for CPE. Clostridium perfringens CPA and CPB2 toxins were detected in 14.5% and 7.2% of fecal samples, respectively, all of which were culture-positive for C. perfringens. No isolates were cpe, etx, netB, or tpeL gene-positive. Atypical cpb2 and consensus cpb2 genes were identified in 15 (13.6%) and 4 (3.6%) of 110 isolates, respectively. All equine C. perfringens isolates showed far milder cytotoxicity effects than a CPB-producing positive control, although cpb2-positive isolates were slightly but significantly more cytotoxic than negative isolates. Based on this studied population, we were unable to confirm our hypothesis that CPE and CPB2-producing C. perfringens are common in horses with colitis in Ontario and we failed to identify cytotoxic activity in vitro in the type A isolates recovered.
Résumé
Jusqu’à 60 % des cas de colite équine n’ont aucune cause connue. Afin d’améliorer la compréhension des causes de colite aigüe chez les chevaux, nous émettons l’hypothèse que les Clostridium perfringens produisant l’entérotoxine (CPE) et/ou la toxine bêta-2 (CPB2) sont des causes courantes et importantes de colites sévères chez les chevaux et/ou qu’une cytotoxine non décrite encore produite par C. perfringens pourrait également causer des colites chez les chevaux. Des échantillons fécaux provenant de 55 chevaux (43 adultes, 12 poulains) avec des évidences cliniques de colite ont été évalués par culture pour la présence de Clostridium difficile, C. perfringens et Salmonella. Les fèces furent également analysées par épreuve immuno-enzymatique (ELISA) pour la présence des toxines A/B de C. difficile et les toxines alpha (CPA), bêta2 (CPB2) et l’entérotoxine (CPE) de C. perfringens. Cinq isolats de C. perfringens par échantillons ont été typés pour les gènes suivants : cpa, cpb, cpb2 consensus, cpb2 atypique, cpe (entérotoxine), etx (toxine epsilon), itx (toxine iota), netB (toxine B de l’entérite nécrotique), et tpeL (cytotoxine large de C. perfringens). Les surnageants de culture de ces isolats ont également été évalués pour leur cytotoxicité envers une lignée cellulaire équine. Tous les échantillons fécaux étaient négatifs pour la présence de Salmonella. Clostridium perfringens et C. difficile furent isolés de 40 % et 5,4 % des échantillons, respectivement. Tous les échantillons de fèces étaient négatifs pour CPE. Les toxines CPA et CPB2 furent détectées à partir de 14,5 % et 7,2 % des échantillons fécaux, respectivement, tous étant positifs pour la présence de C. perfringens en culture. Aucun des isolats n’était positif pour la présence des gènes cpe, etx, netB ou tpeL. Les gènes cpb2 atypiques et cpb2 consensus furent identifiés dans respectivement 15 (13,6 %) et 4 (3,6 %) des 110 isolats. Tous les isolats de C. perfringens équins montraient des effets cytotoxiques nettement plus légers que les témoins positifs produisant CPB, bien que les isolats possédant les gènes cpb2 étaient légèrement mais significativement plus cytotoxiques que les témoins négatifs. En fonction de la population étudiée, il nous est impossible de confirmer notre hypothèse qu’en Ontario des C. perfringens produisant CPE et CPB-2 sont courants chez les chevaux avec colites et nous n’avons pas réussi à identifier une activité cytotoxique in vitro chez les types A isolés.
(Traduit par Docteur Serge Messier)
Introduction
Colitis is an acute and severe disease of horses that is often associated with a high fatality rate (1). Rooney et al (2) coined the term “colitis X” to describe colitis in horses as an independent illness. This is an outdated term that refers to acute fatal disease with unknown etiology, or idiopathic colitis (3). Due to the extensive size of their colon, horses are more seriously affected by colitis than other species without an expanded hind-gut (2). Studying the etiology of acute enterocolitis in horses can be challenging because of the presence of multiple potential pathogens, including Clostridium difficile, Clostridium perfringens, larval cyathostomiasis, Neorickettsia risticii, and Salmonella(1). Among these infectious agents, C. difficile and Salmonella are known to be major contributors to acute colitis in horses (4–6). Although progress has been made in identifying the causes of acute colitis in horses, the cause is still unknown in approximately 60% of cases in horses (6). Acute colitis has some of the classic hallmarks of a clostridial infection, such as rapid onset and development, evidence of toxemia, and development of necrotizing colitis that affects mainly the large intestine. Type A C. perfringens is commonly found in the large intestine of animals including horses and is a candidate as an agent for this often fatal disease. Other than C. difficile, however, the role of type A C. perfringens and other clostridia in equine colitis is not well understood. We hypothesized that C. perfringens producing entero-toxin (CPE) and/or beta2 toxin (CPB2) are common and important causes of severe colitis in horses and/or that C. perfringens producing an as-yet-undescribed cytotoxin may also cause colitis in horses.
Materials and methods
Case definition
The case definition for this study was individual horses presenting to the Large Animal Hospital at the Ontario Veterinary College with acute colitis or enterocolitis, as evidenced by the acute onset of diarrhea with multiple clinical signs of toxemia, such as fever, dehydration, tachycardia, tachypnea, prolonged capillary refill time, and congested mucous membranes, with supporting laboratory data. Changes in clinical pathological parameters, such as hemoconcentration, increased concentration of total solids (plasma protein), and reduced white blood cell count, were also included in the diagnostic assessment of toxemia. Feces were obtained from horses during defecation over a 2-year period (2010 to 2011) and frozen at −70°C within 6 to 12 h for later analysis. Horses had been ill for varying periods before presentation to hospital, but this information was not recorded. In general, horses arrived at the Ontario Veterinary College within 24 h of developing acute colitis. Freezing samples as soon as possible was a priority in the study design.
Salmonella detection
One gram (1 g) of feces was diluted 10-fold in 10 mL of the enrichment broth Selenite Cystine Broth (SCB) (Becton, Dickinson, Sparks, Maryland, USA) and incubated overnight at 37°C. One hundred microliters (100 μL) of SCB was then spread onto Brilliant Green Sulfa (BG) agar (Becton, Dickinson) and incubated overnight at 37°C. Pink colonies were presumptively considered to be Salmonella and cultures were further characterized using the BD BBL Enterotube II system (Becton, Dickinson), according to the manufacturer’s instructions.
Clostridium difficile detection
One gram (1 g) of fecal sample was inoculated in 9 mL of C. difficile moxalactam norfloxacin (CDMN) broth (Oxoid, Nepean, Ontario), enriched with 0.1% sodium taurocholate and incubated anaerobically at 37°C for 5 d. After incubation, 2 mL was transferred into a sterile test tube, mixed with an equal amount of absolute ethanol, and left at room temperature for 60 min. Samples were then centrifuged at 4400 × g for 10 min. The supernatant was discarded and the resulting pellet was plated onto blood agar and incubated anaerobically at 37°C for 48 h. Clostridium difficile was identified on the basis of typical colonial characteristics, Gram-stain morphology, and production of L-proline aminopeptidase. For quantitative culture, 1 g of feces was diluted in 9 mL of phosphate-buffered saline (PBS) at pH 7.2 and 10-fold serial dilutions (10−1 to 10−5) were prepared. One hundred microliter (100 μL) aliquots of the dilutions were cultured on CDMN agar and cell numbers were counted after anaerobic incubation at 37°C for 48 h.
Clostridium difficile toxin detection
Fecal samples were also examined for C. difficile toxins using the C. difficile TOX A/B II ELISA Kit (TECHLAB, Blacksburg, Virginia, USA), according to the manufacturer’s instruction.
Recovery and enumeration of Clostridium perfringens isolates
Feces were inoculated into PBS at a ratio of 1:9 (w/v) and serial dilutions prepared from 10−1 to 10−5. One hundred microliter (100 μL) aliquots of these dilutions were streaked on Shahidi-Ferguson Perfringens (SFP) agar (Becton, Dickinson) containing 5% egg yolk emulsion (Oxoid) and C. perfringens selective supplement (Oxoid) and incubated under anaerobic conditions overnight at 37°C. Plates containing 20 to 200 lecithinase-positive colonies were selected for bacterial enumeration. To confirm the identity of the lecithinase-positive colonies, 5 presumptive C. perfringens colonies were sub-cultured onto blood agar plates and evaluated for the large double zone of hemolysis and the microscopic morphology characteristic of C. perfringens. Bacteria other than C. perfringens were sometimes also isolated on SFP agar in large numbers. Five isolates of each of these were also stored frozen and characterized as described in a later section.
Clostridium perfringens toxins detection
Feces were examined for alpha toxin (CPA) and enterotoxin (CPE) using 2 commercially available ELISA kits [Bio-X Alpha toxin (Biox Diagnostic, Jemelle, Belgium) and C. perfringens Enterotoxin test (TECHLAB)], following the manufacturer’s instructions. An in-house ELISA was used for detecting CPB2 (7).
Genotyping of Clostridium perfringens isolates
The 5 C. perfringens isolates recovered per sample were subjected to multiplex TaqMan real-time polymerase chain reaction (PCR) for detection of: cpa (alpha toxin), cpb (beta toxin), cpb2 (beta2 toxin) consensus, cpb2 (beta2 toxin atypical), cpe (enterotoxin), etx (epsilon toxin), iap (iota toxin), netB (necrotic enteritis toxin B), and tpeL (large C. perfringens cytotoxin). Genotyping was done using primers, probes, and conditions described in a previous study (8). Appropriate genotyping reference strains were included (8).
Identification of non-Clostridium perfringens isolates recovered on SFP medium
Non-C. perfringens isolates recovered on SFP agar were submitted to the Animal Health Laboratory at the University of Guelph for identification using the MALDI-TOF MS (Matrix-assisted laser desorption ionization time-of-flight mass spectrometer) technique (Bruker Daltonics, Billerica, Massachusetts, USA).
Cytotoxicity screening of supernatant of Clostridium spp. isolates
Broth culture supernatants of all C. perfringens and Clostridium spp. other than C. difficile isolates were evaluated for cytotoxicity. For these assays, isolates were streaked onto blood agar and grown anaerobically overnight at 37°C. A single colony of each isolate was then inoculated into 5 mL of TPG (5% tryptone, 0.5% proteose peptone, 0.4% glucose, and 0.1% thioglycolic acid) and grown anaerobically at 37°C for ~ 16 h. Fifty microliters (50 μL) of the overnight culture was inoculated into 5 mL (1:100 v/v) of fresh TPG and grown for ~ 3 h at 37°C to an optical density600 of 0.6 to 0.8. The broth cultures were then centrifuged at 18 000 × g for 15 min before filtering (0.22 μm) to obtain a sterile filtrate. Toxicity was tested using an equine ovarian tumour cell line (EO; Dr. E. Nagy, University of Guelph). One hundred microliters (100 μL) of Eagle’s minimal essential medium (EMEM) complete medium [EMEM 450 mL, 10% fetal calf serum (VWR International, Radnor, Pennsylvania, USA) 50 mL, 2 mM L-glutamine (Sigma-Aldrich, St. Louis, Missouri, USA) 5 mL, 100 IU of penicillin, 2 mL of 0.1 mg/mL streptomycin] with 10% fetal calf serum (FCS) was added to wells of a flat-bottom, 96-well cell culture cluster with lid plates and seeded with ~ 2 μ 105 cells/well. The plate was then incubated at 37°C in an atmosphere of 5% carbon dioxide (CO2) for 24 to 36 h until the cell monolayer was ~ 100% confluent. The medium was then removed from the wells and replaced with 100 μL of complete EMEM containing 5% FCS. One hundred microliters (100 μL) of a 2-fold dilution from 1:2 to 1:64 (v/v) of sterile bacterial culture supernatant was added to wells in duplicate. Cytotoxicity was evaluated microscopically every 2 h for 12 h. Confluent monolayers of the EO cell line with no evidence of toxicity were given a score of “0”. Monolayers where 25%, 50%, 75%, or 100% of cells were rounded or deattached were given scores of 1+, 2+, 3+, and 4+, respectively. Clostridium perfringens strains NCTC3110 (cpb-positive) and CW504 (cpa-positive) were used as positive and negative controls.
Statistical analysis
Linear regression was used to evaluate the association between C. perfringens colony forming units (CFUs) and the presence of CPA and CPB2. Fisher’s exact test was used to evaluate the correlation between the presence of CPA and CPB2, and the relation between possession of cpb2 and cytotoxicity. A P-value of ≤ 0.05 was considered significant for comparisons.
Results
A total of 55 fecal samples was evaluated for known or presumptive bacterial pathogens and their toxins. Forty-three adult horses and 12 foals [8 foals < 1 mo old (5 foals 1 to 3 d old; 1 foal 7 d old; and 2 foals 21 to 22 d old) and 4 foals 1 to 6 mo old] met the case selection criteria. Several breeds were represented among the 55 affected horses, including 23 Thoroughbreds, 7 Standardbreds, 6 mixed breeds, 4 Quarter horses, 4 Dutch Warmbloods, and 1 to 2 horses from 8 other breeds. Common abnormalities detected during physical examination were diarrhea (55/55; 100%), fever (31/40; 77%), cold distal extremities (6/40; 15%), tachycardia (43/50; 86%), tachypnea (33/44; 75%), and clinical dehydration (50/53; 95%). Thirty-eight percent of 42 adults and 9% of 11 foals died or were euthanized due to poor prognosis. A detailed comparison of adult horses and foals is provided in Table I.
Table I.
Comparison of signalment, clinical findings, laboratory results, outcome, presence of C. perfringens and its toxins, as well as C. difficile between adult horses and foals
| Characteristics | Adult horses (n = 43) | Foals (n = 12) |
|---|---|---|
| Gender | M: 24 (55%) | M: 6 (50%) |
| F: 19 (45%) | F: 6 (50%) | |
| Tachycardia | 38/42 (90.5%) | 5/8 (62.5%) |
| Tachypnea | 27/35 (77%) | 6/9 (67%) |
| Fever | 28/35 (80%) | 3/5 (60%) |
| Cold distal extremities | 4/35 (11%) | 2/5 (40%) |
| Capillary refill time (prolonged) | 32/36 (89%) | 9/9 (100%) |
| Dehydrated | 39/42 (93%) | 11/11 (100%) |
| Haematocrit (0.28 to 0.44 L/L) | 0.41 | 0.36 |
| Total solids protein (57 to 75 g/L) | 59 | 60 |
| White blood cells (5.1 to 11 × 109/L) | 5.7 | 7.7 |
| Died/euthanized | 16/42 (38%) | 1/11 (9%) |
| C. perfringens isolated | 17/43 (40%) | 5/12 (42%) |
| C. difficile positive | 3/43 (7%) | 0% |
| CPA toxin positive | 6/43 (14%) | 2/12 (17%) |
| CPB2 toxin positive | 4/43 (9%) | 0/12 (0%) |
| CPA toxin positive Died/euthanized | 3/16 (19%) | 0% |
| CPB2 toxin positive Died/euthanized | 1/16 (6%) | 0% |
Salmonella and Clostridium difficile
Salmonella spp. were not isolated from any horse. Clostridium difficile and its toxins were identified in 3 samples (Table II).
Table II.
Recovery, enumeration, toxin detection (ELISA), and genotyping findings for Clostridium perfringens and Clostridium difficile from cases of equine colitis
| Case number | C. difficile | C. perfringens CFU/g | C. difficile toxins | CPB2 | CPA | Genotype isolate 1 | Genotype isolate 2 | Genotype isolate 3 | Genotype isolate 4 | Genotype isolate 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-567651 | Na | 3.5 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | ||||||||||
| 2-568529 | N | 1.2 × 106 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 3-567923 | N | 1.4 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 4-567811 | N | 9.7 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 5-567516 | N | 7.8 × 105 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 6-566936 | N | 3.2 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| cpb2 | cpb2 | |||||||||
| 7-567673 | N | 4.8 × 105 | N | N | Pb | Type A | Type A | Type A | Type A | Type A |
| 8-567168 | N | 2.5 × 104 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 9-600703 | N | 2.2 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 10-568035 | N | 1.6 × 104 | N | N | P | Type A | Type A | Type A | Type A | Type A |
| 11-568435 | N | 1.0 × 107 | N | N | P | Type A | Type A | Type A | Type A | Type A |
| 12-568644 | N | 2.8 × 103 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | cpb2-aty | cpb2-aty | ||||||||
| 13-105725 | N | 1.3 × 107 | N | N | P | Type A | Type A | Type C | Type A | Type A |
| cpb2 | cpb2 | |||||||||
| 14-568019 | N | 4.5 × 104 | N | P | P | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | cpb2-aty | |||||||||
| 15-568022 | N | 1.8 × 106 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 16-74354C | N | 5.6 × 105 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 17-566032 | N | 2.4 × 105 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| 18-602014 | N | 1.9 × 105 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | ||||||||||
| 19-602122 | N | 3.2 × 105 | N | P | P | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | ||||||||||
| 20-602129 | N | 3.0 × 104 | N | N | N | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | ||||||||||
| 21-568875 | 3.4 × 105 | 2.8 × 107 | P | P | P | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | cpb2-aty | cpb2-aty | cpb2-aty | cpb2-aty | ||||||
| 22-567835 | 8.0 × 105 | 3.5 × 103 | P | P | P | Type A | Type A | Type A | Type A | Type A |
| cpb2-aty | ||||||||||
| 23-565249 | 4.9 × 106 | N | P | N | N | — | — | — | — | — |
Na — Negative; Pb — Positive.
Recovery and enumeration of Clostridium perfringens
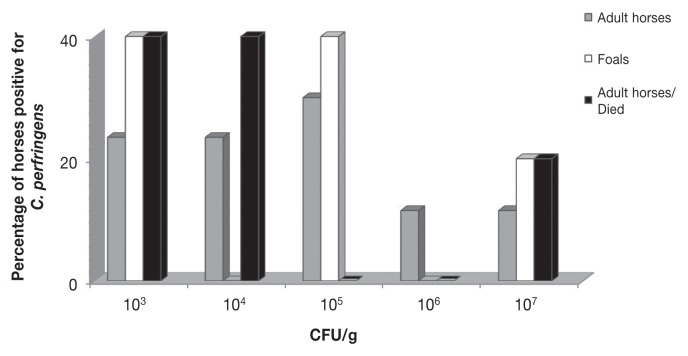
Clostridium perfringens was isolated from 22 (40%) of the 55 samples tested. Quantitative culture and the toxin ELISA results are shown in Table II and Figure 1. Counts of C. perfringens ranged from 1.4 × 103 to 2.8 × 107 CFUs/g. The theoretical minimum detectable CFUs were 1 × 102/g.
Figure 1.
Distribution of colony-forming unit (CFU) counts of C. perfringens recovered from adult horses and foals with colitis.
Clostridium perfringens toxin detection by ELISA
Whereas all fecal samples were negative for CPE, CPA and CPB2 toxins were detected in 36.4% (8 of 22) and 18.2% (4 of 22) of samples, respectively, all of which were positive for C. perfringens isolation (Table II). Using linear regression, a significant association was observed between CFU/g and the presence of CPA (P = 0.05), but no correlation could be demonstrated between CFU/g and the presence of CPB2 (P = 0.8). Fisher’s exact test indicated that the presence of CPA and CPB2 were correlated moderately and that CPB2 toxin was more likely to be identified if CPA was present (P = 0.01).
Genotyping of Clostridium perfringens isolates
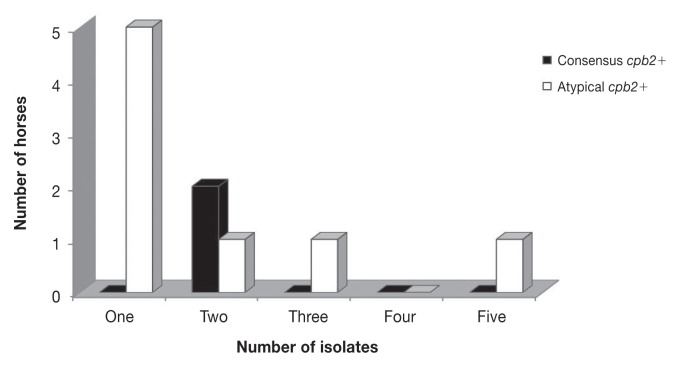
Of 110 C. perfringens isolates, 109 (99%) were type A and 1 was type C. None of the isolates was positive for cpe, netB, or tpeL. Atypical cpb2 and consensus cpb2 were identified in 15 (13.6%) and 4 (3.6%) isolates, respectively. Genotype variation was observed among the 5 isolates from 9 of 22 C. perfringens-positive horses (Table II; Figure 2).
Figure 2.
Genotype variation within 5 C. perfringens isolates per sample subjected to multiplex real-time polymerase chain reaction (PCR); consensus cpb2 or atypical cpb2 were not consistently detected within 5 isolates per cpb2-positive samples.
Recovery and identification of other Clostridium spp
Other Clostridium species isolated on SFP agar from 14 fecal samples were identified by MALDI-TOF MS. Clostridium bifermentans, recovered in 6 samples, showed lecithinase activity on SFP medium that was indistinguishable from that of C. perfringens, but differed by being almost entirely spore-forming and not producing black colonies. Clostridium butryricum, Clostridium tertium, and Clostridium innocuum were isolated from 3, 3, and 2 other samples, respectively.
Cytotoxicity screening of supernatant of Clostridium spp. isolates
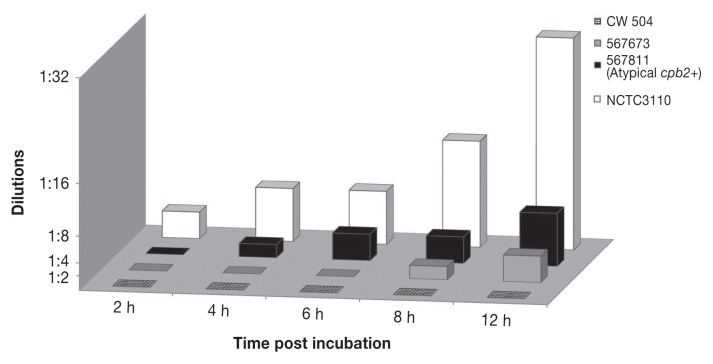
The equine ovarian cell line was found to be the most susceptible to the toxicity of the supernatant of the CPB-producing strain NCTC3110 of cell lines tested (CaCo2-human; MDBK-bovine; MDCK-canine; MIH3T3-mouse; PK3-porcine; 208-rat; although the canine A72 cell line was comparable, data not shown), and was therefore chosen for this study. In the cytotoxicity assay, strains NCTC3110 and CW504 used as positive and negative controls showed consistent toxicity scores. All equine Clostridium spp. were only mildly toxic compared to the CPB-positive control (Figure 3). Using Fisher’s exact test, a significant association was observed between cpb2-positive C. perfringens isolates and cytotoxicity at 6 h (P = 0.016) and at both 8 and 12 h of incubation (P = 0.0001).
Figure 3.
Comparison of supernatant toxicity of atypical cpb2-positive (567811) and type A C. perfringens(567673) strains from cases of colitis with positive control (NCTC3110) and negative control (CW504) strains. Dilutions are those showing 2+ toxicity.
Discussion
This study could not demonstrate that C. perfringens producing enterotoxin (CPE) and/or beta2 toxin (CPB2) are common and important causes of acute enterocolitis in horses in Ontario and/or that C. perfringens producing an as-yet-undescribed cytotoxin may also cause enterocolitis in horses. By following a logical, systematic, and innovative approach to try to implicate C. perfringens as well as other clostridia recovered in large numbers on SFP medium in equine enterocolitis in Ontario, the study reported here contributes to further understanding of an important but poorly understood disease of horses.
Despite this, the study has some limitations. Most importantly, this was not a case-control study and the results were not compared to age-matched healthy horses. Secondly, a relatively small number of horses was evaluated as only 55 cases were available that met the inclusion criteria for the study. Some samples came from animals with different and often incomplete histories. Factors such as antibiotic treatment of these horses, non-infectious causes of colitis, or delay in freezing samples might have influenced the outcome. Postmortem diagnoses of the animals that died were not obtained as part of the study. The inclusion of foals is another possible limitation of this study, since foals may suffer from viral infections, although none was recorded as such. There was no difference between foals and adults in clinical presentation and the presence and numbers of C. perfringens or its toxins (Table I). These limitations should be taken into account, while at the same time recognizing the strength of the multiple approaches used in this study.
The finding that C. perfringens was isolated in variable numbers in nearly half of the fecal samples (Table II) was consistent with the detection of this bacterium in 54% of healthy broodmares and foals in a previous study (9). Also, in agreement with earlier findings (9–12), type A C. perfringens was the most common genotype detected. This study did not support our hypothesis that cpe-positive and CPE-producing isolates of C. perfringens are common and important in equine enterocolitis. Enterotoxin (CPE) was not detected and neither was it found in any isolate. Previous investigations evaluating CPE in feces of horses with diarrhea and enterocolitis have had variable results. For example, C. perfringens was identified in 42 of 233 diarrheic foals; of 24 isolates genotyped, all were type A but cpe was found in only 3. Subsequently, CPE was detected in only 2 of 17 cases with C. perfringens positive culture results (11). By contrast with the results reported here, Weese and colleagues in Ontario detected CPE using ELISA in 19% of 47 adult horses and 29% of 28 foals with colitis and diarrhea (12). In Belgium, however, Van Baelen and Devriese did not find CPE in diarrheic feces from 9 adult horses (13). In another investigation, CPE was detected in 55% of foals with enteric diseases (14). Donaldson and Palmer found CPE in 9 of 57 diarrheic horses, but not in any of 57 control horses (10). Geographical differences, seasonal variations, as well as variation in detection approaches might all contribute to these differences from earlier studies. This is the first time that netB and tpeL have been searched for in C. perfringens isolated from equine enterocolitis. No isolates were positive for these genes, which suggests that neither is involved in equine enterocolitis in Ontario.
Clostridium perfringens alpha toxin was detected in one-third of C. perfringens culture-positive horses, with a significant correlation to the number of C. perfringens isolated. These data identified the presence of CPA in the feces of a small proportion of horses with colitis, but its significance is unclear.
Additionally, cpb2 genes were detected in approximately half the C. perfringens culture-positive cases, which is consistent with the results of other studies (15,16). Our study was not a case-control study using healthy horses as controls. Since cpb2-positive C. perfringens were detected in the feces of 22% of 124 healthy foals and 9% of 128 healthy adult horses in another study (9), it is important to detect CPB2 in feces before implicating cpb2-positive C. perfringens as a possible cause of colitis. In a related retrospective study, CPB2 was identified by immunohistochemistry in 40 of 69 horses with colitis and the results suggested a possible causal relationship between cpb2-positive C. perfringens and the incidence of colitis (16). The current study evaluated, for the first time by ELISA (7), the presence of CPB2 in equine feces. Clostridium perfringens beta2 toxin (CPB2) was detected in slightly less than 10% of samples. Although earlier studies have suggested that CPB2 plays an important role in colitis in hospitalized horses, especially those treated with gentamicin (15,16), it is not possible to conclude from our study that CPB2 is important in severe equine enterocolitis in Ontario. A case-control study, however, would be a better approach on which to base such a conclusion. The atypical cpb2 genotype has been detected in all CPB2 positive cases (7). Interestingly, atypical cpb2 was found 4 times more often than consensus cpb2, which is consistent with a previous study that found that consensus cpb2 is not common in non-porcine isolates (17). A slight cytotoxic effect associated with cpb2-positive isolates was noted in the in-vitro cytotoxicity assay (Figure 3). Fisher and co-workers reported that the atypical CPB2 was less cytotoxic to a CaCo-2 cell line than consensus CPB2 (18).
Although usually a cause of small bowel necrotizing enteritis in neonatal foals, type C infection has occasionally been recorded as a cause of colitis in adult horses (19); in the absence of a CPB ELISA or CPB immunohistochemistry on intestinal mucosa (19), the significance of a single type C isolate cannot be assessed. Others have however, the absence of cpb-positive C. perfringens in horses with necrotizing enteritis that were CPB toxin ELISA positive (19). A diagnosis based on genotyping alone may therefore be inadequate since type C isolates may be missed.
This is the first in-vitro investigation of the cytotoxicity of equine enterocolitis-associated C. perfringens and clostridia other than C. difficile. All equine C. perfringens isolates showed only mild toxicity scores (Figure 3). Toxicity could be the result of alpha toxin, proteases, or the numerous other secreted minor toxins of type A C. perfringens. By contrast, the CPB-producing control strain produced earlier and marked toxicity. Interestingly, a significant association was observed between cpb2-positive C. perfringens isolates and cytotoxicity and cpb2-negative equine isolates, although these isolates were only mildly toxic compared to the positive control; nevertheless, this finding supports the efficacy of the cytotoxicity assay. The equine cell line used was chosen because it was the most susceptible of cell lines tested to the CPB-positive control strain supernatant. It is possible, however, that this cell line might not be susceptible to a cytotoxin and that some equine isolates might produce more subtle, cytotonic effects that would not have been identified by the visual cytotoxicity approach used. Some strains might produce a novel toxin under in vivo conditions when present in the colon, since toxin production might be different in vivo(20).
Importantly, the presence and toxicity of other clostridia were assessed in all samples where their growth occurred on SFP medium. Isolation of a Clostridium spp. from the feces of a diarrheic animal cannot be accepted as prima facie evidence of involvement in disease. The other Clostridium spp. isolated are part of the normal microflora, ubiquitous in soil, sewage, and the environment of animals and humans (21) and they failed to induce toxicity in the cell line. It is therefore concluded that these isolates did not produce a cytotoxin and were not involved in the disease process.
No Salmonella spp. was isolated from horses with enterocolitis. This came as a surprise since, although some other studies have failed to find any association between colitis and Salmonella(10,22), salmonellosis is an important cause of enterocolitis in hospitalized horses (1). Serial sampling might have increased the isolation of Salmonella. The overall apparent culture prevalence of C. difficile was low and approximately 4 times less than that previously reported in Ontario (12). Assessment of a larger number of horses might alter the prevalence of C. difficile colitis.
Based on this sampled population and acknowledging the limitations of the study, including the relatively small number of animals assessed and the absence of a healthy comparison population, it is concluded that there is no evidence that C. perfringens plays an important role in enterocolitis in horses in Ontario. This study essentially provides “negative data” that may form the basis of future work with larger number of samples, since it takes time to build a collection of feces from individual horses with colitis and to develop the methodology to assess the clostridial isolates for cytotoxicity and other characteristics. Extensive effort will be required to identify the unknown causes of severe colitis in horses due to the complexities of case selection, isolating and identifying potential pathogens, and assessing for toxin production. There is considerable scope for an internationally coordinated case-controlled study using both culture- and molecular-based technologies on carefully selected and adequately stored samples, including the methods and approaches applied in this study.
Acknowledgments
The authors thank Equine Guelph and the Natural Sciences and Engineering Research Council of Canada for funding. We thank Jasmina Kircanski, University of Guelph, for assistance with the CPB2 ELISA and Durda Slavic, Animal Health Laboratory, University of Guelph, for assistance with MALDI-TOF identification of Clostridium species.
References
- 1.Feary DJ, Hassel DM. Enteritis and colitis in horses. Vet Clin North Am Equine Pract. 2006;22:437–479. doi: 10.1016/j.cveq.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Rooney JR, Bryans JT, Doll ER. Colitis “X” of horses. J Am Vet Med Assoc. 1963;142:510–511. [PubMed] [Google Scholar]
- 3.Staempfli HR, Townsend HGG, Prescott JF. Prognostic features and clinical presentation of acute idiopathic enterocolitis in horses. Can J Vet Res. 1991;32:232–237. [PMC free article] [PubMed] [Google Scholar]
- 4.Båverud V. PhD dissertation. Uppsala, Sweden: Swedish University of Agricultural Sciences; 2002. Clostridium difficile in horses. [Google Scholar]
- 5.Weese JS, Toxopeus L, Arroyo L. Clostridium difficile associated diarrhea in horses within the community: Predictors, clinical presentation and outcome. Equine Vet J. 2006;38:185–188. doi: 10.2746/042516406776563369. [DOI] [PubMed] [Google Scholar]
- 6.Ruby R, Magdesian KG, Kass PH. Comparison of clinical, microbiologic, and clinicopathologic findings in horses positive and negative for Clostridium difficile infection. J Am Vet Med Assoc. 2009;234:777–784. doi: 10.2460/javma.234.6.777. [DOI] [PubMed] [Google Scholar]
- 7.Kircanski J, Hodgins D, Soltes G, et al. Development of an antigen-capture enzyme-linked immunosorbent assay for Clostridium perfringens beta2-toxin in porcine feces and the neonatal piglet intestine. J Vet Diagn Invest. 2012;24:895–902. doi: 10.1177/1040638712453584. [DOI] [PubMed] [Google Scholar]
- 8.Farzan A, Kircanski J, DeLay J, et al. An investigation into the association between cpb2-encoding Clostridum perfringens type A and diarrhea in neonatal piglets. Can J Vet Res. 2013;77:45–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Tillotson K, Traub-Dargatz JL, Dickinson CE, et al. Population-based study of fecal shedding of Clostridium perfringens in broodmares and foals. J Am Vet Med Assoc. 2002;220:342–348. doi: 10.2460/javma.2002.220.342. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson MT, Palmer JE. Prevalence of Clostridium perfringens enterotoxin and Clostridium difficile toxin A in feces of horses with diarrhea and colic. J Am Vet Med Assoc. 1999;215:358–361. [PubMed] [Google Scholar]
- 11.Frederick J, Giguère S, Sanchez LC. Infectious agents detected in the feces of diarrheic foals: A retrospective study of 233 cases (2003–2008) J Vet Intern Med. 2009;23:1254–1260. doi: 10.1111/j.1939-1676.2009.0383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weese JS, Staempfli HR, Prescott JF. A prospective study of the roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhea. Equine Vet J. 2001;33:403–409. doi: 10.2746/042516401776249534. [DOI] [PubMed] [Google Scholar]
- 13.Van Baelen D, Devriese LA. Presence of Clostridium perfringens enterotoxin in intestinal samples from farm animals with diarrhea of unknown origin. Zentralbl Veterinarmed B. 1987;34:713–716. doi: 10.1111/j.1439-0450.1987.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Kanoe M, Inoue S, Abe T, et al. Isolation of Clostridium perfringens from foals. Microbios. 1990;64:153–158. [PubMed] [Google Scholar]
- 15.Herholz C, Miserez R, Nicolet J, et al. Prevalence of beta2- toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol. 1999;37:358–361. doi: 10.1128/jcm.37.2.358-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacciarini LN, Boerlin P, Straub R, Frey J, Grone A. Immunohistochemical localization of Clostridium perfringens beta2-toxin in the gastrointestinal tract of horses. Vet Pathol. 2003;40:376–381. doi: 10.1354/vp.40-4-376. [DOI] [PubMed] [Google Scholar]
- 17.Jost BH, Billington SJ, Trinh HT, Bueschel DM, Songer JG. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of non-porcine origin. Infect Immun. 2005;73:652–656. doi: 10.1128/IAI.73.1.652-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher DJ, Miyamoto K, Harrison B, Akimoto S, Sarker MR, McClane BA. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol Microbiol. 2005;56:747–762. doi: 10.1111/j.1365-2958.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- 19.Diab SS, Kinde H, Moore J, et al. Pathology of Clostridium perfringens Type C enterotoxemia in horses. Vet Pathol. 2012;49:255–263. doi: 10.1177/0300985811404710. [DOI] [PubMed] [Google Scholar]
- 20.Traub-Dargatz JL, Jones RL. Clostridia-associated enterocolitis in adult horses and foals. Vet Clin North Am Equine Pract. 1993;9:411–421. doi: 10.1016/s0749-0739(17)30407-8. [DOI] [PubMed] [Google Scholar]
- 21.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGorum BC, Dixon PM, Smith DGE. Use of metronidazole in equine acute idiopathic toxaemic colitis. Vet Rec. 1998;142:635–638. doi: 10.1136/vr.142.23.635. [DOI] [PubMed] [Google Scholar]