Abstract
Salmonella hold considerable promise as vaccine delivery vectors for heterologous antigens in chickens. Such vaccines have the potential additional benefit of also controlling Salmonella infection in immunized birds. As a way of selecting attenuated strains with optimal immunogenic potential as antigen delivery vectors, this study screened 20 novel Salmonella Typhimurium vaccine strains, differing in mutations associated with delayed antigen synthesis and delayed attenuation, for their efficacy in controlling colonization by virulent Salmonella Typhimurium, as well as for their persistence in the intestine and the spleen. Marked differences were observed between strains in these characteristics, which provide the basis for selection for further study as vaccine vectors.
Résumé
La bactérie Salmonella est considérée comme un vecteur vaccinal prometteur pour la livraison d’antigènes hétérologues chez les poulets. De tels vaccins ont le potentiel bénéfique supplémentaire de limiter les infections par Salmonella chez les oiseaux immunisés. Comme moyen de sélectionner les souches atténuées avec le potentiel immunogène optimal comme vecteur de livraison d’antigènes, la présente étude a examiné 20 souches vaccinales nouvelles de Salmonella Typhimurium, qui différaient en mutation associées avec une synthèse antigénique retardée et une atténuation retardée, pour leur efficacité à limiter la colonisation par du Salmonella Typhimurium virulent, ainsi que pour leur persistance dans l’intestin et la rate. Des différences marquées furent observées entre les souches pour ces caractéristiques, fournissant ainsi des éléments de sélection pour des études ultérieures comme vecteurs vaccinal.
(Traduit par Docteur Serge Messier)
Introduction
Salmonellosis is one of the most important food-borne zoonotic diseases throughout the world, and poultry products represent an important source of human infection. Chicken meat and eggs have been found to be common food sources of Salmonella enterica infection (1). Among the 2500 serovars of Salmonella, Enteritidis and Typhimurium have been responsible for the majority of food-borne salmonellosis in humans in the past, although the pattern of dominant serovar varies over time and geographically (2,3).
Effective control of food-borne pathogens, including Salmonella, is a major challenge to the poultry industry. Control of Salmonella infection in chicken focuses partly on farm management, although vaccination is sometimes used, for example in Europe to control S. Enteritidis infection (4). For vaccine prevention, both killed virulent and live attenuated Salmonella strains are used (4,5). Although killed and attenuated Salmonella vaccines reduce intestinal colonization, and egg and meat contamination, the level of protection is variable, dependent in part on the serovars involved (6,7). Live attenuated Salmonella vaccines have been shown to induce both humoral and cell-mediated responses similar to those observed after natural infections (8,9), and have the benefits of ease of administration and low cost. Salmonella have promise as delivery vectors for control of bacterial and viral infections in chickens (10–21). In recent years, improvements of attenuated Salmonella as vaccine vehicles have included regulated delayed attenuation (22) and regulated antigen synthesis (23) such that strains express protective antigens at high levels and stimulate strong and lasting immune responses without producing tissue damage, while improving their immunogenicity and safety (23–27).
We have been interested in assessing the value of attenuated Salmonella as vectors for the control of necrotic enteritis (7,16,17,21). If Salmonella vectored vaccines are developed that can effectively control necrotic enteritis, one additional benefit might be the reduction of colonization by Salmonella, so that these vaccines could have an additional benefit to public health. In an earlier study of a Salmonella vaccine vectored control of necrotic enteritis, the unexpectedly disappointing results obtained were attributed to the poor viability of the vector in chickens (7,21). The strain induced only a weak protective effect against Salmonella colonization. There was an association between the efficacy of vaccine strains in protecting against necrotic enteritis and their efficacy in protecting against Salmonella colonization (7,16,21). We concluded (7) that the optimal choice of an attenuated S. Typhimurium vaccine vector for delivery of heterologous antigens in chickens should be based partly on its value in protecting against colonization with serovars within the serogroup B or D.
The purpose of the study reported here was to screen a large number (n = 20) of novel Salmonella vaccine vector strains for their efficacy in controlling colonization by a virulent strain of Salmonella Typhimurium, as the first step in identifying potentially improved vaccine vectors for delivery of heterologous antigens likely to give protection against necrotic enteritis.
Materials and methods
Chickens
White Leghorn mixed-gender fertile specific-pathogen-free (SPF) eggs, obtained from the Animal Disease Research Institute, Canadian Food Inspection Agency (Ottawa, Ontario), were hatched at the Arkell Poultry Research Station, University of Guelph. Two separate experiments, A and B, were carried out under similar conditions. Ten birds in each group were housed in Horsfall isolators to prevent cross-contamination. All chickens were fed an antibiotic–free chicken starter containing 20% protein. Experiments were approved and monitored by the University of Guelph Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
Bacterial strains
Salmonella vaccine strains and the Salmonella Typhimurium wild type are listed in Table I. All Salmonella vaccine strains, except χ3985, had a mutation in the asd gene (28). Briefly, mutants involved genes including combinations of: ara, affecting arabinose utilization (a carbohydrate not available in vivo) and arabinose-controlled expression of other genes; ΔPcrparaC PBADcrp, conferring arabinose-dependent expression of the crp gene that encodes the catabolite regulatory protein (29); ΔPfuraraC PBADfur, giving differential expression of the fur gene dependent on arabinose and causing up-regulation of all Fur-regulated genes for iron and partially for manganese uptake in vivo after colonization of lymphoid tissues; gmd-fcl, eliminating 2 enzymes in colanic acid synthesis pathway for conversion of GDP-mannose to GDP-fucose; lacI, the gene encoding the LacI repressor to confer regulated delayed in vivo synthesis of recombinant protective proteins; ΔpabA, requirement for p-aminobenzoic acid; PphoPQ177::TT araC PBADphoPQ, conferring arabinose-dependent expression of the global virulence regulator genes phoP and phoQ; pmi, phosphomannose isomerase, conferring mannose-dependent LPS O-antigen synthesis and loss of O-antigen in vivo due to a lack of free mannose; Prfc174::TT araC PBADrfc and ΔPrfaH178::TT araC PBADrfaH conferring arabinose-dependent LPS O-antigen synthesis through regulation of either rfc, encoding O-antigen polymerase or rfaH, encoding an antitermination factor required for transcription of the rfb operon; and ΔrelA, uncoupling growth from necessary protein synthesis.
Table I.
Salmonella strains used in this study
| Strains | Genotypes | Description of strains |
|---|---|---|
| χ3985 | Δcrp-11 Δ[zhb ::Tn10] Δcya-12 Δ[zid-62 ::Tn10] | Curtiss et al (22); Hassan and Curtiss (29) |
| χ3987 | Δcrp-11 Δ[zhb ::Tn10] Δcya-12 Δ[zid-62 ::Tn10] ΔasdA1 Δ[zhf::Tn10] (χ3985 derivative, ΔasdA1) | Wyszynska et al. (13) |
| χ9373 | Δpmi-2426 Δ (gmd-fcl)-26 ΔPfur81::TT araC PBADfur ΔPcrp527::TT araC PBADcrp ΔasdA21::TT araC PBADc2 ΔaraE25 ΔaraBAD23.ΔrelA198::araC PBADlacI TT | Li et al. (18) |
| χ9088 | Δpmi-2426 Δ (gmd-fcl)-26. ΔPfur33::TT araC PBADfur | Curtiss et al. (24) |
| χ9241 | ΔpabA1516. ΔpabB232 ΔasdA16 ΔaraBAD23.ΔrelA198::araC PBADlacI TT | Wang et al. (23) |
| χ9852 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT ΔPrfaH178::TT araC PBADrfaH | Kong et al. (19) |
| χ9853 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT Prfc174::TT araC PBADrfc | Kong et al. (27) |
| χ9885 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT Δrfc-48 | Kong et al. (27) |
| χ9894 | PphoPQ177::TT araC PBADphoPQ ΔaraBAD23.ΔasdA33.ΔPcrp527::TT araC PBADcrp | Kader R, Curtiss R, III |
| χ9945 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23ΔrelA198::araC PBADlacI TT ΔrfaH49 | Kong et al. (19) |
| χ11304 | Mutation details proprietary | Kong Q, Shi Z, Curtiss R, III |
| χ11426 | ΔasdA33 ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPcrp527::TT araC PBADcrp Brenneman | K, Curtiss R, III |
| χ11434 | ΔasdA33 ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPfur81::TT araC PBADfur | Brenneman K, Curtiss R, III |
| χ11442 | ΔasdA33.ΔrelA198::araC PBADlacI TT ΔaraBAD23 Δpmi-2426 | Brenneman K, Curtiss R, III |
| χ11437 | ΔasdA33 ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPcrp527::TT araC PBADcrp ΔPfur81::TT araC PBADfur | Brenneman K, Curtiss R, III |
| χ11443 | ΔasdA33.ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPfur81::TT araC PBADfur Δpmi-2426 | Brenneman K, Curtiss R, III |
| χ11444 | ΔasdA33.ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPcrp527::TT araC PBADcrp Δpmi-2426 | Brenneman K, Curtiss R, III |
| χ11445 | Δpmi-2426 Δ(gmd-fcl)-26 ΔPfur33::TT araC PBADfur ΔPcrp527::TT araC PBADcrp ΔasdA21::TT araC PBADc2 ΔaraE25 ΔaraBAD23 ΔrelA198::araC PBADlacI TT | Wanda S-Y, Curtiss R, III |
| χ11447 | Mutation details proprietary | Maddux J, Shi Z, Curtiss R, III |
| χ11464 | ΔasdA33.ΔrelA198::araC PBADlacI TT ΔaraBAD23 ΔPcrp527::TT araC PBADcrp ΔPfur81::TT araC PBADfur Δpmi-2426 | Brenneman K, Curtiss R, III |
| Salmonella Typhimurium | Strain SA 2004-4003; Nalidixic acid resistant; wild type virulent challenge strain. |
Growth curves
All Salmonella vaccine strains carrying the Δasd mutations were complemented by an asd+ plasmid (pYA3342), so that they could grow in the absence of 2–6-diaminopimelic acid (DAP) in vitro and in vivo(30). Complemented Salmonella vaccine vector strains and wild type S. Typhimurium were grown in LB broth (Difco, Detroit, Michigan, USA) only or in LB broth containing 0.05% L-arabinose. Mannose was not added to the media for this experiment. Each overnight culture was adjusted to an optical density at 600 nm (OD600) to 0.5, and 0.5 mL was then inoculated into 50 mL pre-warmed LB or LB containing 0.05% L-arabinose. The OD600 was measured every 60 min.
Growth of Salmonella for vaccination of chickens
The vaccine strains and wild type strain were grown in LB broth supplemented with 0.05% L-arabinose and, for strains carrying a Δpmi mutation, 0.1% mannose (Table I). A 100 mL volume of prewarmed LB broth was inoculated with 2 mL fresh overnight culture and incubated at 37°C until an OD600 of 0.8 was achieved. Cells were pelleted by centrifugation at 8000 × g for 15 min at 4°C. The pellet was suspended with 2 mL phosphate buffered saline, pH 7.2, with 1% gelatin (BSG), and serial dilutions of suspended Salmonella strains were plated onto unsupplemented LB agar to determine the numbers of colony-forming units (CFU). Vaccine strains were diluted to 1 × 109 CFU/mL and the wild type challenge strain was diluted to 2 × 106 CFU/mL.
Vaccination procedure
Two studies were done with the different vaccine strains. The first study (study A) involved 100 birds and the second, study B, involved 120 birds. For these studies, 1-day-old SPF white Leghorn chickens were randomly divided into groups of 10 chickens, which were housed individually in heated Horsfall isolators equipped with high efficiency particulate air filters. Groups were deprived of food and water for 4 h before crop inoculation (gavage) for vaccination or challenge; food and water were returned 1 h later. All, except one group in each study, were vaccinated at 1 d of age and given a booster of the same dose at 7 d of age. The vaccine dose was 1 × 108 CFU of the vaccine strain suspended in BSG, delivered directly into the crop by injection with a 1 mL syringe. One control group was vaccinated on the same schedule with 100 μL BSG. Five birds of each group (10 birds in the control group) were challenged orally at 14 d of age with 2 × 105 CFU of the wild type strain, SA2004-4003.
Enumeration of vaccine strains in spleen, feces, and cecum
Sample collection and processing was similar to that described previously (7). Pooled fecal samples were collected after vaccination on day 14 of age. Five birds per group were euthanized on day 14 of age and processed as described (31). The spleen and cecum were collected aseptically from each bird into sterile preweighed sample tubes, weighed, and kept on ice. The spleen was homogenized 1:10 (w/v) in selenite cystine broth (SCB, Difco) and serially diluted in BSG before plating onto Salmonella-Shigella agar (Difco). Colorless colonies with black centers of typical Salmonella were counted. Day 14 cecal content and the pooled day 14 fecal sample were serially diluted as 1:10 (w/v) in SCB, incubated for 36 to 48 h at 37°C, and 100 μL aliquots were plated onto brilliant green agar (Difco), and incubated at 37°C for 24 h. Pink colonies were considered to be positive and subcultured on MacConkey agar plates supplemented with 0.5% maltose to verify their colorless phenotype.
Enumeration of the wild type challenge strain in tissues and cecum
After challenge with strain SA 2004-4003 at 2 wk of age, a pooled fecal sample was collected on day 28 of age. Five birds per group were also euthanized on day 28. The spleen and cecal contents collection and processing methods for these birds were as described for the vaccine strains, except that 100 μL aliquots of samples serially diluted in SCB were immediately plated onto brilliant green agar and Salmonella-Shigella agar supplemented with 30 μg/mL nalidixic acid, then incubated at 37°C for 24 h. To monitor day 28 fecal shedding of the challenge strain, the feces were processed as described for the vaccine strains except that 100 μL aliquots of samples serially diluted in SCB were immediately plated onto brilliant green agar (Difco) supplemented with 30 μg/mL nalidixic acid and incubated for 24 h.
Statistical analysis
The CFU data were analyzed for significance using a 2-tailed Student’s t-test. The null hypothesis was rejected if P < 0.05.
Results
Growth of vaccine strains in vitro
Eighteen of the 20 vaccine strains have been modified for delayed attenuation and for heterologous antigen expression controlled by arabinose. Therefore, 2 parallel experiments were conducted under the same conditions, except that in the second study 0.05% L-arabinose was added to LB broth. No difference was noted in the presence and absence of arabinose. The growth characters of the 20 strains showed division into 3 types: fast, intermediate, and slow growers. Two strains (χ11304, χ11464) were slow, 9 strains (χ3985, χ3987, χ9373, χ9894, χ11426, χ11434, χ11437, χ11444, χ11447) were intermediate, and 9 strains (χ9088, χ9241, χ9852, χ9853, χ9885, χ9945, χ11442, χ11443, χ11445) were fast growers (data not shown).
Vaccine strains shedding in feces
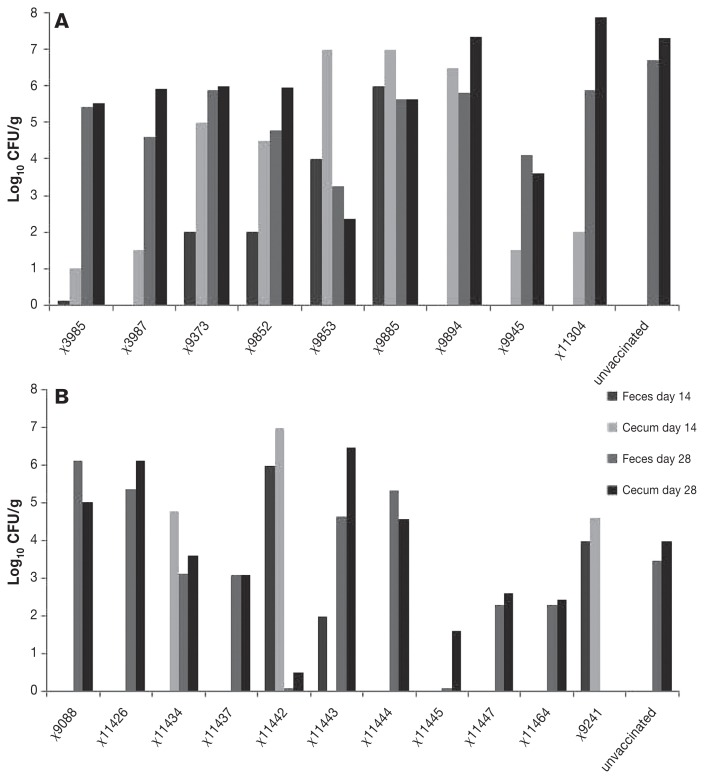
Fecal Salmonella vaccine shedding on day 14 after immunization is summarized in Figure 1. Four of the 20 vaccines strains (χ9241, χ9853, χ9885, χ11442) shed at levels higher than 104 CFU/g, shedding of 13 strains was undetected, and the remaining 3 strains (χ9375, χ9852, χ11443) showed intermediate levels of shedding.
Figure 1.
Isolation of S. Typhimurium vaccine strains (day 14) and wild-type challenge strain (day 28) from pooled feces and ceca (CFU/g) of inoculated birds (5 birds/group). Strain genotypes are given in Table I.
Vaccine strains colonization of the cecum and spleen
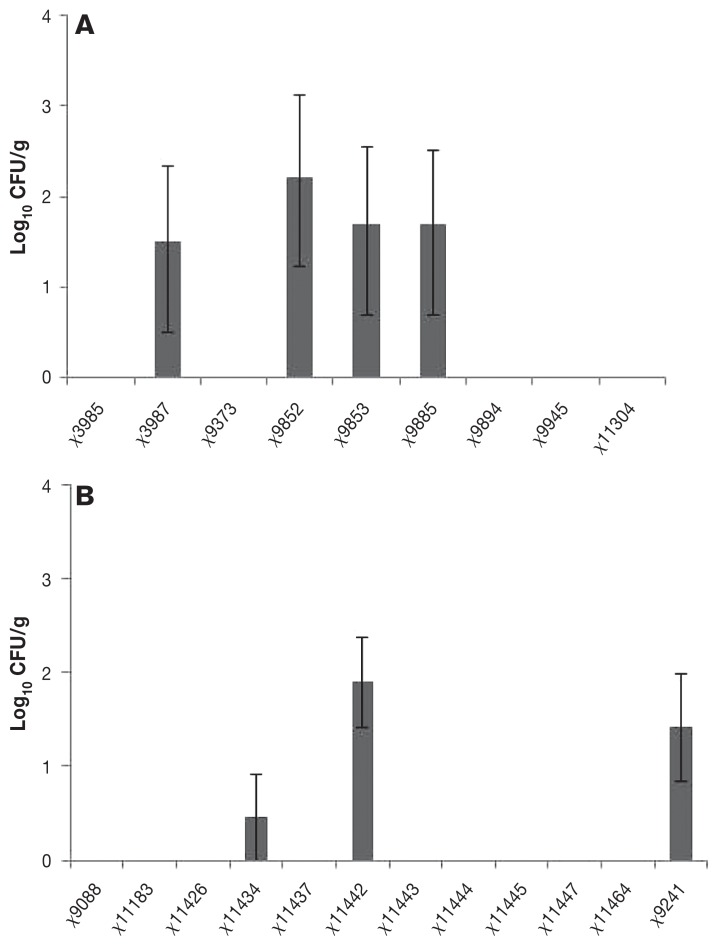
The colonizing ability of the 12 strains that were found in the cecum varied from ≥ 107 to 100.5 CFU/g (Figure 1). The order from highest to lowest was χ11442, χ9885, χ9853, χ9894, χ11434, χ9241, χ9375, χ9852, χ11304, χ3987, χ9945, and χ3985. Cecal colonization of vaccine strains was more marked in birds in study A (9/9) than in study B (3/11). Eight of 20 vaccine strains were not detected in the cecum on day 14 after primary immunization (Figure 1), all of which were detected in study B (Figure 1). In general, slight or absent fecal shedding on day 14 after immunization corresponded with low numbers in the cecum (Figure 1). A pattern generally reflecting high cecal colonization by the different vaccine strains was observed in the spleen (Figure 2). Seven of the 20 vaccine strains were found in the spleen, representing 7 of the 12 vaccine strains present in the cecum at day 14 (Figure 1). The order from the highest to lowest was χ9852, χ11442, χ9885, χ9853, χ3987, χ9241, and χ11434 (Figure 1).
Figure 2.
Isolation of Salmonella enterica serovar Typhimurium vaccine strains in log10 colony-forming units from the spleen on day 14 after primary immunization (5 birds each group).
Challenge strain shedding in feces
The virulent challenge strain was isolated from unvaccinated birds at about 3 logs higher in study A than in study B (Figure 1), and the numbers of wild type bacteria in the feces and cecum were correspondingly higher on day 28 in the immunized birds in study A (Figure 1).
On day 28 after immunization, 3 of the 20 vaccine strains showed reductions in fecal shedding of the wild type challenge strain of approximately 2log10 CFU (χ9945, χ11447, χ11464), 1 strain (χ9853) of approximately 3log10 CFU, and 3 strains (χ9241, χ11442, χ11445) of approximately 4log10 CFU or greater compared to the non-vaccinated control birds (Figure 1).
Challenge strain colonization in cecum and spleen
On day 28 after immunization, numbers of the challenge strain in the cecum were generally similar to those in the feces (Figure 1). Three of the 20 vaccine strains showed cecal reductions of the challenge strain of approximately 2log10 CFU (χ11437, χ11447, χ11464), 3 strains (χ9853, χ9945, χ11445) of approximately 3log10 CFU, and 2 strains (χ9241, χ11442) of approximately 4log10 CFU compared to non-vaccinated control birds (Figure 1). Three of these (χ9241, χ9853, χ11442) showed statistically significant reductions in cecal colonization compared to the non-vaccinated but challenged control chickens (Figure 1).
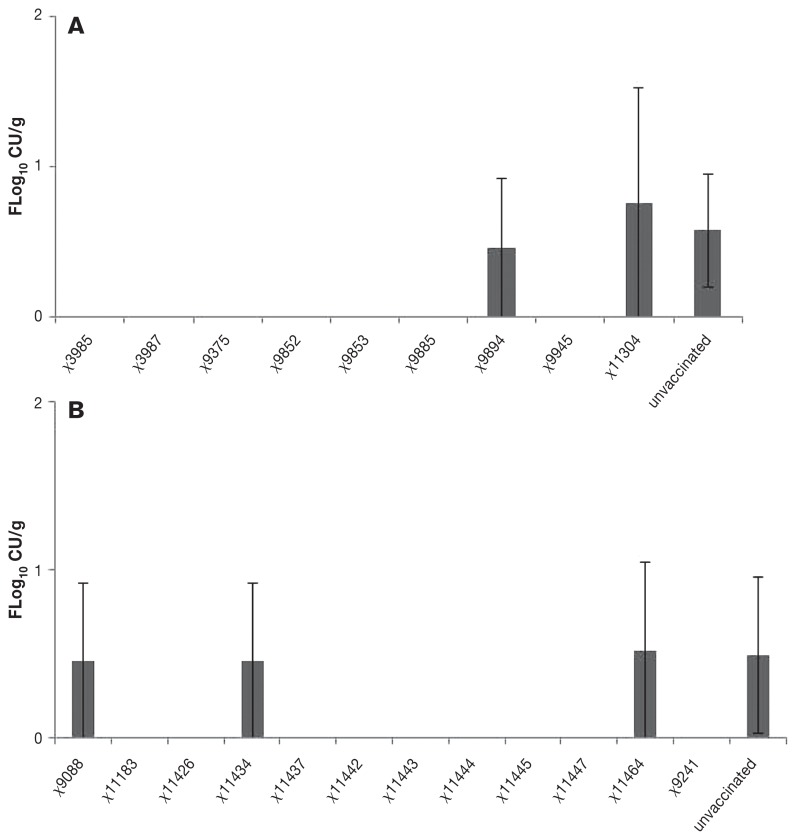
The challenge strain was found in small but variable numbers in the spleen in 7 groups 28 d after initial immunization (Figure 3).
Figure 3.
Isolation of wild type challenge strain of Salmonella enterica serovar Typhimurium in log10 colony-forming units from the spleen on day 28, 14 d after challenge (5 birds each group).
Discussion
There were large differences between the 20 novel genetically modified vaccine strains in persistence in the intestinal tract of vaccinated birds and in protection against colonization by wild type Salmonella. Among these strains, immunization with χ9241, χ9853, χ9945, χ11442, and χ11445 produced the greatest reductions in fecal shedding and cecal colonization of wild type S. Typhimurium. Three of these (χ9241, χ9853, χ11442) showed significant reductions in challenge strain cecal colonization (Figure 1).
The growth curve showed that 11 strains grew slower than the wild type. Two of the slowest growing strains (χ11304, χ11464) showed very limited ability either to persist in the intestine or to invade the spleen (Figures 1, 2, and 3). These slow-growing strains produced poor protection against wild type intestinal colonization and invasion of the spleen (Figures 1, 2, and 3). These strains also grew slowly in vitro, indicating that they are much attenuated. On this basis, these would likely be poor choices as vectors for heterogenous antigens.
In contrast with the strains that grew poorly in vitro, there was no clear pattern discernible between intermediate and fast growers in their ability to persist in the intestine and spleen and to protect against colonization by the wild type challenge strain. For example, one strain that grew fast in vitro, strain χ11442, colonized the intestine well, persisted in the spleen of immunized birds, and reduced intestinal and splenic colonization by the wild type challenge strain. Strain χ9088 also grew fast in vitro, but colonized the intestine and cecum poorly, showed no persistence in the spleen, and produced very poor protection against challenge strain infection. The shedding of vaccine strains in feces on day 14 after immunization was generally consistent with their numbers in the cecum on day 14 (Figure 1), and generally corresponded with their presence in the spleen on day 14 (Figure 2). The vaccine strains found in the spleen (Figure 2) and in the cecum on day 14 after immunization (Figure 1) were, generally, most likely to prevent the presence of the wild type challenge strain in the spleen on day 28 (Figure 3). Notably, 3 of the 4 best vaccine strains (χ9241, χ9853, χ11442) were isolated from the spleen on day 14 after immunization; χ11445 was the exception. With this and one other exception (χ9852), these strains were also most effective in reducing wild type intestinal colonization and presence in the spleen. We did not examine the efficiency of plating of the vaccine strains on brilliant green agar or their growth in SCB; mutations can affect plating efficiency in or on selective media. There was, however, no notable lack of growth of a vaccine strain in feces and cecum with a discrepant ability to control wild type colonization (Figure 1), suggesting that the growth of mutants was efficient.
There was a marked difference in wild type colonization in nonimmunized controls between birds in study A and study B; the reason for this difference is unclear. We, therefore, compared the two groups by examining log10 reduction in CFU in these birds, although this approach may underestimate the efficacy of immunization by some strains in study A, for example χ9945. Unexpectedly, χ3985 and χ3987, which is a DAP mutant of χ3985, were not as effective in preventing wild type colonization of ceca as previously reported (31,32). This discrepancy might be related to the 10-fold reduction in vaccine dose used in the current study and/or to the high challenge that birds in study A experienced, to differences in the challenge strains, or to differences in immunization and challenge schedules.
In general, strains with mutations in pabA and pabB appeared to colonize better than strains with other mutations and give better protection against wild type colonization. However, there was no clear pattern across the range of mutants assessed. Details of the mutations in the 2 least immunogenic strains (χ11304, χ11447), which were the poorest growers, were proprietary, so we cannot comment further on the relationship between specific mutations and colonizing ability. lacI, the gene encoding the LacI repressor confer regulated delayed in vivo synthesis of recombinant protective proteins under the control of the arabinose promoter, was present in 15 of the 20 candidate vaccine strains (Table I). Once the strain is present in the intestine, where arabinose is absent, then antigen expression is no longer repressed (23). There is evidence that LacI synthesis may itself be attenuating (33). At low copy, without control of an antigen, LacI may have a slight detrimental effect on growth, but when an antigen gene under the control of LacI is present then enhanced immunity has been demonstrated (34). There was, however, no clear pattern here of the effect of lacI on immunogenicity of the vaccine strains tested, and no effect on growth of araC PBADlacI TT strains in the presence or absence of arabinose. The lack of apparent effect of arabinose in enhancing growth of the arabinose-regulated (ΔPcrparaC PBADcrp) mutants compared to the unregulated Δcrp mutant χ3985 was unexpected, but may be explained by other genotypic differences.
The ability of vaccine strains to persist in the intestine and to invade the spleen is critical for an effective immune response against Salmonella(20). However, high colonization in the cecum is associated with invasion of the spleen and liver, raising potential food safety issues for consumers. Selection of vaccine strains should be based on efficacy as antigen delivery vectors for the target infection and their potential hazard for consumers. This study indicated that χ9241, χ9853, and χ11442 among other strains, are potential vaccine candidates for control of Salmonella infection in chickens, with χ11445 having an apparent advantage in not persisting in the spleen.
Our goal is to develop an oral Salmonella vaccine against chicken necrotic enteritis while also controlling Salmonella infection in chickens. A previous study indicated that there was an association between the efficacy of vaccine strains in protecting necrotic enteritis and in protecting against Salmonella colonization (7,16,21). The current study was designed to make the selection of potential vaccine vectors less empirical by selecting them on the basis of immunogenicity against Salmonella. Further work using strains selected on this basis from the 20 strains examined here as vectors for Clostridium perfringens antigens is, however, required to determine whether this hypothesis is correct. The strains may behave differently if they express cloned antigens, since they are designed for regulated expression of antigens.
Acknowledgments
This research was supported by a Canadian Poultry Research Council-Agriculture and Agri-Food Canada Poultry Science Cluster grant, as well as by the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA). Construction of Salmonella vector strains was supported by National Institutes of Health Grant R01 AI060557 and the Bill and Melinda Gates Foundation Grant 37863. We thank Karen Brenneman, Soo-Young Wanda, Melha Mellata, Chang-Ho Baek, Zhaoxing Shi, and Qingke Kong for constructing Salmonella vector strains.
References
- 1.Bailey JS, Cason JA, Cox NA. Effect of Salmonella in young chicks on competitive exclusion treatment. Poult Sci. 1998;77:394–399. doi: 10.1093/ps/77.3.394. [DOI] [PubMed] [Google Scholar]
- 2.Voetsch AC, Van Gilder TJ, Angulo FJ, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38(Suppl 3):S127–S134. doi: 10.1086/381578. [DOI] [PubMed] [Google Scholar]
- 3.Government of Canada. Preliminary results. Guelph (Ontario): Public Health Agency of Canada; 2010. Canadian Integrated Program for Antimicrobial Resistance Surveillance. 2009. [Google Scholar]
- 4.Atterbury RJ, Carrique-Mas JJ, Davies RH, Allen VM. Salmonella colonisation of laying hens following vaccination with killed and live attenuated commercial Salmonella vaccines. Vet Rec. 2009;165:493–496. doi: 10.1136/vr.165.17.493. [DOI] [PubMed] [Google Scholar]
- 5.Holt PS, Gast RK, Kelly-Aehle S. Use of a live attenuated Salmonella typhimurium vaccine to protect hens against Salmonella enteritidis infection while undergoing molt. Avian Dis. 2003;47:656–661. doi: 10.1637/7002. [DOI] [PubMed] [Google Scholar]
- 6.McHan F, Shott EB, Brown J. Effect of feeding selected carbohydrates on the in vivo attachment of Salmonella Typhimurium in chick ceca. Avian Dis. 1991;35:328–331. [PubMed] [Google Scholar]
- 7.Jiang Y, Kulkarni RR, Parreira VR, Poppe C, Roland KL, Prescott JF. Assessment of 2 Salmonella enterica serovar Typhimurium-based vaccines against necrotic enteritis in reducing colonization of chickens by Salmonella serovars of different serogroups. Can J Vet Res. 2010;74:264–270. [PMC free article] [PubMed] [Google Scholar]
- 8.Sasai K, Yoshimura K, Lillehoj HS, et al. Analysis of splenic and thymic lymphocyte subpopulations in chickens infected with Salmonella Enteritidis. Vet Immunol Immunopathol. 1997;59:359–367. doi: 10.1016/s0165-2427(97)00082-2. [DOI] [PubMed] [Google Scholar]
- 9.Mastroeni P, Chabalgoity JA, Dunstan SJ, et al. Salmonella: Immune responses and vaccines. Vet J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 10.Medina E, Guzman CA. Use of live bacterial vaccine vectors for antigen delivery: Potential and limitations. Vaccine. 2001;19:1573–1580. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 11.Babu U, Scott M, Myers MJ, et al. Effects of live attenuated and killed Salmonella vaccine on T-lymphocyte mediated immunity in laying hens. Vet Immunol Immunopathol. 2003;91:39–44. doi: 10.1016/s0165-2427(02)00265-9. [DOI] [PubMed] [Google Scholar]
- 12.Roland K, Karaca K, Sizemore D. Expression of Escherichia coli antigens in Salmonella typhimurium as a vaccine to prevent airsacculitis in chickens. Avian Dis. 2004;48:595–605. doi: 10.1637/7178-031004R1. [DOI] [PubMed] [Google Scholar]
- 13.Wyszynska A, Raczko A, Lis M, Jagusztyn-Krynicka EK. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild type Campylobacter. Vaccine. 2004;22:1379–1389. doi: 10.1016/j.vaccine.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Konjufca V, Wanda SY, Jenkins MC, Curtiss R. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun. 2006;74:6785–6796. doi: 10.1128/IAI.00851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong W, Wanda SY, Zhang X, et al. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci U S A. 2008;105:9361–9366. doi: 10.1073/pnas.0803801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni RR, Parreira VR, Sharif S, Prescott JF. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine. 2008;26:4194–4203. doi: 10.1016/j.vaccine.2008.05.079. [DOI] [PubMed] [Google Scholar]
- 17.Zekarias B, Mo H, Curtiss R., III Recombinant attenuated Salmonella enterica serovar typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin Vaccine Immunol. 2008;15:805–816. doi: 10.1128/CVI.00457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wang S, Xin W, et al. A sopB deletion mutation enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Infect Immun. 2008;76:5238–5246. doi: 10.1128/IAI.00720-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong Q, Liu Q, Roland KL, Curtiss R., III Regulated delayed expression of rfaH in an attenuated Salmonella enterica serovar typhimurium vaccine enhances immunogenicity of outer membrane proteins and a heterologous antigen. Infect Immun. 2009;77:5572–5582. doi: 10.1128/IAI.00831-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wang S, Scarpellini G, et al. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc Natl Acad Sci U S A. 2009;106:593–598. doi: 10.1073/pnas.0811697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni RR, Parreira VR, Jiang YF, Prescott JF. A live oral recombinant Salmonella enterica serovar Typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens. Clin Vaccine Immunol. 2010;17:205–214. doi: 10.1128/CVI.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtiss R, III, Porter SB, Munson M, et al. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry. In: Blankenship LC, Bailey JS, Cox NA, Stern NJ, Meinersmann RJ, editors. Colonization Control of Human Bacterial Enteropathogens in Poultry. New York, New York: Academic Press; 1991. pp. 169–198. [Google Scholar]
- 23.Wang S, Li Y, Scarpellini G, et al. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect Immun. 2010;78:3969–3980. doi: 10.1128/IAI.00444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtiss R, Zhang X, Wanda SY, et al. Induction of host immune responses using Salmonella-vectored vaccines. In: Brodgen K, Minion F, Cornick N, et al., editors. Virulence Mechanisms of Bacterial Pathogens. 4th ed. Washington, DC: ASM Press; 2007. pp. 297–313. [Google Scholar]
- 25.Xin W, Wanda SY, Li Y, Wang S, Mo H, Curtiss R., III Analysis of type II secretion of recombinant pneumococcal PspA and PspC in a Salmonella enterica serovar Typhimurium vaccine with regulated delayed antigen synthesis. Infect Immun. 2008;76:3241–3254. doi: 10.1128/IAI.01623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtiss R, III, Wanda SY, Gunn BM, et al. Salmonella enterica serovar Typhimurium strains with regulated delayed attenuation in vivo. Infect Immun. 2009;77:1071–1082. doi: 10.1128/IAI.00693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong Q, Liu Q, Jansen AM, Curtiss R., III Regulated delayed expression of rfc enhances the immunogenicity and protective efficacy of a heterologous antigen delivered by live attenuated Salmonella enterica vaccines. Vaccine. 2010;28:6094–6103. doi: 10.1016/j.vaccine.2010.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtiss R, III, Galan JE, Nakayama K, Kelly SM. Stabilization of recombinant avirulent vaccine strains in vivo. Res Microbiol. 1990;141:797–805. doi: 10.1016/0923-2508(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 29.Hassan JO, Curtiss R., III Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent Δcya Δcrp S. typhimurium. Res Microbiol. 1990;141:839–850. doi: 10.1016/0923-2508(90)90119-b. [published erratum appears in Res Microbiol 1991;142:109] [DOI] [PubMed] [Google Scholar]
- 30.Galan JE, Nakayama K, Curtiss R., III Cloning and characterization of the asd gene of Salmonella typhimurium: Use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 31.Hassan JO, Curtiss R., III Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect Immun. 1994;62:5519–5527. doi: 10.1128/iai.62.12.5519-5527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan JO, Curtiss R., III Efficacy of a live virulent Salmonella typhimurium vaccine in preventing colonization and invasion of laying hens by Salmonella typhimurium and Salmonella enteritidis. Avian Dis. 1997;41:783–791. [PubMed] [Google Scholar]
- 33.Eswarappa SM, Karnam G, Nagarajan AG, et al. lac repressor is an antivirulence factor of Salmonella enterica: Its role in the evolution of virulence of Salmonella. PLOSOne. 2009;4:e5789. doi: 10.1371/journal.pone.0005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Li Y, Shi H, et al. Comparison of a regulated delayed antigen synthesis system with in vivo-inducible promoters for antigen delivery by live attenuated Salmonella vaccines. Infect Immun. 2001;79:937–949. doi: 10.1128/IAI.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]