Abstract
Few studies have investigated the efficacy of extended ceftiofur therapy and none have focused on extended therapy for naturally occurring clinical mastitis. The objective of this study was to compare the efficacy of extended intramammary ceftiofur therapy of 8 d duration with a standard 2-day regimen for the treatment of naturally occurring mild to moderate clinical mastitis in lactating dairy cows. Holstein cows from 22 dairy herds (n = 241) were randomly allocated to the 2 treatment groups. For each case of mastitis, 125 mg of ceftiofur hydrochloride was administered intramammary once a day for 2 or 8 d. Clinical cure, 21 d after the last treatment, was 89% (98/110) in each group. Bacteriological cure 21 d after the last treatment for the 2- and 8-day regimens were 32% (15/47) and 61% (25/41), respectively, for all bacteria (P = 0.007), 64% (9/14) and 82% (9/11), respectively, for streptococci (P = 0.50), and 0% (0/20) and 47% (9/19), respectively, for Staphylococcus aureus (P = 0.0004). There were no statistical differences between groups for new intramammary infections. Overall, ceftiofur extended therapy increased cure when compared to a 2-day regimen for the treatment of naturally occurring mild to moderate clinical mastitis in lactating dairy cows.
Résumé
Peu d’études ont investigué l’efficacité d’une thérapie prolongée avec du ceftiofur et aucune n’a examiné attentivement une thérapie prolongée dans le cas de mammite clinique se produisant naturellement. L’objectif de la présente étude était de comparer l’efficacité d’une thérapie intra-mammaire prolongée d’une durée de 8 jours avec du ceftiofur à la thérapie standard de 2 jours pour le traitement de mammite clinique légère à modérée survenant naturellement chez des vaches laitières en lactation. Des vaches de race Holstein provenant de 22 troupeaux laitiers (n = 241) ont été réparties de manière aléatoire dans les 2 groupes de traitement. Pour chaque cas de mammite, 125 mg d’hydrochlorure de ceftiofur furent administrés par voie intra-mammaire une fois par jour pour 2 ou 8 jours. La guérison clinique, 21 jours après le dernier traitement, était de 89 % (98/110) dans chaque groupe. La guérison bactériologique 21 jours après le dernier traitement pour les groupes 2 et 8 jours était respectivement 32 % (15/47) et 61 % (25/41) pour toutes les bactéries (P = 0,007), 64 % (9/14) et 82 % (9/11), respectivement, pour les streptocoques (P = 0,50) et 0 % (0/20) et 47 % (9/19), respectivement pour Staphylococcus aureus (P = 0,0004). Il n’y avait aucune différence statistiquement significative entre les groupes pour les nouvelles infections intra-mammaires. Globalement, une thérapie prolongée au ceftiofur augmenta la guérison lorsque comparée à une thérapie de 2 jours pour le traitement de mammite clinique légère à modérée survenant naturellement chez les vaches laitières en lactation.
(Traduit par Docteur Serge Messier)
Introduction
Mastitis is one of the most common diseases in dairy cattle and one of the most frequent causes for antibiotic use in dairy herds (1). The incidence of clinical mastitis was reported to be 19.8% at the individual cow level in a survey in Ontario, Canada (2). Due in part to its frequency, mastitis is one of the most costly diseases for the dairy industry (3). In a review on economic aspect of mastitis, the cost was estimated between CDN $83 and CDN $132 per cow per year (4).
Extended therapy has been introduced to help manage this costly disease, first for the treatment of subclinical mastitis (5–8), then for treating clinical mastitis (9–11). The principle of extended therapy is to increase the duration of treatment beyond the traditional 2- to 3-day treatment regimen. As the activity of all labeled intramammary antibiotics is time-dependant, an increased frequency of cure is expected. Extended therapy has been evaluated for the treatment of subclinical mastitis and experimentally induced clinical mastitis (5–11), but not for treating naturally occurring clinical mastitis.
Ceftiofur therapy is a third generation cephalosporin effective against a wide range of Gram-positive and Gram-negative bacterial pathogens that cause mastitis. It has been labeled to treat clinical mastitis caused by Escherichia coli, Streptococcus dysgalactiae, and coagulase negative staphyloccoci (8,10,12). Extended intramammary therapy with ceftiofur was reported to provide a greater probability of cure for experimentally induced Str. uberis mastitis (10) and for subclinical mastitis (8). When compared to the standard 2-day treatment, extended therapy increased cure by 57% and 27%, respectively.
The objective of the current study was to compare the efficacy of an intramammary ceftiofur extended therapy of 8 d duration with a standard 2-day regimen for the treatment of naturally occurring mild to moderate clinical mastitis in lactating dairy cows. The hypothesis was that extended therapy would increase cure frequency.
Materials and methods
A randomized clinical trial was conducted between January and December 2009 on 241 Holstein cows coming from 22 dairy herds located in Ontario and Québec, Canada. Four of them were free-stall barns and 18 were tie-stall barns. The number of cows per herd was between 40 and 300. The experiment protocol was accepted by the Animal Care and Use Committee of the Faculté de médecine vétérinaire of the Université de Montréal (08-rech-1447).
Cows with a mild to moderate clinical case of mastitis as diagnosed by the producer during the study period were included in the trial. Mild clinical mastitis (score 1) was defined as the presence of abnormal milk only. Moderate clinical mastitis (score 2) was defined as the presence of abnormal milk combined with signs of inflammation of the affected quarter (redness, swelling, heat, and/or pain) without signs of systemic illness. Severe clinical mastitis (score 3) was defined as the presence of signs identical to score 2 and associated with signs of systemic illness (such as, elevated rectal temperature, heart rate, and respiratory rate; decreased rumen motility or absence of ruminal contraction; and dehydration) (13). Severe mastitis cases were not included in the study to avoid any bias caused by systemic treatments needed to treat those cases.
Cows diagnosed with mild to moderate clinical mastitis (score 1 and 2) were randomly allocated to receive one of the 2 experimental treatments: intramammary infusion of 125 mg of ceftiofur hydrochloride (Spectramast LC; Pfizer Animal Health, Kirkland, Quebec) every 24 h for 2 consecutive days (2d group), or the same treatment every 24 h for 8 consecutive days (8d group). The targeted allocation ratio was 50% in each group. Randomization was done by researchers using sequentially numbered envelopes designating the treatment allocation. A randomized sequence of treatments was generated for each herd. Each sequence of 10 treatments was divided in equal proportions into the 2d or 8d treatment groups. Treatments were each assigned a random number using a random number table and then sorted in ascending order and placed in individual envelopes with the treatment number shown on the outside. After having verified that the case meets the inclusion and exclusion criteria, producers opened the next envelope in the sequence to find the treatment assignment. Validation that the predetermined order followed was carried out continuously during the study was done one of the authors (GT). No negative control group was used in this trial as mastitis is a painful condition and a protocol to not treat would be unethical.
Treatments were done by dairy producers. Producers were given a written form explaining the protocol and the protocol was explained individually to each producer. Before treatments, teats were cleaned thoroughly with 70% alcohol. For both experimental treatment groups, ceftiofur was infused using aseptic technique by full insertion of the plastic canula into the teat canal. In case of multiple quarter infections, the individual quarters received the same treatment, but only one quarter was randomly selected for the study to avoid clustering infections within cow.
Cows were excluded from the trial if they received an antibiotic or anti-inflammatory therapy within 14 d prior to enrolment. Cows receiving antibiotic or anti-inflammatory therapy other than intramammary ceftiofur, as described in the protocol during experimental treatment period, were also excluded. Cows were eligible for only one period of enrolment. Subsequent case events more than 21 d after the last treatment were excluded. For each case of mild to moderate clinical mastitis, the data collected included cow identification, the infected quarter, dates of treatments and samples, days in milk (DIM) at first treatment, severity of mastitis (score 1 or 2), and the day milk returned to normal. The time necessary to return to normal milk was calculated from the day of diagnosis to the day milk returned normal.
Duplicate milk samples for bacteriological culture were collected from affected quarters by dairy producers prior to the first ceftiofur treatment, and at 7, 14, and 21 d after the last treatment. All milk samples were collected prior to regular milking using a standardized procedure. Before sample collection, teats were dipped in a premilking teat disinfectant solution if usually used by the producer, cleaned thoroughly, dried with paper towels, and teat ends were sanitized with gauzes containing 70% isopropyl alcohol. A second alcohol application was made before taking the duplicate sample. Immediately after sampling, milk samples were frozen (−20°C).
Frozen milk samples were picked up every month and sent to the bacteriological laboratory at the Faculté de médecine vétérinaire, Université de Montréal (St-Hyacinthe, Quebec). Milk samples were thawed by immersion in cold water for 30 min. Samples (10 μL) from each infected quarter were plated onto a Columbia agar plate supplemented with 5% defibrinated sheep blood (PML Microbiological, Mississauga, Ontario). The plates and the remaining milk were incubated to enrich them at 35°C and bacteriological growth was observed and recorded at 24 h and 48 h of incubation. Bacteria found on culture media were identified according to colony morphology and appropriate identification tests. In the absence of bacterial growth at 24 h, the enriched milk samples were plated using the same techniques. Gram coloration was done on every bacterial isolate. Gram-positive cocci were first tested with a catalase test. Gram-positive, catalase-positive cocci were then tested for coagulase and DNase. Growth evaluations in 6.5% sodium chloride and Lancefield groups (group B and C) were done if needed for the identification of enterococci and Str. dysgalactiae and Str. agalactiae, respectively. Streptococci were presumptively identified down to species level (API 20 Strep System; BioMérieux, Marcy l’Étoile, France). Gram-negative bacteria were plated (MacConkey agar; PML Microbiological, Mississauga, Ontario) and evaluated with the following tests: triple sugar iron, urea, citrate, motility, and indole. Yeasts, Nocardia spp., and Prototheca spp. were identified based on their appearance after Gram coloration. Laboratory technicians doing the bacteriological analysis were blinded to treatment groups.
Bacteriological culture interpretation was based on the National Mastitis Council (NMC) guidelines (14). Bacterial growth from enrichment samples were considered significant for S. aureus, Str. agalactiae, and Pasteurella spp. A sample was considered contaminated if 3 or more different bacterial species grew. A quarter was considered infected by a bacteria when growth was significant (highly and probably significant as in the NMC guidelines) in both duplicate samples. Quarters were considered non-infected if both samples were free from bacteria or if the growth was considered non significant using the NMC guidelines. If a quarter was infected by 2 bacteria, only one, chosen randomly by tossing a coin, was included for the bacteriological cure to avoid having 2 infections for the same cow. Clinical cure was defined as a return to a milk of normal appearance at 21 days after the last treatment. Reappearance of clinical signs of mastitis within 21 days was considered a treatment failure. A quarter was considered bacteriologically cured if the quarter was non-infected, for the previously identified bacteria, at 7, 14, and 21 d following the last treatment. A new intramammary infection (NIMI) was defined as isolation, using the above criteria, in any of the follow-up duplicate samples of a bacterial species different than the previously identified ones.
Mixed logistic regression was used to evaluate the association between treatment and clinical and bacteriological cure and NIMI. Only cows having NIMI or having all the samples complete without data loss could be included in the analysis for NIMI. A mixed linear model was used to compare the time for a return to normal milk between the 2 groups. In both types of analysis, the fixed factor was the treatment regimen and herd was the random factor. The covariate DIM (as a continuous quantitative variable) was forced into the model because of assumed biological relevance. Severity of mastitis (mild or moderate) and the affected quarter were forced into the model to test for subgroup effects. Cofactors were included because of assumed biologically plausible effects and were tested for significance. To compare the degree of severity of mastitis between treatment groups, the exact Chi-square test was used. To compare bacteriological cure for S. aureus infections, the exact Chi-square test was used a posteriori, as cure was 0% in one of the groups, preventing the use of the mixed logistic regression. The proportion of S. aureus and streptococci versus the other bacterial strains was compared between the groups using the exact Chi-square test. Cases of clinical mastitis caused by Prototheca spp. and yeasts were not included for statistical analysis, as they are not bacteria and treatment was not expected to be efficacious. Based on the report by Olde Riekerink et al (10), the expected proportion of clinical mastitis caused by Staphylococcus aureus and by streptococci was 15% and 10%, respectively. The expected incidence rate of mastitis in dairy herds in Québec was 30%, with a 20% proportion of acute mastitis. Based on previous ceftiofur studies (5,9) the expected difference in the cure rate between a standard 2 d treatment and an extended 8 d treatment was 30% for mastitis caused by S. aureus, streptococci, or any pathogen. In those conditions, the number of cases of mastitis needed to have a confidence level of 95% and a power of 80% was 400. To have those 400 cases of mastitis in one year, 2000 cows needed to be included with an expected lost of data was 15%. Results were considered statistically significant when P < 0.05.
Results
A total of 241 cases of mild to moderate clinical mastitis (score 1: n = 65; score 2: n = 176) were included in this study; 122 in the 2d group and 119 in the 8d group. There were 121 cases of clinical mastitis in the front quarters and 120 in the rear quarters. There was no difference between the 2 groups for DIM (average: 136.3 and 151.7 for 2d and 8d groups, respectively; P = 0.30) or severity of mastitis (36 mild mastitis cases and 86 moderate mastitis cases in the 2d group, and 29 mild mastitis cases and 90 moderate mastitis cases in the 8d group; P = 0.39).
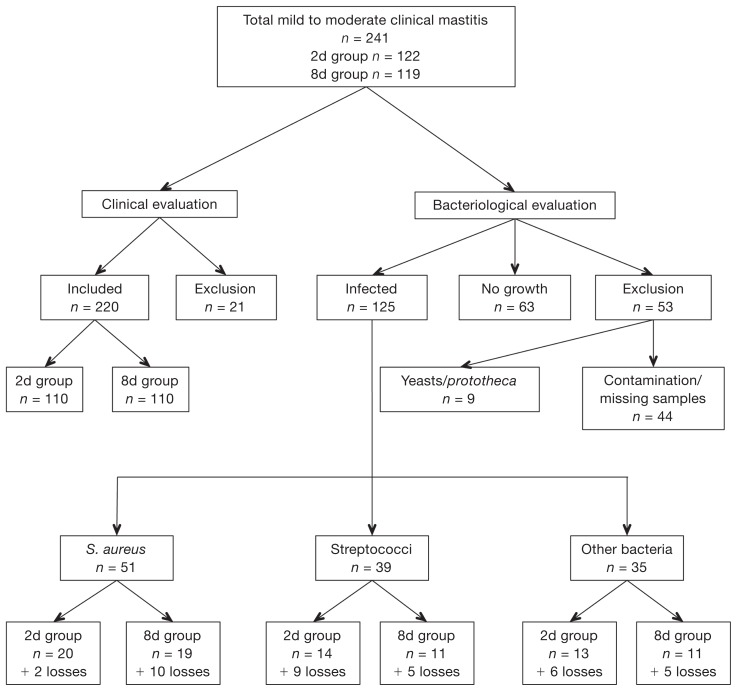
The numbers of included, excluded, or lost cases of clinical mastitis for clinical and bacteriological evaluations are summarized in Figure 1. The distribution of pathogens isolated is summarized in Table I. In 2 cases, 2 different pathogen species were isolated: one was S. aureus and streptococci, and the other one was Enterococcus spp. and S. aureus. For the first, streptococci infection was randomly chosen for study and for the second the Enteroccus spp. infection was randomly chosen. There was no significant difference in the distribution of S. aureus or streptococci between the two treatment groups (P = 0.72 and P = 0.76, respectively).
Figure 1.
Number cases of clinical mastitis included and excluded for clinical and bacteriological evaluation.
Table I.
Distribution of initial bacteriological culture results among clinical cases of mastitis for 2 ceftiofur intramammary treatment regimens: Standard 2-day therapy (2d group) and extended therapy (8d group)
| Frequency (%) | |||
|---|---|---|---|
|
|
|||
| Bacterial species | 2d group | 8d group | Total |
| S. aureus | 22 (22) | 29 (29.9) | 51 (25.9) |
| Streptococci | 23 (23) | 15 (15.5) | 38 (19.8) |
| Enterococcus spp. | 7 (7) | 3 (3.1) | 10 (5.1) |
| E. coli | 4 (4) | 4 (4.1) | 8 (4.1) |
| Klebsiella spp. | 2 (2) | 3 (3.1) | 5 (2.5) |
| CNS | 2 (2) | 3 (3.1) | 5 (2.5) |
| Yeasts | 3 (3) | 2 (2.1) | 5 (2.5) |
| Prototheca | 1 (1) | 3 (3.1) | 4 (2.0) |
| Mixed infectionsa | 0 (0) | 2 (2.1) | 2 (1.0) |
| Enterobacter spp. | 1 (1) | 1 (1.0) | 2 (1.0) |
| Citrobacter spp. | 1 (1) | 0 (0) | 1 (0.5) |
| Corynebacterium spp. | 1 (1) | 0 (0) | 1 (0.5) |
| Mannheimia spp. | 0 (0) | 1 (1.0) | 1 (0.5) |
| Pasteurella spp. | 1 (1) | 0 (0) | 1 (0.5) |
| No growth or insignificant growth | 32 (32) | 31 (31.9) | 63 (32.0) |
| Total | 100 | 97 | 197 |
Mixed infections were S. aureus/streptococci and Enterococcus spp./S. aureus infections.
CNS — coagulase negative staphyloccocci.
For clinical cure, 21 of the 241 cases of mild to moderate clinical mastitis were excluded, 9 because they were yeasts or Prototheca spp. mastitis, 9 because follow-up was stopped without a reason given, 2 because another treatment was administered to treat another disease, and 1 because the cow was dried off before the end of the follow-up period. The clinical cure for the 2d and 8d groups were identical (98/110 for each) with no effect of the affected quarter (P = 0.56) or severity of mastitis (P = 0.95). There was a significant difference between the 2 groups for the delay in the return to normal milk (2.8 d for the 2d group, 3.7 d for the 8d group, P = 0.007), with no effect of the affected quarter (P = 0.51) or severity of mastitis (P = 0.07).
Of the 125 initially infected cases, 37 could not be analyzed for bacterial cure, so 88 cases were included. The causes of the 37 cases lost to observation are summarized in Table II. Bacteriological cures are presented in Table III. Bacteriological cure was significantly greater in the 8d group than in the 2d group for all bacteria combined [odds ratio (OR) = 3.75, 95% confidence interval (CI): 1.81 to 7.75; P = 0.007], for S. aureus (0% and 47.4% for the 2d and 8d groups, respectively; exact Chi-square P = 0.0004), but not for streptococci (OR = 2.24, 95% CI: 0.21 to 23.42; P = 0.50). For each of these analyses (all bacteria combined, S. aureus and streptococci), there were no effects of the affected quarter and severity of mastitis (P > 0.07). Of the 48 bacteriological cure failures, 34 were considered clinically cured.
Table II.
Causes of loss of data for bacteriological analysis of the initially infected cases of mastitis
| Causes of loss of data | Number | % |
|---|---|---|
| Contamination of at least one sample | 17 | 45.9 |
| At least 1 sample missing | 10 | 27.0 |
| Samples not taken at the right time | 6 | 16.2 |
| Other treatments | 2 | 5.4 |
| Monitoring stopped | 1 | 2.7 |
| Cow dried off | 1 | 2.7 |
| Total | 37 | 100 |
Table III.
Bacteriological cure for 2 ceftiofur intramammary treatment regimens: Standard 2-day therapy (2d group) and extended therapy (8d group)
| Bacteriological cure rates | ||
|---|---|---|
|
|
||
| Bacteria | 2d group (n) | 8d group (n) |
| Staphylococcus aureus | 0% (0/20)a | 47.4% (9/19)b |
| Streptococci | 64.3% (9/14)a | 81.8% (9/11)a |
| Overall | 31.9% (15/47)a | 61% (25/41)b |
Data within the same row with different letters are statistically different (P < 0.05).
Out of the 241 cases, 155 cases could be included for the NIMI analysis (78 in the 2d group, 77 in the 8d group). A total of 86 cases was excluded for the analysis because of missing (n = 42), or contaminated samples (n = 44). Of the 155 cases of mastitis, 16 were followed by NIMI during the observation period, 10 in the 2d group and 6 in the 8d group. There was no significant difference between treatment groups (OR = 0.60, CI: 0.23 to 1.53; P = 0.30) with no effect on the severity of mastitis (P = 0.25) and DIM (P = 0.16). The distribution and identification of NIMI for the 2 treatment regimens are presented in Table IV. Note that only one bacteria was Gram-negative. The risk of NIMI varied significantly with the location of the infected quarter (P = 0.04). There were 3 NIMI in left front quarters, 1 in a left rear quarter, 8 in right front quarters, and 4 in the right rear quarter. There were 11 NIMI in front quarters and 5 NIMI in the rear quarters.
Table IV.
Distribution and identification of new intramammary infections for the 2 intramammary treatment regimens using ceftiofur: Standard 2 day therapy (2d group) and extended therapy (8d group) at 7, 14, and 21 d after the end of the treatments
| Treatment group | Follow-up period | ||
|---|---|---|---|
|
| |||
| 7 d | 14 d | 21 d | |
| 2d | 1 S. aureus | 1 Serratia spp. | 1 CNS |
| 1 CNS | 1 Corynebacterium spp. | 1 Enterococcus spp. | |
| 1 Corynebacterium spp. | 1 CNS | 1 Corynebacterium spp. | |
| 1 Str. dysgalactiae | |||
| 8d | 1 S. aureus | 1 S. aureus | 1 Corynebacterium spp. |
| 1 CNS | 2 CNS | ||
CNS — coagulase negative staphylococci.
Discussion
The objective of this study was to compare clinical and bacteriological cures with ceftiofur intramammary extended therapy to a standard 2-day regimen for treating naturally occurring clinical mastitis. Extended therapy significantly increases bacteriological cure for all bacteria combined and for S. aureus, but not for streptococci as a group. No improvement of clinical cure was noted. Improvements in bacteriological cure using extended ceftiofur therapy reported in previous studies (8,10) were similar to our results. Clinical cure has not been reported in the study on experimentally induced clinical mastitis (10).
Seventy-one percent (34/48) of bacteriological cure failures were considered clinically cured during the study. Similar findings have been reported and this situation can result in recurrence of clinical mastitis and high somatic cell counts (15–17). In one of those studies (16), a persistent intramammary infection, caused by the same bacteria, was found one year after the first culture in 5% of all episodes of clinical mastitis. This result points out that, for any intramammary therapy, a bacteriological cure, and not only clinical cure, should be the goal to maximize the benefit of treatment.
No improvement in bacteriological cure for streptococci infections was found, unlike results reported by Oliver et al (10). However, in that study, the infections were experimental [same strain and infectious dose of the same species (Str. uberis UT 888)] and only 2 herds were included. Whereas, in our study, there were multiple strains of streptococci coming from the 22 different herds. This major difference in study protocol can lead to a different sensitivity profile and increase the variation within each group, masking differences between groups. More cases would have been needed. To find a 30% difference between groups, with a significance level of 0.05 and a power of 0.8, at least 30 cases in each group would be necessary. For streptococci infections, only 14 and 11 cases in the 2d and 8d group, respectively, were recruited.
More cases of moderate mastitis (n = 176) than mild mastitis (n = 65) were observed in this study. As previously reported (2,17), a greater proportion of mild mastitis was expected. It is possible that some mild cases of mastitis were not included in the study, even though producers were clearly instructed to collect samples from any quarter with abnormal milk, regardless of intention to treat. Thus, the number of cases of mild mastitis may be underestimated.
There was the same number of mastitis in the front quarters as in the rear quarters. The distribution of enrolled affected quarters did not agree with reports of higher incidence in rear quarters for naturally occurring intramammary infections (IMI) (16–20). However, a possible under-detection of mild cases and exclusion of severe cases may have distorted the distribution of cases among mammary quarters.
More than 70% of the infected cases were caused by S. aureus (40.8%) and streptococci (31.2%). In a Canadian study (18), S. aureus and streptococci were responsible for 21.7% and 26.4% of the infections, respectively; however, cases of severe mastitis were included. The population of bacteria responsible for severe clinical mastitis may be different from the population of bacteria responsible for mild to moderate cases. Moreover, the study by Olde Riekerink et al (18) was conducted on dairy farms from all over Canada, whereas this study was done in Québec and Ontario. As reported by Olde Riekerink et al (18), differences in the distribution of bacterial- specific clinical mastitis throughout provinces in Canada exist. Finally, the method used for diagnosis was different, which might change the measured prevalence of each pathogen. In this study, duplicates of each sample were used, whereas one sample for each time-point was used in Olde Riekerink et al (18) study.
No other study reports an increase in the delay for a return to normal milk associated with extended therapy. As the formulation of ceftiofur that was used has been labeled for intrammamary usage, it is very unlikely that it could have been irritating for the udder. The increased delay may relate to the fact that producers were not blinded to the treatment: the follow-up examinations may have been more thorough during the 6 additional days of treatment in the 8d group compared to the 2d group. Interaction of effects between treatment groups and severity on time to normality was not tested because severity was not statistically different between the 2 groups, but because the 8d group had a modestly higher proportion of moderate severity cases than did the 2d group, the longer average time to normality may be in part a result of case severity.
A potential increase in risk of NIMI is one of the reported drawbacks of extended therapy. Previous studies reported NIMI that were clinical (6,19–21) or subclinical mastitis (21). In this study, only subclinical NIMI were observable because no milk samples were taken for bacterial culture of clinical relapses occurring during the study period. New intramammary infections have been reported following pirlimycine (6,19,21) and cephapirin (20) extended therapy. With pirlimycine, NIMI were mainly due to E. coli and Klebsiella spp., whereas NIMI following cephapirin therapy were all due to yeasts. In our study, only one NIMI was caused by a Gram-negative bacteria (Serratia spp.) and none were caused by yeasts. Cephapirin and ceftiofur are members of the cephalosporin group of beta-lactam drugs. Their spectrum is broader than pirlimycine. First generation cephalosporin drugs, such as cephapirine, are generally considered to be active against streptococci, staphylococci, E. coli, Klebsiella spp., and Proteus spp., but not against Enterococcus spp. Third generation cephalosporins such as ceftiofur are less active than 1st generation cephalosporins against Gram-positive cocci but more active against the enterobacteriaceae, including strains producing beta-lactamase. Those differences in spectrum could explain the differences of incidence of NIMI when using different antibiotics. In addition, instructions were given to the producers concerning the infusion technique, to reduce the risk of infusing bacteria in the quarter. Finally, the previously reported NIMI were diagnosed after a treatment for subclinical infections, unlike this study. The population of cows having subclinical infections could be different from the population of cows having clinical infections in terms of sensitivity to new infections. Furthermore, the study reporting NIMI was not performed in the same geographical location. The population of our study is representative of dairy cows from Québec and Ontario and, as reported in the study of Olde Riekerink, et al (18), population of cows and bacteria are different between countries.
In this study, the probability of NIMI varied significantly depending on the affected quarter. The right front quarter seems to be affected more than the others, and the left rear quarter less. Front quarters also seem to be more affected than rear quarters. There is no data available in the literature concerning the distribution of NIMI according to quarter. This distribution of affected quarters does not agree with reports of a higher incidence in rear quarters for naturally occurring IMI (16–20). This kind of distribution is thought to be due to the higher contamination risk of rear quarter by feces. As the distribution of affected quarter does not follow the distribution of naturally occurring IMI, NIMI following extended therapy may not be caused by a contamination by feces. Introduction of new pathogen in the udder during the infusion could be responsible for NIMI.
In this study, 153 of the 241 cases of mastitis could not be analyzed for the bacteriological cure. Of the 241 samples, 63 were culture negative, which is similar to what has been reported (22–24). The expected losses of data for other reasons were expected to reach approximately 15% of all samples versus the 37% loss we observed. One study (25) reported 41% of loss of data with a protocol less strict than the one in the present study. Requiring 2 samples to have the same bacteriological results probably increased the losses of data of this study. Of the 37 losses of data for bacteriological evaluation 27 were lost because at least one sample could not be analyzed, either because of contamination or because one of the samples was missing. Moreover, taking 2 samples at each of the time points increased the workload of the producers, which may account for the loss of data due to non-compliance with the protocol. However, even if duplicate sampling increased the numbers of data lost, false negative and false positive results were minimized. This fact and the strict definition of infection and cure aimed to decrease selection bias in the study.
Finally, the interval between days of detection of mastitis to post-treatment samples is not the same in both groups. However, in this study, the intervals between the end of treatment to post-treatment samples are identical in both groups. This methodology has been previously described (8,10,20). The authors recognized that it could introduce a bias, such as allowing more time to cure in the 8d group. However, having the same interval between days of detection of mastitis to post-treatments samples in the groups could introduce some bias too, such as potential antibiotic residue in the extended therapy group compared to the 2d group depending on the intervals chosen, and higher risk of new infections in the 2d group compared to the 8d group, since the interval between the end of the treatment period and the sampling is longer for the 2d group than the 8d group.
This study reports the clinical and bacteriological efficacy of intramammary extended ceftiofur therapy for naturally occurring mild to moderate clinical mastitis. Bacteriological cures were higher with the extended therapy for all mastitis cases combined and for S. aureus mastitis cases. However, no differences were found for streptococci infection. Therefore, extended therapy could be considered an efficient treatment for naturally occurring clinical mastitis and S. aureus clinical mastitis. Further studies are needed concerning the efficacy of extended therapy for treatment of natural streptococci clinical mastitis cases.
Acknowledgments
The authors thank Guy Beauchamp for his help with the statistical analysis, Pfizer Animal Health Canada for funding this study, and the 22 producers and their veterinarian for their participation.
References
- 1.Erskine RJ, Wagner S, DeGraves FJ. Mastitis therapy and pharmacology. Vet Clin North Am Food Anim Pract. 2003;19:109–138. vi. doi: 10.1016/s0749-0720(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 2.Sargeant JM, Scott HM, Leslie KE, Ireland MJ, Bashiri A. Clinical mastitis in dairy cattle in Ontario: Frequency of occurrence and bacteriological isolates. Can Vet J. 1998;39:33–38. [PMC free article] [PubMed] [Google Scholar]
- 3.DeGraves FJ, Fetrow J. Economics of mastitis and mastitis control. Vet Clin North Am Food Anim Pract. 1993;9:421–434. doi: 10.1016/s0749-0720(15)30611-3. [DOI] [PubMed] [Google Scholar]
- 4.Hogeveen H, Huijps K, Lam TJ. Economic aspects of mastitis: New developments. N Z Vet J. 2011;59:16–23. doi: 10.1080/00480169.2011.547165. [DOI] [PubMed] [Google Scholar]
- 5.Deluyker HA, Michaenek P, Wuyts N, VanOye SN, Chester ST. We Treat Sick Cows Don’t We?. The Case of Subclinical Mastitis National Mastitis Council Annual Meeting Proceedings; 2001. pp. 170–174. [Google Scholar]
- 6.Gillespie BE, Moorehead H, Lunn P, et al. Efficacy of extended pirlimycin hydrochloride therapy for treatment of environmental Streptococcus spp. and Staphylococcus aureus intramammary infections in lactating dairy cows. Vet Ther. 2002;3:373–380. [PubMed] [Google Scholar]
- 7.Taponen S, Dredge K, Henriksson B, et al. Efficacy of intramammary treatment with procaine penicillin G vs. procaine penicillin G plus neomycin in bovine clinical mastitis caused by penicillin-susceptible, Gram-positive bacteria — A double blind field study. J Vet Pharmacol Ther. 2003;26:193–198. doi: 10.1046/j.1365-2885.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 8.Oliver SP, Gillespie BE, Headrick SJ, et al. Efficacy of extended ceftiofur intramammary therapy for treatment of subclinical mastitis in lactating dairy cows. J Dairy Sci. 2004;87:2393–2400. doi: 10.3168/jds.S0022-0302(04)73361-5. [DOI] [PubMed] [Google Scholar]
- 9.Hillerton JE, Kliem KE. Effective treatment of Streptococcus uberis clinical mastitis to minimize the use of antibiotics. J Dairy Sci. 2002;85:1009–1014. doi: 10.3168/jds.S0022-0302(02)74161-1. [DOI] [PubMed] [Google Scholar]
- 10.Oliver SP, Almeida RA, Gillespie BE, et al. Extended ceftiofur therapy for treatment of experimentally-induced Streptococcus uberis mastitis in lactating dairy cattle. J Dairy Sci. 2004;87:3322–3329. doi: 10.3168/jds.S0022-0302(04)73468-2. [DOI] [PubMed] [Google Scholar]
- 11.Oliver SP, Almeida RA, Gillespie BE, et al. Efficacy of extended pirlimycin therapy for treatment of experimentally induced Streptococcus uberis intramammary infections in lactating dairy cattle. Vet Ther. 2003;4:299–308. [PubMed] [Google Scholar]
- 12.Hornish RE, Kotarski SF. Cephalosporins in veterinary medicine — ceftiofur use in food animals. Curr Top Med Chem. 2002;2:717–731. doi: 10.2174/1568026023393679. [DOI] [PubMed] [Google Scholar]
- 13.Roberson JR. Establishing treatment protocols for clinical mastitis. Vet Clin North Am Food Anim Pract. 2003;19:223–234. viii. doi: 10.1016/s0749-0720(02)00071-3. [DOI] [PubMed] [Google Scholar]
- 14.National Mastitis Council. Laboratory and Field Handbook on Bovine Mastitis. 1st ed. Arlington, Virginia: Natl. Mastitis Counc; 1987. [Google Scholar]
- 15.Cattel MB. An outbreak of Streptococci uberis as a consequence of adopting a protocol of no antibiotic therapy for clinical mastitis. Proceeding of the 35th National Mastitis Council Annual Meeting; 1996. p. 123. [Google Scholar]
- 16.Dopfer D, Barkema HW, Lam TJ, Schukken YH, Gaastra W. Recurrent clinical mastitis caused by Escherichia coli in dairy cows. J Dairy Sci. 1999;82:80–85. doi: 10.3168/jds.S0022-0302(99)75211-2. [DOI] [PubMed] [Google Scholar]
- 17.Morin DE, Shanks RD, McCoy GC. Comparison of antibiotic administration in conjunction with supportive measures versus supportive measures alone for treatment of dairy cows with clinical mastitis. J Am Vet Med Assoc. 1998;213:676–684. [PubMed] [Google Scholar]
- 18.Olde Riekerink RG, Barkema HW, Kelton DF, Scholl DT. Incidence rate of clinical mastitis on canadian dairy farms. J Dairy Sci. 2008;91:1366–1377. doi: 10.3168/jds.2007-0757. [DOI] [PubMed] [Google Scholar]
- 19.Middleton JR, Luby CD. Escherichia coli mastitis in cattle being treated for Staphylococcus aureus intramammary infection. Vet Rec. 2008;162:156–157. doi: 10.1136/vr.162.5.156. [DOI] [PubMed] [Google Scholar]
- 20.Roy JP, DesCoteaux L, DuTremblay D, Beaudry F, Elsener J. Efficacy of a 5-day extended therapy program during lactation with cephapirin sodium in dairy cows chronically infected with Staphylococcus aureus. Can Vet J. 2009;50:1257–1262. [PMC free article] [PubMed] [Google Scholar]
- 21.Deluyker HA, Oye SNV, Boucher JF. Factors affecting cure and somatic cell count after pirlimycin treatment of subclinical mastitis in lactating cows. J Dairy Sci. 2005;88:604–614. doi: 10.3168/jds.S0022-0302(05)72724-7. [DOI] [PubMed] [Google Scholar]
- 22.Bishop H, Erkelens J, Van Winden S. Predictors for successful bacteriological culture from milk samples. Vet Rec. 2010;166:322–324. doi: 10.1136/vr.b4789. [DOI] [PubMed] [Google Scholar]
- 23.Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet Rec. 2007;160:253–257. doi: 10.1136/vr.160.8.253. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson JD, Azzaro G, Gambina M, Licitra G. Prevalence of mastitis pathogens in Ragusa, Sicily, from 2000 to 2006. J Dairy Sci. 2007;90:5798–5813. doi: 10.3168/jds.2006-903. [DOI] [PubMed] [Google Scholar]
- 25.Reyher KK, Dufour S, Barkema HW, et al. The National Cohort of Dairy Farms — A data collection platform for mastitis research in Canada. J Dairy Sci. 2010;94:1616–1626. doi: 10.3168/jds.2010-3180. [DOI] [PubMed] [Google Scholar]