Abstract
Impaired abomasal motility is common in cattle with abomasal disorders. The macrolide erythromycin has been demonstrated to be an effective prokinetic agent in healthy calves and in adult cattle with abomasal volvulus or left displaced abomasum. We hypothesized that 2 structurally related macrolides, spiramycin and tulathromycin, would also be effective prokinetic agents in cattle. Six milk-fed, male, Holstein-Friesian calves were administered each of the following 4 treatments: spiramycin, 75 000 IU/kg BW, IM, this dose approximates 25 mg/kg BW, IM; tulathromycin, 2.5 mg/kg BW, SC; 2 mL of 0.9% NaCl (negative control); and erythromycin, 8.8 mg/kg BW, IM (positive control). Calves were fed 2 L of cow’s milk containing acetaminophen (50 mg/kg body weight) 30 min after each treatment was administered and jugular venous blood samples were obtained periodically after the start of sucking. Abomasal emptying rate was assessed by the time to maximal plasma acetaminophen concentration. Spiramycin, tulathromycin, and the positive control erythromycin increased abomasal emptying rate compared to the negative control. We conclude that the labeled antimicrobial dose of spiramycin and tulathromycin increases the abomasal emptying rate in healthy milk-fed calves. Additional studies investigating whether spiramycin and tulathromycin exert a prokinetic effect in adult cattle with abomasal hypomotility appear indicated.
Résumé
Un dérèglement de la motilité de l’abomasum est fréquent chez les bovins avec des troubles de l’abomasum. L’érythromycine, un antibiotique de la famille des macrolides, a été reconnu comme un agent procinétique efficace chez les veaux en santé et chez les bovins adultes avec un volvulus de l’abomasum ou un déplacement à gauche de la caillette. Nous avons émis l’hypothèse que deux autres macrolides apparentés structurellement, la spiramycine et la tulathromycine, seraient également des agents procinétiques efficaces chez les bovins. Six veaux mâles de race Holstein-Friesian nourris au lait ont reçu chacun des quatre traitements suivants : spiramycine, 75 000 IU/kg de poids corporel (BW), par voie intramusculaire (IM), cette dose équivaut approximativement à 25 mg/kg BW, IM; tulathromycine, 2,5 mg/kg BW, par voie sous-cutanée (SC); 2 mL d’une solution de NaCl 0,9 % (témoin négatif); et érythromycine 8,8 mg/kg BW, IM (témoin positif). Les veaux ont reçu 2 L de lait de vache contenant de l’acétaminophène (50 mg/kg de poids corporel) 30 min après l’administration de chaque traitement et des échantillons de sang veineux ont été obtenus périodiquement après le début de la tétée. Le rythme de vidange de l’abomasum a été évalué par le temps requis pour atteindre la concentration plasmatique maximale d’acétominophène. La spiramycine, la tulathromycine, et le témoin positif érythromycine ont fait augmenter le rythme de vidange de l’abomasum comparativement au témoin négatif. Nous concluons que la dose antimicrobienne de spiramycine et de tulathromycine mentionnée sur l’étiquette augmente le rythme de vidange de l’abomasum chez des veaux en santé nourris au lait. Des études supplémentaires semblent indiquées pour évaluer si la spiramycine et la tulathromycine ont un effet procinétique chez les bovins adultes avec hypomotilité de l’abomasum.
(Traduit par Docteur Serge Messier)
Introduction
Impaired abomasal motility is common in dairy cattle and is suspected to play a major role in the development of left displaced abomasum, abomasal volvulus, and abomasal impaction in adult cattle, and abomasal tympany in calves (1). A number of factors, such as hypocalcemia, endotoxemia, alkalemia, hyperglycemia, and increased abomasal luminal osmolality and energy content, decrease the abomasal emptying rate in cattle (2–8). The abomasal emptying rate is decreased in cows with left displaced abomasum (9–11) and abomasal volvulus (9,12), and is further decreased immediately after surgical correction of left displaced abomasum (9). It would, therefore, be clinically helpful to identify effective prokinetic agents that stimulate, coordinate, and restore abomasal motility in cattle suspected to have abomasal hypomotility. Erythromycin, neostigmine, bethanechol, and metoclopramide have been used as part of the treatment of cattle suspected to have gastrointestinal hypomotility (10,12–17), with the macrolide erythromycin exerting the strongest prokinetic effect (17,18).
Macrolides are a group of closely related antimicrobials that are categorized according to the number of lactone ring components as 12-membered, 13-membered, 14-membered, 15-membered, and 16-membered groups, with one or more amino sugars or deoxy sugars being attached to the macrocyclic lactone ring (19). There are no macrolides with a 12-membered lactone ring currently in clinical use in humans or animals. Tulathromycin is a semi-synthetic equilibrated mixture of a 13-membered (10%) and 15-membered (90%) macrolide ring structure with a unique chemical structure consisting of 3 polar amine groups (20–22). Erythromycin, oleandomycin, and clarithromycin belong to the 14-membered group, with erythromycin and oleandomycin being of natural origin. Erythromycin is widely used in humans and domestic animals and is considered the representative macrolide. The 15-membered ring macrolides are named azalides, with the semisynthetic antimicrobials azithromycin, gamithromycin, and the predominant isomer of tulathromycin being in widespread clinical use. Josamycin, midemycin, miokamycin, rokitamycin, spiramycin, tildipirosin, tilmicosin, and tylosin are the only 16-membered macrolides in clinical use (19); spiramycin, tilmicosin, and tylosin have been widely used in veterinary medicine, and tildipirosin was first made available in the summer of 2012. Erythromycin, gamithromycin, spiramycin, tildipirosin, tilmicosin, tulathromycin, and tylosin are currently approved and marketed for veterinary use, with availability varying from country to country.
As a group, macrolides generally act as bacteriostatic agents by reversibly binding to 50S subunits of the ribosome and inhibiting the transpeptidation and translocation process, resulting in premature detachment of incomplete polypeptide chains (19). Macrolides have pharmacodynamic properties beyond their antimicrobial effects, including anti-inflammatory and immunomodulatory properties that are perceived to be clinically beneficial (19,23,24). An additional pharmacodynamic property of macrolides is a prokinetic effect, which has been documented extensively for erythromycin (10,12,16,25–36) and, to a lesser extent, for clarithromycin (37), azithromycin (38), tilmicosin (30), and tylosin (30). Previous studies have failed to demonstrate any effect of spiramycin on gastrointestinal motility (34,35,39). Based on structural similarities to erythromycin, particularly the presence of an amino-sugar at C-5 of the lactone ring, we hypothesized that parenteral administration of spiramycin and tulathromycin would increase the abomasal emptying rate in milk-fed calves. Preliminary support for this hypothesis was provided by a recent study that demonstrated 2 structurally related macrolides to spiramycin (tylosin and tilmicosin) exerted a prokinetic effect in milk-fed calves (30). We investigated our hypothesis in milk-fed calves by using 2 methods to assess abomasal emptying rate, acetaminophen (paracetamol) absorption and glucose absorption, as well as a negative and positive control treatment.
Materials and methods
Animals
Six 5- to 8-day-old Holstein-Friesian bull calves, ranging in body weight from 38 to 46 kg (mean, 42 kg) were obtained from a local dairy farm. Calves were bottle-fed fresh cow’s milk and housed at the University of Shahid Chamran unrestrained in separate stalls that were bedded with wood shavings. Calves had access to fresh water at all times, but a calf starter ration was not fed. Approval of the study protocol was not required by the institutional animal care and use committee because institutional guidelines indicated approval was not needed if commercially available formulations were administered at the labeled dose and route of administration, and because of the minimally invasive nature of the procedures in the study (IV, IM, and SC injections and periodic IV collection of blood samples).
Experimental design
Calves were at least 10 d of age when they entered the treatment phase of the study. At least 18 h before each experiment, calves were sedated using xylazine hydrochloride (0.2 mg/kg BW, IV) to facilitate placement of a jugular venous catheter. The hair over the right jugular vein was clipped and the skin aseptically prepared. One milliliter of lidocaine hydrochloride was injected SC over the right jugular vein, and the skin was incised (1 cm in length) with a scalpel blade to assist in catheter placement. A 16- or 18-gauge catheter was inserted in the jugular vein; an extension set was attached to the catheter and extension set were secured to the neck. The catheter was flushed every 12 h with heparinized saline solution (40 U of heparin/mL).
Calves were administered each of 4 treatments in a crossover study. A minimum of 36 h was allowed to elapse between subsequent treatments. Treatments were not initiated until at least 12 h had elapsed since a calf had consumed the preceding feeding. Each calf was weighed and then assigned to receive one of the following treatments: spiramycin (Suanovil 20; Mérial, Lyon, France), 75 000 IU/kg BW, this dose approximates 25 mg/kg BW, IM); tulathromycin (Draxxin; Zoetis, Florham Park, New Jersey, USA), 2.5 mg/kg BW, SC; 2 mL of 0.9% NaCl solution IM (negative control treatment); and erythromycin (Hospira, Royal Leamington Spa, United Kingdom), 8.8 mg/kg BW, IM, which was the positive control treatment. The first 3 treatments administered were control, erythromycin, and spiramycin and they were randomly assigned using a random number generator (Excel spreadsheet; Microsoft Corp, Redmond, Washington, USA). Tulathromycin was administered as the last treatment for each calf because it is slowly cleared after SC administration (40).
Thirty minutes after administration of each treatment, the calves were allowed to suckle 2 L of fresh cow’s milk at room temperature (19°C to 22°C) that contained a dose of acetaminophen (Jalinous Pharmaceutical Company, Tehran, Iran), 50 mg/kg BW. Abomasal emptying rate was measured by use of acetaminophen and glucose absorption techniques as previously described (6,41). Venous blood samples for determination of plasma acetaminophen and glucose concentrations were obtained at −30, 0, 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 300, 360, 420, and 480 min (start of suckling was designated as time 0). These time points for obtaining samples were selected in an attempt to provide at least 6 data points before and after the time of maximal acetaminophen concentration (Tmax) in order to facilitate nonlinear regression analysis for pharmacokinetic modeling. Blood samples were collected into 6-mL partially evacuated tubes containing sodium fluoride and potassium oxalate and centrifuged at 1000 × g for 15 min. Three milliliters of plasma was harvested and stored at −20°C until analysis was done.
Laboratory analysis
Plasma was thawed at 19°C to 22°C and the acetaminophen concentration analyzed spectrophotometrically (Convergys-100, Convergent Technologies GmbH & Co. KG Frankenberg Germany) by use of a colorimetric nitration assay as described elsewhere (41). Actual maximal concentration (Cmax) and actual Tmax were derived from a plot of the plasma acetaminophen concentration versus time data. The first derivative of Siegel’s modified power exponential formula was used to model the acetaminophen time curve (6,41,42). The equation was derived from the fact that the acetaminophen concentration versus time curve represented as a cumulative dose curve is an inverse analogue of the scintigraphic curve with the following equation:
where: C(t) is the acetaminophen concentration in plasma at a specified time point, t is time, m [units of (μg/mL) × min] is the area under acetaminophen concentration-time curve when time is infinite, k (units of min−1) is an estimate of the rate constant for abomasal emptying, β is a constant that provides an estimate of the duration of the lag phase before an exponential rate of emptying is reached, and e is the natural logarithm. Nonlinear regression (PROC NLIN, SAS, version 9.2; SAS Institute, Cary, North Carolina, USA) was used to estimate values for m, k, and β as described (41,42). Values for model Cmax and model Tmax were obtained by fitting the estimated values for k, β, and m in the nonlinear equation to the cumulative dose curve equation for acetaminophen.
Plasma glucose concentration was determined using an automatic analyzer (Convergys-100, Convergent Technologies GmbH & Co. KG, Frankenberg, Germany). Actual Cmax and actual Tmax were derived from a plot of the plasma glucose concentration versus time data, and the area under the plasma glucose concentration–time curve was calculated from 0 to 6 h by using the trapezoid method; this area provides a crude index of the amount of glucose absorbed for each treatment (6–8).
Statistical analysis
Data were expressed as mean ± SD and a value of P < 0.05 was considered significant for all statistical analyses. The primary variable of interest was the mean value for Tmax calculated by modeling the acetaminophen concentration-time relationship (model Tmax). A secondary variable of interest was the mean value for the actual Tmax for the glucose concentration-time relationship. A repeated-measures analysis of variance (ANOVA) (PROC MIXED, SAS, version 9.2; SAS Institute) was used to determine the main effects of treatment using a compound symmetry covariance matrix. Post hoc tests were conducted to compare spiramycin and tulathromycin with the negative control whenever the value for the F-test for treatment was significant. Previous studies by the investigators have indicated that a study involving 6 calves in a crossover design provided adequate statistical power to detect a prokinetic effect at α = 0.05 and β = 0.80 (17,30–32).
Results
Acetaminophen absorption
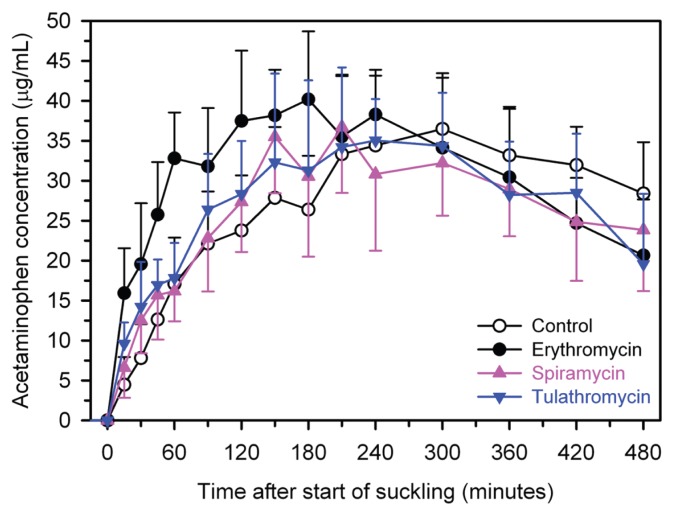
Spiramycin significantly increased the rate of abomasal emptying, as assessed by actual Tmax (P = 0.023) and model Tmax (P = 0.024; Figure 1, Table I). Tulathromycin significantly increased the rate of abomasal emptying, as assessed by model Tmax (P = 0.022; Figure 1, Table I), but not by actual Tmax (P = 0.41). The positive control treatment, erythromycin, significantly increased the rate of abomasal emptying, as assessed by actual Tmax (P = 0.0002) and model Tmax (P < 0.0001; Figure 1, Table I).
Figure 1.
Mean ± standard deviation (SD) plasma concentration of acetaminophen in 6 calves after treatment with spiramycin (75 000 IU/kg BW, IM, pink triangles), tulathromycin (2.5 mg/kg BW, SC, blue triangles), a negative control (2.0 mL of 0.9% NaCl solution IM, open circles), or a positive control (erythromycin, 8.8 mg/kg BW, IM, black circles) using a crossover design. Calves were allowed to suckle 2 L of fresh cow’s milk containing acetaminophen (50 mg/kg BW) 30 min after treatments were administered.
Table I.
Abomasal emptying rate indices (mean ± SD) of 6 calves suckling 2 L of fresh cow’s milk containing acetaminophen (50 mg/kg BW). Calves were administered one of the following 4 treatments 30 min before suckling in a cross-over design; spiramycin (75 000 IU/kg BW, IM), tulathromycin (2.5 mg/kg BW, SC), a negative control (2.0 mL of 0.9% NaCl solution, IM), or a positive control (erythromycin, 8.8 mg/kg BW, IM) using a crossover design. Abomasal emptying rate was assessed by acetaminophen absorption and glucose absorption. Actual Cmax is the maximal plasma acetaminophen or glucose concentration and actual Tmax is the time at which actual Cmax occurred. Model Cmax and Tmax for acetaminophen were obtained by fitting a nonlinear equation to the first derivative of Siegel’s modified power exponential formula for acetaminophen. For glucose absorption, area under the curve is the area under the plasma glucose concentration-time relationship for the 6-hour period after suckling
| Factor | Spiramycin | Tulathromycin | Negative control | Positive control (erythromycin) | P-value: F-test treatment |
|---|---|---|---|---|---|
| Acetaminophen absorption | |||||
| Actual Cmax (μg/mL) | 37.7 ± 4.8 | 37.4 ± 6.6 | 37.6 ± 7.5 | 42.0 ± 6.4 | 0.58 |
| Actual Tmax (minutes) | 220 ± 53a | 255 ± 53 | 273 ± 43 | 170 ± 31a | 0.0009 |
| Model Cmax (μg/mL) | 34.5 ± 6.0 | 34.1 ± 5.8 | 35.2 ± 7.6 | 39.7 ± 6.9 | 0.52 |
| Model Tmax (minutes) | 226 ± 48a | 225 ± 52a | 285 ± 38 | 165 ± 15a | 0.0015 |
| k (minutes−1) | 0.0032 ± 0.0009 | 0.0032 ± 0.0011 | 0.0027 ± 0.0009 | 0.0034 ± 0.0009 | 0.35 |
| β | 2.05 ± 0.34 | 1.96 ± 0.18 | 2.17 ± 0.48 | 1.76 ± 0.27a | 0.035 |
| m (μg/mL) × minutes | 22 645 ± 6388 | 22 844 ± 6968 | 27 425 ± 5798 | 23 374 ± 6609 | 0.28 |
| Glucose absorption | |||||
| Actual Cmax (mg/dL) | 133 ± 10 | 124 ± 8 | 124 ± 9 | 124 ± 12 | 0.15 |
| Actual Tmax (minutes) | 195 ± 146 | 180 ± 118 | 245 ± 119 | 83 ± 42 | 0.18 |
| Area under the curve (g × minutes/dL) | 56.8 ± 3.1 | 52.9 ± 2.8 | 53.9 ± 4.5 | 52.5 ± 3.2 | 0.042 |
Significantly different (P < 0.05) from control value.
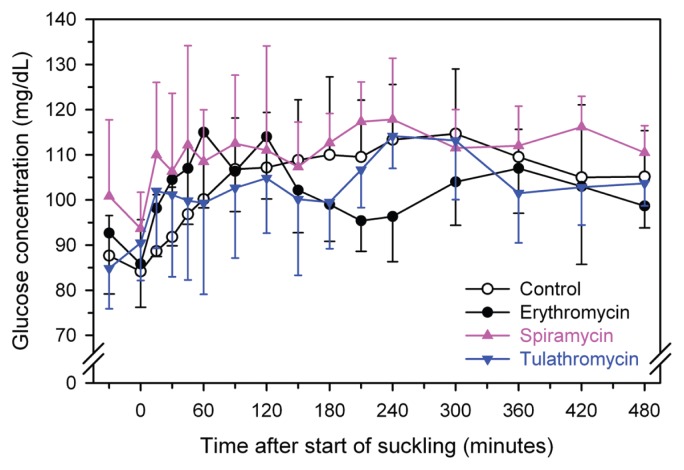
Glucose absorption
There was no significant effect of treatment on the glucose absorption curve (Figure 2, Table I); however, the mean value for actual Tmax was numerically shorter for spiramycin, tulathromycin, and erythromycin than control.
Figure 2.
Mean ± SD plasma concentration of glucose in 6 calves after treatment with spiramycin (75 000 IU/kg BW, IM, pink triangles), tulathromycin (2.5 mg/kg BW, SC, blue triangles), a negative control (2.0 mL of 0.9% NaCl solution IM, open circles), or a positive control (erythromycin, 8.8 mg/kg BW, IM, black circles) using a crossover design. Calves were allowed to suckle 2 L of fresh cow’s milk containing acetaminophen (50 mg/kg BW) 30 min after treatments were administered.
Discussion
The major new findings of the present study were that spiramycin and tulathromycin increased the abomasal emptying rate in suckling calves. We believe this report is the first to demonstrate a prokinetic effect of spiramycin or tulathromycin in any species, although the prokinetic effect was not marked. Our findings are contrary to long held beliefs that only 14-membered macrolides (such as erythromycin) have prokinetic activity (34–36).
Erythromycin was administered as a positive control in this study because it has been documented to produce a prokinetic effect in calves (17,30–32) and adult cows (10,12,16), probably by acting as a motilin-receptor agonist via binding to motilin receptors in the pyloric antrum and proximal portion of the small intestine (33,43). Motilin is a peptide consisting of 22 amino acids that is periodically released from endocrine cells in the duodenojejunal mucosa, thereby initiating the migrating motor complex of the mammalian gastrointestinal tract during the interdigestive period. There is considerable interest in the group of nonpeptide motilin agonists, referred to as the motilides (i.e., motilin-like macrolides), that interact with the motilin receptor and promote gastric emptying (43).
Structure-activity studies have indicated that motilides have 3 main structural requirements that enable them to interact strongly with the motilin receptor and thereby induce changes in gastrointestinal motility: a ring structure [typically a 14-member lactone (cyclic ester) ring], an amino sugar (desosamine) bound at C-5 of the ring in a glycosidic linkage, and a neutral sugar (such as cladinose) bound at C-3 of the ring in a glycosidic linkage (44,45). From this 3-part structure, the potency of the motilide is influenced primarily by modifications to the N-dimethylamino group at the 3′ position of the amino sugar bound at C-5 of the ring and, to a lesser extent, the configuration of the lactone ring structure (C-6 through C-9) and by the presence of a neutral sugar at C-3 that is parallel to the amino sugar at C-5 (46,47). Erythromycin has a 14-membered enol ether lactone ring with a dimethylamino sugar (desosamine) at C-5 and a neutral sugar (cladinose) at C-3 in parallel with desosamine and, therefore, possesses excellent potency as a prokinetic agent. Spiramycin has a 16-membered lactone ring with 2 double bonds, an amino sugar at C-5 with a neutral sugar attached in serial glycosidic linkage, a hydroxyl group instead of a neutral sugar at C-3, and a side-chain sugar at C-14. Tulathromycin is a semi-synthetic macrolide that contains a regioisomeric, equilibrated mixture of a 15-membered (90%) and 13-membered (10%) macrocyclic ring 15-membered lactone ring structure and 3 polar amine groups (20–22). The results of the study reported here regarding spiramicin and tulathromycin, combined with the results of our previous study in calves investigating the prokinetic effects of tilmicosin and tylosin (30), and those in humans involving clarithromycin (37) and azithromycin (38) provide strong support to the concept that the binding of an amino sugar (desosamine) to C-5 of the lactone ring plays an important role in producing a prokinetic effect. Based on the results of the study reported here and current knowledge of structure-activity relationships for macrolides, we speculate that of the 2 new macrolides released in 2012 for administration to cattle, tildipirosin (which is derived from tylosin) will exert a weak prokinetic effect, whereas gamithromycin should be a much stronger prokinetic agent. We suspect that gamithromycin may increase abomasal emptying rate in cattle to the same extent as erythromycin and to a greater extent than tulathromycin. This supposition requires experimental verification.
Acetylspiramycin did not alter gastric emptying or motility in dogs when administered intravenously at 10 to 25 mg/kg BW (34,35,48) or orally at 60 mg/kg BW (49). However, spiramycin is suspected to produce a gastrointestinal effect in dogs, as oral administration of spiramycin (500 mg or 1000 mg, BW not stated) increased intestinal contractions and induced vomiting in 2 of 5 dogs (48), and IV administration of spiramycin adipate (50 mg/kg BW) induced vomiting in 4/4 dogs (50). The relevance of these dog studies to the prokinetic effect of spiramycin in cattle is not clear, but the acetylspiramycin studies in dogs have been used as a basis for long-held beliefs that spiramycin does not alter gastric emptying or motility. In contrast, we demonstrated a statistically significant effect of spiramycin (25 mg/kg BW, IM) on abomasal emptying rate in calves. The milk-fed calf may, therefore, provide a more sensitive in vivo model for evaluating prokinetic agents than the adult dog as the calf’s abomasum can be rapidly primed with a large fluid volume (approximately 4% of body weight within 3 min), and the ingested meal is fluid and not semisolid or solid.
The study reported here was conducted in milk-fed calves instead of adult cattle because abomasal emptying studies are technically much easier and less expensive to conduct in milk-fed calves, and because we have validated acetaminophen absorption as an index of abomasal emptying against the reference method, scintigraphy, in milk-fed calves (41). Abomasal emptying studies in adult cattle most commonly employ percutaneous injection of a marker substance into the abomasum using ultrasonographic guidance (9), surgically implanted wires to record the electrical activity of abomasal smooth muscle (14–16), the surgical placement of a rumen or abomasal fistula in order to administer a marker substance directly into the abomasum (51), or surgical placement of a T cannula in the proximal duodenum so that abomasal effluent can be collected during timed intervals (4,52). All of these techniques are more invasive and expensive than the acetaminophen absorption test in milk-fed calves, and many have not been validated against a reference method.
We believe that the results of this study conducted in calves suckling fresh cow’s milk can be extrapolated to adult cattle with a functional forestomach for 3 reasons. First, the abomasal volume of adult cattle contains approximately 2 to 3 L of fluid (53), which is similar to the 2 L volume of fresh cow’s milk suckled by the calves in the study reported here. Second, abomasal emptying in both suckling calves and adult cattle is best characterized as liquid phase emptying (30). Third, we have obtained similar increases in abomasal emptying rate when erythromycin has been administered to adult cattle (10,12,16) as in milk-fed calves (17,18,30,31). Spiramycin is labeled in France and many other countries for treating adult cattle and calves with respiratory disease, foot rot, metritis, and mastitis. Tulathromycin is labeled in many countries for the treatment of respiratory disease in cattle. Therefore, we administered spiramycin and tulathromycin in an extra label manner. It is clearly inappropriate to administer an antimicrobial for a non-antimicrobial effect (such as increasing abomasal emptying rate), as such use may unnecessarily promote the development of antimicrobial resistance (10,12,23,30).
Our finding that spiramycin and tulathromycin exerts a prokinetic effect in milk-fed calves suggests an additional potential therapeutic advantage for these drugs in the treatment of infectious diseases in adult cattle beyond the elimination of infection, in that spiramycin and tulathromycin may also mitigate gastrointestinal tract hypomotility that is commonly associated with anorexia in diseased cattle. We have previously documented the prokinetic effect of erythromycin in healthy milk-fed calves (17,30–32) also occurs in adult dairy cows with abomasal hypomotility due to left displaced abomasum (10) or abomasal volvulus (12). It is important to note that erythromycin increased milk production and rumen contraction rate in the immediate post-operative period in dairy cattle undergoing surgical correction of left displaced abomasum or abomasal volvulus (10,12). Therefore, it is likely that the documented prokinetic effect of spiramycin and tulathromycin is clinically important. Additional studies investigating whether spiramycin and tulathromycin exert a prokinetic effect in adult cattle with abomasal hypomotility appear to be indicated.
Acknowledgment
This work was supported, in part, by a grant from the University of Shahid Chamran, Ahvaz, Iran.
References
- 1.Constable PD, Miller GY, Hoffsis GF, Hull BL, Rings DM. Risk factors for abomasal volvulus and left abomasal displacement in cattle. Am J Vet Res. 1992;53:1184–1192. [PubMed] [Google Scholar]
- 2.Madison JB, Troutt HF. Effects of hypocalcaemia on abomasal motility. Res Vet Sci. 1988;44:264–266. [PubMed] [Google Scholar]
- 3.Vlaminck K, Van Meirhaeghe HV, Den Hende C. Einfluss von Endotoxinen auf die Labmagenetleerung beim rind. Deutsche Tierarztliche Wochenschrift. 1985;92:392–395. [PubMed] [Google Scholar]
- 4.Poulsen JSD, Jones BEV. The influence of metabolic alkalosis and other factors on abomasal emptying rate in goats and cows. Nord Vet Med. 1974;26:22–30. [PubMed] [Google Scholar]
- 5.Holtenius K, Jacobson MSO, Holtenius P. Effects of intravenous infusion of glucose and pancreatic glucagon on abomasal function in dairy cows. Acta Vet Scand. 1998;39:291–300. doi: 10.1186/BF03547801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nouri M, Constable PD. Comparison of two oral electrolyte solutions and route of administration on the abomasal emptying rate of Holstein-Friesian calves. J Vet Intern Med. 2006;20:620–626662. doi: 10.1892/0891-6640(2006)20[620:cotoes]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Sen I, Constable PD, Marshall TS. Effect of suckling isotonic or hypertonic solutions of sodium bicarbonate or glucose on abomasal emptying rate in calves. Am J Vet Res. 2006;67:1377–1384. doi: 10.2460/ajvr.67.8.1377. [DOI] [PubMed] [Google Scholar]
- 8.Constable PD, Grünberg W, Carstensen L. Comparative effects of two oral oral rehydration solutions on milk clotting, abomasal luminal pH, and abomasal emptying rate in suckling calves. J Dairy Sci. 2009;92:296–312. doi: 10.3168/jds.2008-1462. [DOI] [PubMed] [Google Scholar]
- 9.Wittek T, Schreiber K, Furll M, Constable PD. Use of D-xylose absorption test to measure abomasal emptying rate in healthy lactating Holstein-Friesian cows and in cows with left displaced abomasum or abomasal volvulus. J Vet Intern Med. 2005;19:905–913. doi: 10.1892/0891-6640(2005)19[905:uotdat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Wittek T, Tischer K, Gieseler T, Fürll M, Constable PD. Effect of preoperative administration of erythromycin or flunixin meglumine on postoperative abomasal emptying rate in dairy cows undergoing surgical correction of left displacement of the abomasum. J Am Vet Med Assoc. 2008;232:418–423. doi: 10.2460/javma.232.3.418. [DOI] [PubMed] [Google Scholar]
- 11.Wittek T, Locher L, Alkaassem A, Constable PD. Effect of surgical correction of left displaced abomasum by means of omentopexy via right flank laparotomy or two-step laparoscopy-guided abomasopexy on postoperative abomasal emptying rate in lactating dairy cows. J Am Vet Med Assoc. 2009;234:652–657. doi: 10.2460/javma.234.5.652. [DOI] [PubMed] [Google Scholar]
- 12.Wittek T, Tischer K, Körner I, Sattler T, Constable PD, Fürll M. Effect of preoperative erythromycin or dexamethasone/Vitamin C on postoperative abomasal emptying rate in dairy cows undergoing surgical correction of abomasal volvulus. Vet Surg. 2008;37:537–544. doi: 10.1111/j.1532-950X.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- 13.Braun SA, Kaegi B. Clinical, hematological and biochemical findings and the results of treatment in cattle with acute functional pyloric stenosis. Vet Rec. 1990;126:107–110. [PubMed] [Google Scholar]
- 14.Dardillat C, Ruckebusch Y. Aspects fonctionnels de la junction gastro-duodenale chez le veau nouveau-ne. Ann Rech Veter. 1973;4:31–56. [Google Scholar]
- 15.Roussel AJ, Brumbaugh GW, Waldon RC, Baird AN. Abomasal and duodenal motility in yearling cattle after administration of prokinetic drugs. Am J Vet Res. 1994;55:111–115. [PubMed] [Google Scholar]
- 16.Huhn JC, Nelson DR, Constable PD, Morin DE. Prokinetic properties of erythromycin lactobionate in cattle. Proceedings World Buiatrics Cong. 1998:177–180. [Google Scholar]
- 17.Wittek T, Constable PD. Assessment of the effects of erythromycin, neostigmine, and metoclopramide on abomasal motility and emptying rate in calves. Am J Vet Res. 2005;66:545–552. doi: 10.2460/ajvr.2005.66.545. [DOI] [PubMed] [Google Scholar]
- 18.Constable PD, Nouri M, Sen I, Baird AN, Wittek T. Evidence-based use of prokinetic drugs for abomasal disorders in cattle. In: Buczinski S, Vandeweerd JM, editors. Evidence-based medicine for the bovine veterinarian. Veterinary Clinics of North America, Food Animal Practice. 1. Vol. 28. Philadelphia, Pennsylvania: WB Saunders Company; 2012. pp. 50–70. [DOI] [PubMed] [Google Scholar]
- 19.Giguère S. Macrolides, azalides, and ketolides. In: Giguère S, Prescott JF, Baggot JD, Walker RD, Dowling PM, editors. Antimicrobial Therapy in Veterinary Medicine. 4th ed. Blackwell Publishing; Ames, Iowa: 2006. pp. 191–206. [Google Scholar]
- 20.EMEA: European Medicines Agency, Veterinary Medicines and Inspections. Committee for Veterinary Medicinal Products: Tulathromycin, Summary Report; 2002. [Last accessed September, 2013]. pp. 1–10. EMEA/MRL/842/02-FINAL. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015700.pdf. [Google Scholar]
- 21.Benchaoui HA, Nowakowski M, Sherington J, Rowan TG, Sunderland SJ. Pharmacokinetics and lung tissue concentrations of tulathromycin in swine. J Vet Pharmacol Ther. 2004;27:203–210. doi: 10.1111/j.1365-2885.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 22.Evans NA. Tulathromycin: An overview of a new triamilide antimicrobial for livestock respiratory disease. Vet Ther. 2005;6:83–95. [PubMed] [Google Scholar]
- 23.Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: Benefits versus risks. J Antimicrob Chemotherapy. 2007;59:347–358. doi: 10.1093/jac/dkl537. [DOI] [PubMed] [Google Scholar]
- 24.Buret AG. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin. Can J Vet Res. 2010;74:1–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Tomomasa T, Kuroume T, Arai H, Wakabayski K, Itoh Z. Erythromycin induced migratory motor complex in human gastrointestinal tract. Dig Dis Sci. 1986;31:157–161. doi: 10.1007/BF01300701. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Z, Nakay M, Suzuki T, Arai H, Wakabayski K. Erythromycin mimics exogenous motilin in gastrointestinal contractile activity in the dog. Am J Physiol. 1984;247:G688–G694. doi: 10.1152/ajpgi.1984.247.6.G688. [DOI] [PubMed] [Google Scholar]
- 27.Fraser R, Shearer T, Fuller J, Horowitz M, Dent J. Intravenous erythromycin overcomes small intestinal feedback on antral, pyloric, and duodenal motility. Gastroentrerology. 1992;103:114–119. doi: 10.1016/0016-5085(92)91103-b. [DOI] [PubMed] [Google Scholar]
- 28.Annese V, Jenssens J, Vantrapen G, et al. Erythromycin accelerates gastric emptying by inducing antral contractions and improved gastroduodenal coordination. Gastroenterology. 1992;102:823–828. doi: 10.1016/0016-5085(92)90164-t. [DOI] [PubMed] [Google Scholar]
- 29.Ringger C, Lester GD, Neuwirth L, Merritt AM, Vetro T, Harrison J. Effect of bethanechol or erythromycin on gastric emptying in horses. Am J Vet Res. 1996;57:1771–1775. [PubMed] [Google Scholar]
- 30.Nouri M, Constable PD. Effect of parenteral administration of erythromycin, tilmicosin, and tylosin on abomasal emptying rate in suckling calves. Am J Vet Res. 2007;68:1392–1398. doi: 10.2460/ajvr.68.12.1392. [DOI] [PubMed] [Google Scholar]
- 31.Nouri M, Hajizolaee MR, Constable PD, Omidi A. Effect of erythromycin and gentamicin on abomasal emptying rate in suckling calves. J Vet Intern Med. 2008;22:196–201. doi: 10.1111/j.1939-1676.2007.0027.x. [DOI] [PubMed] [Google Scholar]
- 32.Afshari G, Nouri M, Hassan EB, Mokhber-Dezfooli MR, Constable PD. Effect of parenteral administration of ivermectin and erythromycin on abomasal emptying rate in suckling calves. Am J Vet Res. 2009;70:527–531. doi: 10.2460/ajvr.70.4.527. [DOI] [PubMed] [Google Scholar]
- 33.Coulie B, Tack J, Peeters T, Janssens J. Involvement of two different pathways in the motor effects of erythromycin on the gastric antrum in humans. Gut. 1998;43:395–400. doi: 10.1136/gut.43.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh Z, Suzuki T, Nakaya M, Inoue M, Arai H, Wakabayashi K. Structure-activity relation among macrolide antibiotics in initiation of interdigestive migrating contractions in the canine gastrointestinal tract. Am J Physiol. 1985;248:G320–G325. doi: 10.1152/ajpgi.1985.248.3.G320. [DOI] [PubMed] [Google Scholar]
- 35.Itoh Z, Suzuki T, Nakaya M, Mitsuhashi S. Gastrointestinal motor-stimulating activity of macrolide antibiotics and analysis of their side effects on the canine gut. Antimicrob Agents Chemother. 1984;26:863–869. doi: 10.1128/aac.26.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catnach SM, Fairclough PD. Erythromycin and the gut. Gut. 1992;33:397–401. doi: 10.1136/gut.33.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bortolotti M, Mari C, Brunelli F, Sarti P, Miglioli M. Effect of intravenous clarithromycin on interdigestive gastroduodenal motility of patients with functional dyspepsia and Helicobacter pylori gastritis. Dig Dis Sci. 1999;44:2439–2442. doi: 10.1023/a:1026674719476. [DOI] [PubMed] [Google Scholar]
- 38.Larson JM, Tavakkoli A, Drane WE, Toskes PP, Moshiree B. Advantages of azithromycin over erthromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil. 2010;16:407–413. doi: 10.5056/jnm.2010.16.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin XY, Pilot MA, Thompson HH, Maskell JP. Comparison of the side effects and gastrointestinal motility observed after administration of erythromycin and josamycin to dogs. J Antimicrob Chemother. 1986;18:747–756. doi: 10.1093/jac/18.6.747. [DOI] [PubMed] [Google Scholar]
- 40.Nowakowski MA, Inskeep PB, Risk JE, et al. Pharmacokinetics and lung tissue concentrations of tulathromycin, a new triamilide antibiotic, in cattle. Vet Ther. 2004;5:60–74. [PubMed] [Google Scholar]
- 41.Marshall T, Constable PD, Crochik S, Wittek T. Comparison of acetaminophen absorption and scintigraphy as methods for studying abomasal emptying rate in suckling dairy calves. Am J Vet Res. 2005;66:364–374. doi: 10.2460/ajvr.2005.66.364. [DOI] [PubMed] [Google Scholar]
- 42.Maes BD, Ghoos YF, Geypens BJ, et al. Combined carbon-13-glycine/carbon-14-octanoic acid breath test to monitor gastric emptying rates of liquids and solids. J Nucl Med. 1994;35:824–831. [PubMed] [Google Scholar]
- 43.Itoh Z. Motilin and clinical application. Peptides. 1997;18:593–608. doi: 10.1016/s0196-9781(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 44.Tsuzuji K, Sunazuka T, Marui S, et al. Motilides, macrolides with gastrointestinal motor stimulating activity. I. O-substituted and tertiary N-substituted derivatives of 8,9-anhydroerythromycin A 6,9-hemiacetal. Chem Pharm Bull (Tokyo) 1989;37:2687–2700. doi: 10.1248/cpb.37.2687. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Depoortere I, Vertongen P, Waelbroeck M, Robberecht P, Peetes TL. Motilin and erythromycin-A share a common binding site in the third transmembrane segment of the motilin receptor. Biochem Pharmacol. 2005;70:879–887. doi: 10.1016/j.bcp.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Pilot MA. Macrolides in roles beyond antibiotic therapy. Br J Surg. 1994;81:1423–1429. doi: 10.1002/bjs.1800811006. [DOI] [PubMed] [Google Scholar]
- 47.Sunazuka T, Tsuzuki K, Marui S, et al. Motilides, macrolides with gastrointestinal motor stimulating activity. II. Quaternary N-substituted derivatives of 8,9-anhydroeryhtromycin A 6,9-hemiacetal and 9,9-dihydroerythromycin A 6,9-epoxide. Chem Pharm Bull (Tokyo) 1989;37:2701–2709. doi: 10.1248/cpb.37.2701. [DOI] [PubMed] [Google Scholar]
- 48.Qin X, Pilot M, Thompson H, Maskell J. Effects of spiramycin on gastrointestinal motility. Chemioterapia. 1987;6:319–320. [PubMed] [Google Scholar]
- 49.Nakayoshi T, Izumi M, Tatsuta K. Effects of macrolide antibiotics on gastrointestinal motility in fasting and digestive states. Drugs Exptl Clin Res. 1992;18:103–109. [PubMed] [Google Scholar]
- 50.Woodward KN. Spiramycin. [Last accessed September 2013]. (JECFA — Monographs and Evaluations #737, WHO Food Additives Series 29). Available from: http://www.inchem.org/documents/jecfa/jecmono/v29je07.htm.
- 51.Ahmed AF, Constable PD, McCallister MM, Misk NA. Abomasal cannulation in the milk-fed calf using a 7 mm polyurethane tube. J Vet Med A Physiol Pathol Clin Med. 2005;52:39–42. doi: 10.1111/j.1439-0442.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 52.Koenig GJ, Constable PD, Firkins J, Hull BL. Limitations and safety hazards of the propionate loading test: assessing hepatic function in the dairy cow. Large Anim Vet. 1999;20:24–29. [Google Scholar]
- 53.Wittek T, Constable PD, Morin DE. Ultrasonographic assessment of change in abomasal position during the last three months of gestation and first three months of lactation in Holstein-Friesian cows. J Am Vet Med Assoc. 2005;227:1469–1475. doi: 10.2460/javma.2005.227.1469. [DOI] [PubMed] [Google Scholar]