Abstract
The objective of this pilot study was to determine the efficacy of inactivated (1 or 2 dose) and live-attenuated chimeric porcine circovirus (PCV)1-2 vaccines in sows using the PCV2-spiked semen model. Thirty-five sows were randomly divided into 6 groups: negative and positive controls, 1 dose inactivated PCV1-2 vaccine challenged (1-VAC-PCV2), 2 dose inactivated PCV1-2 vaccine challenged (2-VAC-PCV2), 1 dose live-attenuated PCV1-2 vaccine unchallenged (1-LIVE-VAC), and 1 dose live-attenuated PCV1-2 vaccine challenged (1-LIVE-VAC-PCV2). The inactivated PCV1-2 vaccine induced higher levels of PCV2-specific antibodies in dams. All vaccination strategies provided good protection against PCV2 viremia in dams, whereas the majority of the unvaccinated sows were viremic. Four of the 35 dams became pregnant: a negative control, a positive control, a 2-VAC-PCV2 sow, and a 1-LIVE-VAC-PCV2 sow. The PCV2 DNA was detected in 100%, 67%, and 29% of the fetuses obtained from the positive control, inactivated vaccinated, or live-attenuated vaccinated dams, respectively. The PCV2 antigen in hearts was only detectable in the positive control litter (23% of the fetuses). The PCV1-2 DNA was detected in 29% of the fetuses in the litter from the 1-LIVE-VAC-PCV2 dam. Under the conditions of this pilot study, both vaccines protected against PCV2 viremia in breeding age animals; however, vertical transmission was not prevented.
Résumé
L’objectif de cette étude pilote était de déterminer l’efficacité de vaccins inactivés (1 ou 2 doses) et vivants atténués chimériques du circovirus porcin (PCV)1-2 chez des truies en utilisant le modèle de semence inoculée avec PCV2. Trente-cinq truies ont été réparties de manière aléatoire en six groupes : témoins négatif et positif, 1 dose de vaccin PCV1-2 inactivé et challengé (1-VAC-PCV2), 2 doses de vaccin PCV1-2 inactivé et challengé (2-VAC-PCV2), 1 dose de vaccin PCV1-2 vivant atténué non-challengé (1-LIVE-VAC), et 1 dose de vaccin PCV1-2 vivant atténué et challengé (1-LIVE-VAC-PCV2). Le vaccin PCV1-2 inactivé a induit des niveaux plus élevés d’anticorps anti-PCV2 spécifiques chez les truies. Toutes les stratégies de vaccination ont entrainé une bonne protection contre une virémie par PCV2 chez les truies, alors que la majorité des truies non-vaccinées étaient virémiques. Quatre des 35 truies sont devenues gestantes : une truie témoin négatif, une truie témoin positif, une truie 2-VAC-PCV2, et une truie 1-LIVE-VAC-PCV2. L’ADN du PCV2 a été détecté chez, respectivement, 100 %, 67 %, et 29 % des fœtus obtenus des truies témoin positif, vaccin inactivé ou vaccin vivant atténué. L’antigène PCV2 dans le cœur n’était détectable que dans les portées des témoins positifs (23 % des fœtus). L’ADN de PCV1-2 a été détecté chez 29 % des fœtus dans la portée d’une truie 1-LIVE-VAC-PCV2. Dans les conditions de cette étude pilote, les deux vaccins étaient protecteurs contre une virémie par PCV2 chez des animaux en âge de se reproduire; toutefois ils n’empêchaient pas la transmission verticale.
(Traduit par Docteur Serge Messier)
Introduction
Porcine circovirus (PCV) is a member of the Circoviridae family in the genus Circovirus. It is a non-enveloped, single stranded DNA virus with a circular genome. The genome of PCV2 contains 2 major open reading frames (ORF): ORF1 encodes for a protein essential for viral replication, and ORF2 encodes for the capsid protein (1,2). Two main types of PCV have been identified: PCV type 1 (PCV1) and PCV type 2 (PCV2), which share approximately 83% nucleotide sequence identity in ORF1, but only 67% identity in ORF2 (3).
Porcine circovirus type 1 was first identified as a contaminate of a continuous porcine kidney cell line (PK-15) in 1974 (4,5). Despite being widespread in the pig population, PCV1 has been shown to be non-pathogenic in pigs (6,7). However, PCV2 is associated with a group of diseases collectively called PCV-associated disease (PCVAD), including PCV2-associated reproductive failure in mature animals (8).
Porcine circovirus type 2-associated reproductive failure is typically characterized by increased numbers of abortions, mummified and stillborn fetuses, and weakborn piglets (9). Confirmation of PCV2 as the causative agent of reproductive failure is done by identification of myocardial fibrosis, lymphoplasmacytic myocarditis, and association of PCV2 antigen with the fetal heart lesions by immunohistochemistry (IHC) (10). Newborn piglet serum or fetal thoracic fluid may also be positive for PCV2 DNA or PCV2-specific antibodies (10).
It has been shown that a chimeric PCV1-2 strain with the capsid gene of PCV2 in the backbone of the non-pathogenic PCV1 was non-pathogenic under experimental conditions. The inactivated commercial PCV2 vaccine “FosteraTM PCV” was recently reintroduced to the global market and is based on the PCV1-2 chimera. The non-pathogenic chimeric PCV1-2 virus may also have potential for use as a live-attenuated chimeric PCV2 vaccine (11,12).
Porcine circovirus type 2 can be further divided into at least 3 subtypes. The most important subtypes are prevalent worldwide and include PCV2a, which was the predominant strain in the pig population before the year 2000; and PCV2b, which has replaced PCV2a in most herds and is currently the predominant PCV2 genotype in North America (13,14). One of the main differences between PCV2a and PCV2b is in the signature motif in ORF2. Comparative pathogenicity studies among the 2 PCV2 subtypes side by side in experimentally infected pigs have failed to show any differences in virulence (15,16).
Vaccination against PCV2 has been shown to be highly effective in decreasing losses associated with PCVAD. There are currently several types of commercial vaccines available including an inactivated PCV2 vaccine for use in dams or piglets (Circovac; Merial, Lyon, France), 2 subunit vaccines based on PCV2-ORF2 expressed in baculovirus for use in growing pigs (Circumvent PCV; Merck Animal Health Summit, New Jersey, USA and Ingelvac CircoFLEX; Boehringer Ingelheim, Vetmedica, Ingelheim, Germany), and an inactivated chimeric PCV1-2 vaccine for use in growing pigs (Fostera PCV; Zoetis, Florham Park, New Jersey USA, formerly Suvaxyn PCV2 One Dose; Fort Dodge Animal Health, Fort Dodge, Iowa, USA). All these vaccines are based on PCV2a with differences in dosage and recommended timing for use of these products (8).
All commercially available PCV2 vaccines are inactivated or subunit vaccines. Another type of vaccine currently in the experimental stage of development is a live-attenuated PCV2 vaccine based on a chimeric PCV1-2. One concern with any live-attenuated vaccine is the development of vaccine virus viremia in immunized pigs and spread of the vaccine virus among pigs and herds. It has been previously shown that a live-attenuated chimeric PCV1-2 vaccine prevented viremia and decreased macroscopic and microscopic lesions caused by PCV2 infection (12). Interestingly, a chimeric PCV1-2 was recovered from clinically healthy pigs on Canadian farms with no signs of PCVAD (17). Another concern with a live-attenuated chimeric vaccine based on the PCV1 backbone is the disease causing potential of PCV1. It has been previously shown that PCV1 is associated with congenital tremors in newborn fetuses (18), but other authors failed to reproduce these initial findings (19,20). Recently, PCV1 has been associated with hemorrhages in lung tissues of fetuses experimentally inoculated with the PK-15 cell-derived PCV1 isolate but not a field isolate of PCV1 (21).
Porcine circovirus type 2 is shed in oral, nasal, and fecal excretions (22–24), and has been found in boar semen without damaging sperm morphology (25). It has also been shown that insemination of naïve dams with semen containing low levels of PCV2 DNA did not result in virus transmission (viremia, seroconversion) or reproductive failure (26); however, insemination with semen spiked with high levels of PCV2 was capable of inducing reproductive failure in naïve dams (10).
The objective of this pilot study was to determine efficacy of a commercial inactivated (administered as 1 or 2 dose) vaccine and an experimental live-attenuated chimeric PCV1-2 vaccine to prevent viremia in sows using the PCV2-spiked semen model. Four of the 35 sows became pregnant, including 2 vaccinated sows (2 dose inactivated and 1 dose live-attenuated PCV1-2 vaccine), a negative control and a positive control sow, and their litters were utilized to further determine the efficacy of sow vaccination in preventing or reducing vertical transmission.
Materials and methods
Animals and housing
Thirty-five dams, ranging in age from 792 to 2389 d (Table I) and in parity from 1 to 7, were obtained from a herd confirmed to be free of PCV2 by serology and polymerase chain reaction (PCR) testing. The dams were transported over a 3-day interval approximately 250 km to Iowa State University in Ames, Iowa, and housed in groups of 1 to 6 in a total of 17 rooms depending on room size. Each room was equipped with one nipple drinker and sows were fed daily with a pelleted feed ration, which contained whey, but was free of other animal proteins and antibiotics (Nature’s Made; Heartland Co-op, Cambridge, Iowa, USA). Fifteen of the 35 dams were pregnant on arrival (between 15 to 116 d of gestation) and the pregnancies were terminated in all dams less than 52 d into gestation by using 2 mL cloprostenol sodium (Estrumate; Merck Animal Health, Summit, New Jersey, USA).
Table I.
Experimental design
| Group | Animals | Mean age in days (95% CI) | PCV2 vaccine | PCV2 inoculation | |
|---|---|---|---|---|---|
|
| |||||
| 35* | 14* | ||||
| Positive control | 6 | 1174 (1421; 928) | — | — | Spiked semen |
| 2-VAC-PCV2a | 6 | 1247 (1654; 841) | YES | YES | Spiked semen |
| 1-VAC-PCV2a | 6 | 1291 (1836; 746) | YES | — | Spiked semen |
| 1-LIVE-VAC-PCV2b | 6 | 1462 (1991; 935) | YES | — | Spiked semen |
| 1-LIVE-VACb | 6 | 1363 (1719; 1008) | YES | — | — |
| Negative control | 5 | 1048 (1290; 806) | — | — | — |
Dams vaccinated with a commercial vaccine (Suvaxyn PCV).
Dams vaccinated with an experimental live-attenuated chimeric PCV1-2 vaccine.
— days before insemination or PCV2 infection; PCV — porcine circovirus; CI — confidence interval.
Experimental design
The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee. The experimental design is summarized in Table I. After arrival, the dams were blocked by age and randomly assigned to groups and rooms. After an acclimation period of approximately 3 mo, 12 of the 35 dams were vaccinated with one 2 mL dose of an experimental live-attenuated chimeric PCV1-2 vaccine (1-LIVE-VAC, and 1-LIVE-VAC-PCV2), 6 of the 35 dams were vaccinated with one 2 mL dose of an inactivated chimeric PCV1-2 vaccine (1-VAC-PCV2), 6/35 dams were vaccinated with 2 doses of an inactivated chimeric PCV1-2 vaccine, 1 mL, 3 wk apart (2-VAC-PCV2), and 11 of the 35 dams remained unvaccinated as controls. Estrus cycles were synchronized for all dams as previously described (10), followed by artificial insemination 35 d post one dose inactivated chimeric or live vaccination or 14 d post 2 dose inactivated chimeric vaccination. Extended semen obtained from 9 boars all of the same breed and confirmed to be PCV2 negative was used for artificial insemination. Each dam in the negative control group and in the 1-LIVE-VAC group received 80 mL of PCV2-free semen. All dams in the other groups were inseminated with 75 mL of semen spiked with 5 mL of PCV2b immediately before insemination. Inseminations were repeated in 24 h intervals for 3 d. Dams were monitored for signs of estrus, and if any recycled they were re-inseminated using 80 mL PCV2-free semen in 24 h intervals for 3 d. After vaccination, the dams were bled weekly until necropsy at 105 d post inoculation (DPI). All dams tested negative for specific antibodies against porcine reproductive and respiratory syndrome virus and porcine parvovirus prior to initiation of the study and at termination of the study (data not shown). At necropsy all fetuses were removed from the uterus, euthanized, and samples were collected.
Vaccination
Dams in the 1-VAC-PCV2 group were vaccinated with 2 mL of PCV2 vaccine (Suvaxyn PCV2; Fort Dodge Animal Health Inc. Fort Dodge, Iowa, USA; now known as Fostera PCV; Zoetis, Florham Park, New Jersey, USA), dams in the 2-VAC-PCV2 group were vaccinated with 2 doses (Suvaxyn PCV2), 1 mL, 3 wk apart, and dams in the 1-LIVE-VAC and 1-LIVE-VAC-PCV2 groups were vaccinated with one 2 mL dose of an experimental live-attenuated chimeric PCV1-2 vaccine at a dose of 104 median tissue culture infective dose (TCID50), as previously described (27).
Clinical observation
All dams were examined daily for signs of illness such as lethargy, respiratory disease, inappetence, and lameness, as well as signs of return to estrus.
Inoculation
The PCV2b isolate NC-16845 (16) used for the inoculation was propagated in PCV1-free PK-15 cells to an infectious titer of 104.5 TCID50. Five weeks after vaccination of the one dose groups and 2 wk after booster vaccination of the 2 dose group, 24 of the 35 dams (1-VAC-PCV2; 2-VAC-PCV2, 1-LIVE-VAC-PCV2, positive controls) were artificially inseminated with semen spiked with 5 mL PCV2b.
Sample collection
Blood was collected from dams in 8.5 mL serum separator tubes (BD vacutainer; BD Biosciences; San Jose, California, USA) on a weekly basis from the time of vaccination until necropsy at 105 DPI (except on 63 DPI). At necropsy, fetal blood was collected from all live fetuses and fetal thoracic fluid was collected from all dead or mummified fetuses. If no serum or fetal thoracic fluid could be obtained from a fetus, tissue homogenates were collected. The blood was centrifuged at 3220 × g for 10 min at 4°C and the serum was aliquoted into 5 mL polystyrene round bottom tubes (Thermo Fisher Scientific, Hampton, New Hampshire, USA) and stored at −20°C until testing.
Serology
All serum samples were tested for PCV2-specific IgG antibodies using an indirect PCV2 ORF2-based enzyme-linked immunosorbent assay (ELISA), as previously described (28). The results were expressed as sample-to-positive (S/P) ratio. Samples were considered to be negative if the S/P ratio was < 0.2 and positive if the S/P ratio was ≥ 0.2. In addition, all serum samples from dams at DPI 0 were tested for PCV2-specific neutralizing antibodies using a fluorescence focus neutralization (FFN) assay (29). Virus neutralizing titers are expressed as the highest serum dilution resulting in a 90% reduction in virus replication compared to the virus control.
Porcine circovirus type 2 DNA detection
The PCV2 viral DNA was extracted from the serum samples, fetal thoracic fluid samples, or tissue homogenates using a viral isolation kit (MagMax Viral Isolation Kit; Applied Biosystems, Carlsbad, California, USA) on an automated magnetic particle processor (KingFisher Flex System; ThermoFisher Scientific, Pittsburgh, Pennsylvania, USA). The PCV2 DNA detection, to verify and quantify the presence of PCV2 in all samples, was done using a quantitative real-time PCR (TaqMan Fast Universal PCR Master Mix; Applied Biosystems) using the same primers and probe at the same concentrations, as previously described (30). The total PCR reaction volume, including 2.5 μL of DNA extract, was 25 μL. The thermal cycle conditions were 95°C for 2 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. The sensitivity of the PCV2 real-time PCR was 1 × 103 copies/mL (2.5 copies per reaction). Samples were considered negative if no signal was observed during the 40 amplification cycles.
The PCV1-2 PCR
The PCV1-2 DNA detection was done to verify the presence of any PCV1-2 using the same DNA extracts as for the PCV2 PCR. The DNA quantification was done using quantitative real-time PCR (TaqMan Universal PCR Master Mix; Applied Biosystems) with the same primers and probes at the same concentrations, as previously described (27). The total PCR reaction volume was 25 μL. The thermal cycle conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, and 60°C for 1 min. The sensitivity of the PCV1-2a real-time PCR was 8.13 × 104 copies/mL (204 copies per reaction). Samples were considered negative if no signal was observed during the 40 amplification cycles.
Necropsy
All dams were humanely euthanized by intravenous pentobarbital sodium overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, Michigan, USA) and necropsied at 105 DPI. The extent of total macroscopic lung lesions (ranging from 0% to 100%) was scored by a veterinary pathologist in a blinded fashion, as previously described (31). Fetuses were surgically removed from the uterus immediately after euthanasia of the dam. Fetal blood was collected from all live fetuses, and the piglets were then humanely euthanized by intravenous pentobarbital sodium overdose (Vortech Pharmaceuticals) and necropsied. If blood collection was not possible (mummified or dead fetuses), fetal thoracic fluid was collected if available. Fetal heart tissues were collected at necropsy, a section stored fresh at −20°C until further testing and a section fixed in 10% neutral buffered formalin and then routinely processed for histological examination, as previously described (10).
Immunohistochemistry (IHC)
Immunohistochemistry for detection of PCV2-specific antigen was done on fetal hearts using a rabbit polyclonal antiserum (32). Antigen scoring was done by a veterinary pathologist blinded to treatment groups and the scores were reported from 0 (no antigen detected) to 3 (abundant PCV2 antigen), as previously described (33).
Statistical analysis
Statistical analysis of the data was done using computer software (SAS and JMP softwares, version 9.0.0; SAS Institute, Cary, North Carolina, USA). Summary statistics were calculated for all groups to assess the overall quality of the data, including normality. Real-time PCR results and FFN results were log10 transformed prior to statistical analysis. Continuous repeated measured data (PCV2 viremia, ELISA S/P ratios) were assessed using the repeated measures analysis of variance (ANOVA) method. In each repeated measures ANOVA analysis, group, time, and their interaction were fixed effects whereas animal was the subject of repeated measures. Differences in response among groups were assessed using F-tests by time. Non-repeated measurements (FFN results) were assessed using one-way ANOVA. If an ANOVA F-test was significant (P < 0.05), then pairwise t-tests with the Tukey’s adjustment were used to assess specific group differences.
Results
Clinical observation
One 1-LIVE-VAC-PCV2 dam and one negative control dam developed severe lameness and were humanely euthanized at 84 DPI. The overall conception rate of the sows was 11.4% (4/35 sows) after insemination during the first estrus cycle following synchronization and 37.1% (13/35) after 16 sows were rebred during subsequent estrus cycles. Among all pregnant animals (first or second cycle of insemination), none displayed recognizable signs of early abortions.
Reproductive measures
Of the 4 sows that were pregnant after initial insemination using PCV2-spiked semen, the litter compositions were as follows: 1 mummified and 12 live fetuses (positive control dam), 4 mummified and 11 live fetuses (2-VAC-PCV2), 2 mummified and 12 live fetuses (1-LIVE-VAC-PCV2), and 0 mummified and 10 live fetuses (negative control dam). Fetuses that were not full-term at necropsy (pregnant upon second insemination after returning to estrus) were excluded from analysis. The litter distribution of these dams was as follows: 27 fetuses (positive control dams, n = 2), 18 fetuses (2-VAC-PCV2 dams, n = 2), 37 fetuses (1-VAC-PCV2 dams, n = 3), 17 fetuses (1-LIVE-VAC-PCV2 dams, n = 1), and 10 fetuses (1-LIVE-VAC dam, n = 1). Viability data at the time of extraction from the uterus was not obtained.
Seroconversion to PCV2
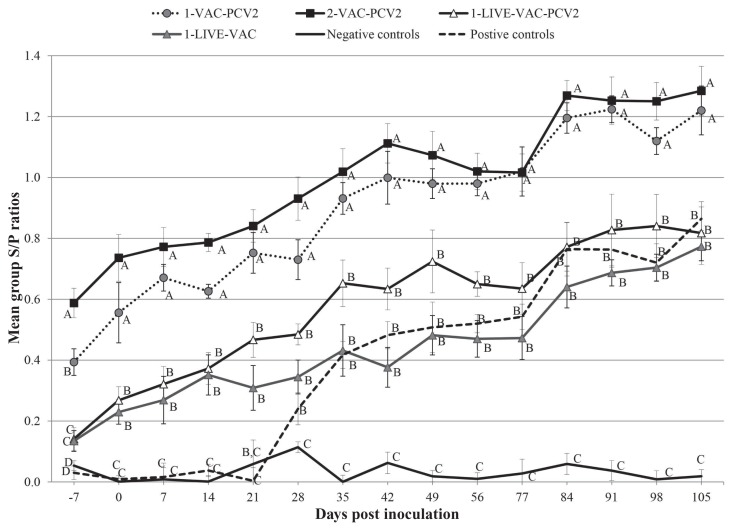
Negative control dams remained negative for PCV2-specific antibodies throughout the study (Figure 1; Table II). All 1-VAC-PCV2 and 2-VAC-PCV2 dams had seroconverted to PCV2 prior to the first blood collection at −7 DPI (Table II). Five of the 6 1-LIVE-VAC and 5 of the 6 1-LIVE-VAC-PCV2 dams had seroconverted by DPI 7, respectively. There was a significant group by time interaction (P < 0.001). There was no significant difference in antibody levels between the 1-VAC-PCV2 and 2-VAC-PCV2 groups at any time points, except at −7 DPI (Figure 1). Also, there was no significant difference in antibody levels at any time points between the 1-LIVE-VAC and 1-LIVE-VAC-PCV2 groups (Figure 1). Dams vaccinated with the inactivated vaccine had significantly higher (P < 0.05) S/P ratios as detected by ELISA, compared to those vaccinated with the live-attenuated vaccine at every DPI throughout the course of the study. Positive controls had similar S/P ratios as the LIVE-VAC and 1-LIVE-VAC-PCV2 groups from 28 DPI through 105 DPI (Figure 1). All 45 live fetuses from all 4 litters were negative for PCV2-specific antibodies at derivation (data not shown).
Figure 1.
Mean group PCV2 ELISA sample-to-positive (S/P) ratios (± standard error) in serum at different days post inoculation. Negative values are included in the group means. An S/P ratio ≥ 0.2 was considered positive. Groups with different letters (A,B,C) on a certain day post inoculation have significantly (P < 0.05) different group S/P ratios.
Table II.
Group prevalence of porcine circovirus type 2 (PCV2)-specific antibodies in serum. Groups that contain seropositive pigs are shaded in grey. Vaccines were administered at −35 DPI and in the case of the 2 dose product a second dose was given at −14 DPI. Dams were challenged at 0 days post inoculation with PCV2 free or PCV2 spiked semen
| DPI | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Group | −7 | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 77 | 84 | 91 | 98 | 105 |
| Positive controls | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 4/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| 2-VAC-PCV2 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| 1-VAC-PCV2 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| 1-LIVE-VAC-PCV2 | 2/6 | 4/6 | 5/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 5/5a | 5/5a | 5/5a |
| 1-LIVE-VAC | 1/6 | 3/6 | 5/6 | 5/6 | 5/6 | 5/6 | 5/6 | 5/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 |
| Negative controls | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/4a | 0/4a | 0/4a |
One negative control dam and a 1-LIVE-VAC-PCV2 dam developed severe lameness and were euthanized at DPI 84.
Neutralizing antibodies
On the day of challenge, the log10 transformed FFN titers were 2.71 ± 0.08 units for 1-VAC-PCV2, 2.96 ± 0.24 units for 2-VAC-PCV2, 1.57 ± 0.17 units for 1-LIVE-VAC, 1.52 ± 0.13 units for 1-LIVE-VAC-PCV2, 0.28 ± 0.17 units for the negative control group, and 0.28 ± 0.18 units for the positive control group. The inactivated chimeric PCV1-2 vaccine induced significantly higher (P < 0.05) levels of neutralizing antibodies compared to the live-attenuated vaccine prior to challenge.
The PCV2 viremia
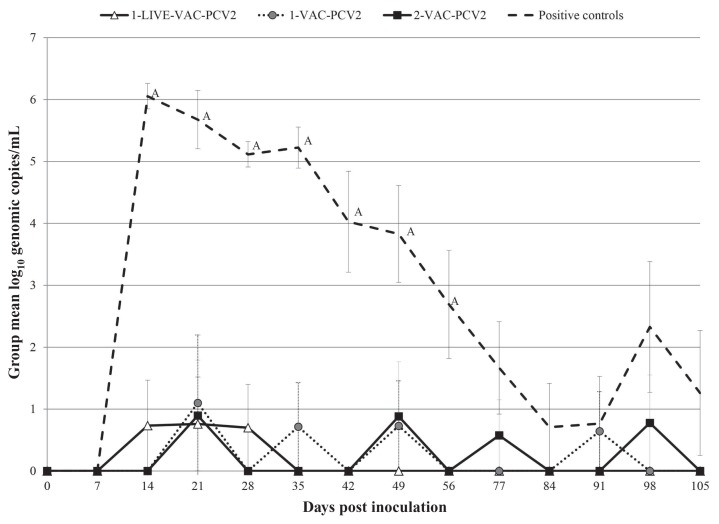
Negative control dams, as well as the 1-LIVE-VAC dams, remained negative for PCV2 viremia throughout the study (Table III). There was a significant group by time interaction (P < 0.001). Specifically, positive control dams developed PCV2 viremia by 14 DPI and had significantly higher (P < 0.05) levels of viremia from 14 DPI through 56 DPI compared with vaccinated groups (Figure 2). Challenged, vaccinated dams had detectable PCV2 DNA at varying DPI during the study (Table III) without significant differences among the vaccinated groups at any time point (Figure 2). Of the 4 dams that became pregnant after initial insemination, the positive control dam was viremic from 14 through 49 DPI, the 2-VAC-PCV2 sow was not viremic, the 1-LIVE-VAC-PCV2 sow was viremic at 105 DPI, and PCV2 viremia was not detected in the negative control dam. The prevalence and concentration of PCV2 DNA detected in the fetuses obtained after initial insemination are summarized in Table IV. Due to the length of time after inoculation of the dam with PCV2, PCV2 DNA detection was not attempted on the fetuses resulting from the second insemination.
Table III.
Group prevalence of porcine circovirus type 2 (PCV2) DNA in serum. Groups that contain viremic pigs are shaded in grey. Vaccines were administered at −35 DPI and in the case of the 2 dose product a second dose was given at −14 DPI. Dams were challenged at 0 DPI with PCV2 free or PCV2 spiked semen
| DPI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Group | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 77 | 84 | 91 | 98 | 105 |
| Positive controls | 0/6 | 0/6 | 6/6 | 6/6 | 6/6 | 6/6 | 5/6 | 5/6 | 4/6 | 3/6 | 1/6 | 1/6 | 3/6 | 3/6 |
| 2-VAC-PCV2 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 1/6 | 0/6 | 0/6 | 1/6 | 0/6 |
| 1-VAC-PCV2 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 1/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 |
| 1-LIVE-VAC-PCV2 | 0/6 | 0/6 | 1/6 | 1/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5a | 0/5a | 0/5a |
| 1-LIVE-VAC | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| Negative controls | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/4a | 0/4a | 0/4a |
One negative control dam and a 1-LIVE-VAC-PCV2 dam developed severe lameness and were euthanized at 84 DPI.
Figure 2.
Mean group amount of log10 PCV2 DNA (± standard error) in serum at different days post inoculation. Negative values are included in the group means. A sample with no threshold cycle (CT) value during the 40 amplification cycles was considered negative. An “A” next to the positive control group on a certain day post inoculation indicates significantly (P < 0.05) different group log10 PCV2 DNA compared to the PCV2 challenged, vaccinated groups (1-LIVE-VAC-PCV2, 1-VAC-PCV2, 2-VAC-PCV2).
Table IV.
Mean group amount of log10 porcine circovirus type 2 (PCV2) DNA (± standard error) and prevalence in serum or fetal thoracic fluid of piglets born to dams vaccinated or unvaccinated and infected with PCV2. A sample with no threshold cycle (CT) value during the 40 amplification cycles was considered negative
| Treatment | PCV2 DNA | Prevalence |
|---|---|---|
| Positive control | 6.72 ± 0.57 | 13/13 |
| 2-VAC-PCV2 | 3.09 ± 0.67 | 10/15 |
| 1-LIVE-VAC-PCV2 | 1.65 ± 0.74 | 4/14 |
| Negative control | 0.00 ± 0.00 | 0/10 |
The PCV1-2 viremia
All dams remained negative for PCV1-2 DNA throughout the course of the study. Three of 14 fetuses from the 1-LIVE-VAC-PCV2 litter were positive for PCV1-2 DNA with an average log10 transformed mean amount PCV1-2 DNA of 6.45 ± 0.31 per mL. All other piglets were negative for PCV1-2 DNA (data not shown).
Macroscopic lesions
Lungs, lymph nodes, and the reproductive tract of the dams were examined for gross lesions and all were macroscopically normal.
Immunohistochemistry
All 10 piglets from the negative control dam and all 29 piglets from the 2-VAC-PCV2 and 1-LIVE-VAC-PCV2 dams were negative for PCV2 antigen in heart tissues. Low-to-abundant amounts of PCV2 antigen were detected in the hearts of 3 of 13 fetuses from the positive control dam.
Discussion
Evidence from the field supports an association of PCV2 with reproductive failure. Although limited experimental trials have been conducted in breeding age animals, it has been previously demonstrated that dams develop PCV2 viremia when inseminated with PCV2-positive semen (10). In this pilot study, sows were inseminated with PCV2-spiked semen and viremia was detected in the positive control group further confirming that PCV2 is infectious when administered via artificial insemination. The limited information available in the literature on the efficacy of inactivated PCV2 vaccines in breeding age animals indicates that PCV2-vaccinated dams may deliver PCV2 positive piglets (34), potentially an important source of PCV2 transmission in pig production systems.
Currently there are 3 commercial vaccines on the market approved for use in healthy pigs (3 wk of age or older), and one vaccine is approved for use in both dams and growing pigs. All of these commercial vaccines are inactivated. Experimental and field trial evidence clearly demonstrates that use of these vaccines reduces losses associated with PCVAD in growing pigs (35,36). In this study, an experimental attenuated live PCV1-2 vaccine was utilized for the first time in breeding age animals, and compared to 2 different dose regimens of the inactivated version of the vaccine. The results indicate that the use of the 3 different vaccination protocols all decreased PCV2 viremia and increased anti-PCV2-specific antibody production in dams when compared to the positive control group.
The exact mechanism for the decrease of PCV2 viremia in vaccinated animals is unknown, but likely due to development of a combination of cellular and humoral immune responses. Interestingly, the groups vaccinated with the inactivated chimeric PCV1-2 vaccine (1-VAC-PCV2 and 2-VAC-PCV2) developed detectable PCV2-specific antibody levels earlier and maintained higher levels throughout the study compared to those vaccinated with the live-attenuated chimeric vaccine (1-LIVE-VAC and 1-LIVE-VAC-PCV2). In contrast to these results in breeding age animals, a previous study comparing several commercial and experimental vaccines in young pigs found no significant differences between any of the commercial vaccines (27).
In Canada, PCV1-2 was recently isolated from 3 different pigs in 3 different herds vaccinated with a commercial PCV2 vaccine based on an inactivated chimeric PCV1-2 (17). Several studies have shown that the PCV1-2 infectious DNA clones and the live-attenuated PCV1-2 vaccine are capable of inducing an antibody response, but are attenuated in pigs and not capable of producing any characteristic lesions of PCV2 infection (11,12,37). In this study, the 1-LIVE-VAC dams showed no evidence of PCV2 or PCV1-2 viremia; however, they did develop a PCV2-specific antibody response. The 1-LIVE-VAC-PCV2 dams developed PCV2 viremia at selected time points at low levels, which quickly resolved. As the sensitivity of the PCV1-2 PCR assay was low, it is possible that PCV1-2 DNA was present but at too low a level to be detected. Overall, the results are similar to previous studies in which some PCV1-2 vaccinated animals developed viremia, while others did not; however, as in this study, the majority of the animals seroconverted and had no or minimal lesions characteristic of PCV2 infection (12,37). These results further confirm that the live PCV1-2 chimeric vaccine is capable of inducing a humoral immune response and remains attenuated.
In this study, only 11.4% (4/35) of the sows became pregnant during the first artificial insemination and only 37.1% (13/35) sows became pregnant overall. Low reproductive rates are not uncommon in older parity sows. In addition, PCV2 has been implicated in early embryonic death (38) and re-absorption leading to irregular or regular return to estrus. Several sows arrived at the research facility with varying stages of pregnancy, which were purposely terminated, potentially causing complications in their ability to conceive during the subsequent artificial insemination attempts. However, previously, using the same insemination technique and PCV2 dose but a different sow source, conception rates of 88.9% (8/9) (39) and 66.7% (6/9) (10) were observed. Among all pregnant animals (first or second cycle of insemination) none displayed signs of early abortions under the study conditions.
Among the animals that became pregnant during the first cycle, PCV2-positive piglets were detected in all 3 challenged sows regardless of vaccination status, further confirming that dam vaccination does not prevent vertical PCV2 transmission. The exact mechanism of how and when fetuses became infected with PCV2 remains unknown. Possibilities include (I) directly via the semen at the time of fertilization, (II) localized intrauterine infection via the semen and spread to fetuses at any time during gestation, or (III) re-circulating PCV2 from the blood of the dam and cross-placental infection at any time during gestation. In this study, PCV2 viremia was detected in the positive control dam from 14 through 49 DPI, but not in any of the other 2 challenged dams. All dams were tested for presence of PCV2 viremia once a week. Therefore, a short viremia (less than 7 d) could have remained undetected under the study conditions. The fact that vaccinated dams can still deliver PCV2-positive piglets causes some to question the value of vaccination in breeding age females; however, while the vaccines did not entirely prevent PCV2 spread to fetuses in the current study, vaccination did decrease the overall PCV2 load in the dam and fetuses.
The vaccination protocols in the pregnant sows (1-LIVE-VAC-PCV2 and 2-VAC-PCV2) resulted in a decrease in PCV2 DNA detection in piglets (28.6% and 66.7%, respectively) when compared to the positive control group (100%), indicating both types of the PCV1-2 chimeric vaccine are efficacious in breeding age animals. However, due to the low numbers of pregnancies in this study, these results should be interpreted with caution. In fetuses, PCV2 antigen detection was done on fetal heart tissues since PCV2 antigen in fetuses was previously shown to be found predominantly in the heart and tonsil of piglets (34,39). The PCV2 antigen was only detected in the positive control piglets in the current study. This is further confirmation that both vaccines are efficacious in decreasing levels of PCV2.
To further evaluate the safety of the live-attenuated PCV1-2 vaccine, all 45 live piglets and 7 mummified fetuses from pregnant sows at first insemination cycle (n = 4) were tested for presence of PCV1-2 DNA. Three of 14 piglets from the 1-LIVE-VAC-PCV2 dam were positive for PCV1-2 DNA at birth; however, no lesions were noted and no PCV2 antigen was detected in the fetal myocardium. Only fetal heart tissues were investigated in the current study; however, the tissue tropism of PCV1-2 is unknown. Porcine circovirus type 1 has been previously identified in fetal lung tissues (21). Therefore, PCV1-2, although unlikely, could have been present elsewhere in the fetal tissues. As a PCV1-2 chimeric virus was identified in Canadian swine herds, the risks of introduction of a live-attenuated PCV2 vaccine for use in breeding age females needs to be seriously considered before approval.
To our knowledge, this is the first study investigating the use of a live PCV2 vaccine in breeding animals. While the pilot data obtained indicate that the inactivated chimeric PCV1-2 vaccine was capable of inducing higher levels of PCV2-specific antibodies throughout the course of this study when compared to the live-attenuated experimental chimeric vaccine, no significant differences among vaccinated groups in levels and duration of PCV2 viremia were found. All vaccines were capable of reducing viremia in dams. The piglets obtained from the 2 vaccinated dams had lower levels of PCV2 in comparison to those obtained from the non-vaccinated dam. Live-vaccine virus viremia was detected in selected dams and piglets at low levels of viral load, which was not associated with myocardial lesions or antigen.
Acknowledgments
Funding for this study was provided by the National Pork Board Checkoff Dollars. The authors thank Dr. J. C. Gomes-Neto for assistance with the animal work.
References
- 1.Cheung AK. The essential and nonessential transcription units for viral protein synthesis and DNA replication of porcine circovirus type 2. Virology. 2003;313:452–459. doi: 10.1016/s0042-6822(03)00373-8. [DOI] [PubMed] [Google Scholar]
- 2.Lekcharoensuk P, Morozov I, Paul PS, Thangthumniyom N, Wajjawalku W, Meng XJ. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J Virol. 2004;78:8135–8145. doi: 10.1128/JVI.78.15.8135-8145.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morozov I, Sirinarumitr T, Sorden SD, et al. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J Clin Micro. 1998;36:2535–2541. doi: 10.1128/jcm.36.9.2535-2541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tischer I, Rasch R, Tochtermann G. Characterization of papovavirus- and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol. 1974;226:153–167. [PubMed] [Google Scholar]
- 5.Tischer I, Gelderblom H, Vettermann W, Koch MA. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- 6.Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- 7.Allan GM, Ellis JA. Porcine circoviruses: A review. J Vet Diagn Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med. 2009;23:1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor B, Gauvreau H, West K, et al. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can Vet J. 2001;42:551–553. [PMC free article] [PubMed] [Google Scholar]
- 10.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng XJ, Opriessnig T. Reproductive failure experimentally induced in sows via artificial insemination with semen spiked with porcine circovirus type 2. Vet Pathol. 2009;46:707–716. doi: 10.1354/vp.08-VP-0234-O-FL. [DOI] [PubMed] [Google Scholar]
- 11.Fenaux M, Opriessnig T, Halbur PG, Meng XJ. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J Virol. 2003;77:11232–11243. doi: 10.1128/JVI.77.20.11232-11243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–6303. doi: 10.1128/JVI.78.12.6297-6303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenaux M, Halbur PG, Gill M, Toth TE, Meng XJ. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J Clin Micro. 2000;38:2494–2503. doi: 10.1128/jcm.38.7.2494-2503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon CA, del C, Jr, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. 2008;20:545–558. doi: 10.1177/104063870802000503. [DOI] [PubMed] [Google Scholar]
- 15.Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008;26:1063–1071. doi: 10.1016/j.vaccine.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Opriessnig T, Ramamoorthy S, Madson DM, et al. Differences in virulence among porcine circovirus type 2 isolates are unrelated to cluster type 2a or 2b and prior infection provides heterologous protection. J Gen Virol. 2008;89:2482–2491. doi: 10.1099/vir.0.2008/001081-0. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon CA, Music N, Fontaine G, Tremblay D, Harel J. Emergence of a new type of porcine circovirus in swine (PCV): A type 1 and type 2 PCV recombinant. Vet Microbiol. 2010;144:18–23. doi: 10.1016/j.vetmic.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 18.Hines RK, Lukert PD. Porcine circovirus as a cause of congenital tremors in newborn pigs. Proc Am Assoc Swine Pract. 1994:344–345. [Google Scholar]
- 19.Kennedy S, Segalés J, Rovira A, et al. Absence of evidence of porcine circovirus infection in piglets with congenital tremors. J Vet Diagn Invest. 2003;15:151–156. doi: 10.1177/104063870301500209. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson GW, Kiupel M, Mittal SK, Choi J, Latimer KS, Kanitz CL. Tissue distribution and genetic typing of porcine circoviruses in pigs with naturally occurring congenital tremors. J Vet Diagn Invest. 2001;13:57–62. doi: 10.1177/104063870101300111. [DOI] [PubMed] [Google Scholar]
- 21.Saha D, Lefebvre DJ, Ducatelle R, Doorsselaere JV, Nauwynck HJ. Outcome of experimental porcine circovirus type 1 infections in mid-gestational porcine foetuses. BMC Vet Res. 2011;7:64. doi: 10.1186/1746-6148-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segalés J, Calsamiglia M, Olvera A, Sibila M, Badiella L, Domingo M. Quantification of porcine circovirus type 2 (PCV2) DNA in serum and tonsillar, nasal, tracheo-bronchial, urinary and faecal swabs of pigs with and without postweaning multisystemic wasting syndrome (PMWS) Vet Microbiol. 2005;111:223–229. doi: 10.1016/j.vetmic.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Caprioli A, McNeilly F, McNair I, et al. PCR detection of porcine circovirus type 2 (PCV2) DNA in blood, tonsillar and faecal swabs from experimentally infected pigs. Res Vet Sci. 2006;81:287–292. doi: 10.1016/j.rvsc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Patterson AR, Ramamoorthy S, Madson DM, Meng XJ, Halbur PG, Opriessnig T. Shedding and infection dynamics of porcine circovirus type 2 (PCV2) after experimental infection. Vet Microbiol. 2011;149:91–98. doi: 10.1016/j.vetmic.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Madson DM, Ramamoorthy S, Kuster C, et al. Characterization of shedding patterns of porcine circovirus types 2a and 2b in experimentally inoculated mature boars. J Vet Diagn Invest. 2008;20:725–734. doi: 10.1177/104063870802000603. [DOI] [PubMed] [Google Scholar]
- 26.Madson DM, Ramamoorthy S, Kuster C, et al. Infectivity of porcine circovirus type 2 DNA in semen from experimentally-infected boars. Vet Res. 2009;40:10. doi: 10.1051/vetres:2008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen HG, Beach N, Huang YW, Halbur PG, Meng XJ, Opriessnig T. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV) Vaccine. 2010;28:5960–5966. doi: 10.1016/j.vaccine.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Nawagitgul P, Harms PA, Morozov I, et al. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin Diagn Lab Immunol. 2002;9:33–40. doi: 10.1128/CDLI.9.1.33-40.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogranichniy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. 2000;13:143–153. doi: 10.1089/vim.2000.13.143. [DOI] [PubMed] [Google Scholar]
- 30.Opriessnig T, Yu S, Gallup JM, Evans RB, et al. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529. doi: 10.1354/vp.40-5-521. [DOI] [PubMed] [Google Scholar]
- 31.Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 32.Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 1999;11:528–530. doi: 10.1177/104063879901100607. [DOI] [PubMed] [Google Scholar]
- 33.Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640. doi: 10.1354/vp.41-6-624. [DOI] [PubMed] [Google Scholar]
- 34.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng X, Opriessnig T. Effect of porcine circovirus type 2 (PCV2) vaccination of the dam on PCV2 replication in utero. Clin Vaccine Immunol. 2009;16:830–834. doi: 10.1128/CVI.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pejsak Z, Podgorska K, Truszczynski M, Karbowiak P, Stadejek T. Efficacy of different protocols of vaccination against porcine circovirus type 2 (PCV2) in a farm affected by postweaning multisystemic wasting syndrome (PMWS) Comp Immunol Microbiol Infect Dis. 2010;33:e1–e5. doi: 10.1016/j.cimid.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Kixmöller M, Ritzmann M, Eddicks M, Saalmüller A, Elbers K, Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451. doi: 10.1016/j.vaccine.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie J, Juhan NM, DiCristina J, Key KF, Ramamoorthy S, Meng XJ. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) is genetically stable in vitro and in vivo. Vaccine. 2008;26:4231–4236. doi: 10.1016/j.vaccine.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Mateusen B, Maes DG, Van SA, Lefebvre D, Nauwynck HJ. Effect of a porcine circovirus type 2 infection on embryos during early pregnancy. Theriogenology. 2007;68:896–901. doi: 10.1016/j.theriogenology.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng XJ, Opriessnig T. Effect of natural or vaccine-induced porcine circovirus type 2 (PCV2) immunity on fetal infection after artificial insemination with PCV2 spiked semen. Theriogenology. 2009;72:747–754. doi: 10.1016/j.theriogenology.2009.04.024. [DOI] [PubMed] [Google Scholar]