Abstract
We cloned and analyzed Legionella pneumophila Corby homologs of rpoN (encoding σ54) and fleQ (encoding σ54 activator protein). Two other genes (fleR and pilR) whose products have a σ54 interaction domain were identified in the genome sequence of L. pneumophila. An rpoN mutant strain was nonflagellated and expressed very small amounts of the FlaA (flagellin) protein. Like the rpoN mutant, the fleQ mutant strain of L. pneumophila was also nonflagellated and expressed only small amounts of FlaA protein compared to the amounts expressed by the wild type. In this paper we show that the σ54 factor and the FleQ protein are involved in regulation of flagellar gene operons in L. pneumophila. RpoN and FleQ positively regulate the transcription of FliM and FleN, both of which have a σ54-dependent promoter consensus sequence. However, they seemed to be dispensable for transcription of flaA, fliA, or icmR. Our results confirmed a recently described model of the flagellar gene regulation cascade in L. pneumophila (K. Heuner and M. Steinert, Int. J. Med. Microbiol. 293:133-145, 2003). Flagellar gene regulation was found to be different from that of Enterobacteriaceae but seems to be comparable to that described for Pseudomonas or Vibrio spp.
Legionella pneumophila, the causative agent of Legionnaires' disease, is a ubiquitous microorganism inhabiting man-made water systems and freshwater biotopes. Legionella infection occurs after inhalation of aerosolized bacteria. The organism invades and proliferates in alveolar macrophages of the human lung. In the environment, Legionella replicates intracellularly in amoebae and other protozoans (13).
Previous studies have demonstrated that the flagellum positively affects the establishment of infection but is not required for intracellular replication (9, 28). On the other hand, it has been shown that the flagellar system is needed for full fitness of L. pneumophila (20). Furthermore, it is known that the complex flagellar regulon is coordinately regulated with the expression of other virulence-associated factors (4, 6, 20, 32). Studies in our laboratory have demonstrated that the flaA gene (encoding the major subunit flagellin) is positively regulated by the alternative σ28 factor (FliA) and seems to be negatively regulated by the transcriptional regulator FlaR (16, 18, 20). Furthermore, flaA expression is modulated by various environmental factors (17); for a review see reference 21).
Genome analysis revealed the presence of putative σ54 promoter sites upstream of most of the flagellar operons, and we hypothesized that RpoN and FleQ may regulate these operons (21). For Pseudomonas aeruginosa it has been shown that σ54 and a factor containing a σ54 interaction domain are at the top of the cascade of flagellar gene regulation (2, 7, 8, 25). In order to obtain support for our hypothesis and to further characterize the cascade of flagellar gene expression, we screened the genome sequence of L. pneumophila for a homolog of rpoN and for factors having a σ54 interaction domain. In this paper we describe identification of the σ54 factor and the transcriptional regulator FleQ and the role of this factor and this regulator in flagellar gene regulation in L. pneumophila.
MATERIALS AND METHODS
L. pneumophila Corby serogroup 1 (S1) (22) was used to clone the rpoN and fleQ genes. The legionellae used in a Southern blot analysis were L. pneumophila Philadelphia I (= ATCC 33152) (S1), L. pneumophila Msp19 (S1) (3), L. pneumophila 685 (S1) (3), L. pneumophila U22 (S3) (3), L. pneumophila U21 (S6) (3), L. pneumophila 664 (S6) (3), L. pneumophila type strains (S7, S10, S12, and S13) (P. C. Lück, Dresden, Germany), L. pneumophila Bloomington (= ATCC 33155) (S3), L. pneumophila Los Angeles (= ATCC 33156) (S4), L. pneumophila Chicago-2 (= ATCC 33215) (S6), Legionella anisa (12), Legionella bozemanii ATCC 33217, Legionella dumoffii ATCC 33279, Legionella feelei ATCC 35072 (S1), Legionella gormanii ATCC 33297, Legionella hackeliae ATCC 33250 and ATCC 35999 (S1 and S2), Legionella israeliensis ATCC 43119, Legionella jordanis ATCC 33623, Legionella longbeachae ATCC 33462 and ATCC 33484 (S1 and S2), Legionella micdadei ATCC 33218, Legionella oakridgensis ATCC 33761, Legionella erythra (34), and Sarcobium (Legionella) lyticum PCM 2298 (Polish Culture of Microorganisms). Escherichia coli DH5α was used for cloning of recombinant plasmid DNA. Plasmid pUC18 or pUC19 (Pharmacia LKB, Freiburg, Germany) was used for subcloning of DNA fragments, and the vector pBC KS (Stratagene) was used to construct plasmids for complementation of L. pneumophila mutants.
Media and chemicals.
E. coli was cultivated in Luria-Bertani medium. The antibiotics used in E. coli cultures were chloramphenicol (20 mg ml−1) and ampicillin (100 mg ml−1). L. pneumophila was grown in YEB medium, which contained 1% yeast extract and was supplemented with 1% N-(2-acetamido)-2-aminoethanesulfonic acid, 0.025% ferric PPi, and 0.04% l-cysteine, or on buffered charcoal yeast extract (BCYE) agar (10). Enzymes were purchased from MBI Fermentas (Vilnius, Lithuania), Amersham, Boehringer GmbH (Mannheim, Germany), and Invitrogen GmbH (Karlsruhe, Germany). AmpliTaq polymerase was purchased from Invitrogen GmbH. Chemicals and oligonucleotides were obtained from MWG-Biotech (Ebersberg, Germany).
DNA techniques and nucleotide sequencing analysis.
Preparation of chromosomal or plasmid DNA, DNA manipulation, and Southern hybridization were performed by using standard protocols (33). PCR was carried out by using a TRIO-Thermoblock thermocycler (Biometra, Göttingen, Germany) and AmpliTaq polymerase (Invitrogen GmbH). Introduction of foreign DNA into bacterial strains by electroporation was performed by using a Bio-Rad gene pulser (Bio-Rad, Munich, Germany) according to the manufacturer's specifications. Electroporation of E. coli strains was carried out by using 1.8 kV, 200 Ω, and 25 mF, and electroporation of Legionella strains was carried out by using 2.3 kV, 100 Ω, and 25 mF.
Both strands of plasmid DNA were sequenced with infrared-dye-labeled primers by using an automated DNA sequencer (LI-COR-DNA 4000; MWG-Biotech). Sequences were analyzed by using the Genetics Computer Group package, Pendant (htp://pendant.gfs.de), and SMART (http://smart.embl-heidelberg.de) programs, as well as data available on the website of the Legionella genome project (http://genome3.cpmc.columbia.edu/∼legion).
Generation of σ54 (rpoN) and fleQ mutant strains of L. pneumophila Corby.
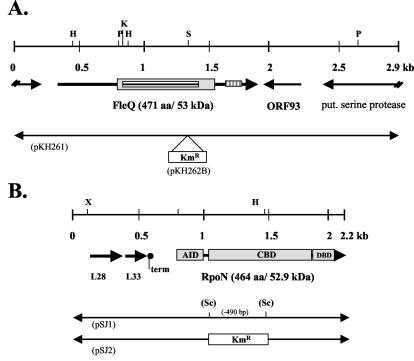
Mutant strains were generated as described recently (9). In brief, the gene of interest was inactivated by introduction of a kanamycin resistance cassette into the chromosomal gene by using an SnaBI restriction site (fleQ) or a PCR-introduced SacII restriction site (rpoN) (Fig. 1). Mutants were generated by using the natural competence of L. pneumophila (35). Correct insertion of the resistance gene cassette into the chromosome was verified by PCR and Southern blot analysis (data not shown). For complementation studies, the complete gene was cloned in the vector pBCKS (fleQ, pKH262C; rpoN, pKH268) and introduced into the mutant strain by electroporation.
FIG. 1.
Genetic maps of the FleQ-encoding region (A) and the RpoN-encoding region (B) of L. pneumophila Corby. (A) The gene was inactivated by inserting a kanamycin resistance cassette into the SnaBI site of pKH261 and was subcloned into vector pBCKS, resulting in plasmid pKH262B. For a description of the method used for integration of the fleQ::Kmr resistance cassette into the chromosome, see Materials and Methods. Functional domains encoded by fleQ are indicated. The deduced protein contains a σ54 activation domain (grey box), a putative ATP binding site (AAA) (box with horizontal lines), and a C-terminal HTH_8 motif (box with vertical lines). (B) The rpoN gene was inactivated by inserting a kanamycin resistance cassette into the SacII site of pSJ1, resulting in pSJ2. For a description of the methods used for integration of the rpoN::Kmr resistance cassette into the chromosome, see Materials and Methods Typical σ54 functional domains that are encoded by rpoN are indicated and included the AID, CBD, and DBD. Genes are indicated by arrows. L28, region encoding 50S ribosomal protein L28 homolog (78 amino acids); L33, region encoding 50S ribosomal protein L33 homolog (54 amino acids); term, putative rho-independent termination site; aa, amino acids; put., putative. Restriction endonuclease sites: H, HindIII; K, KpnI; P, PstI; S, SnaBI; Sc, SacII.
Southern hybridization.
Chromosomal DNA from various Legionella strains were digested with SacII and HindIII and electrophoresed, and fragments were transferred to nylon membranes (Pall, Dreieich, Germany) by capillary blotting. DNA probes containing the complete L. pneumophila fleQ or rpoN gene were used as fleQ- or rpoN-specific probes. DNA probes were labeled and detected by using a nonradioactive enhanced chemiluminescence detection kit (ECL; Amersham). Hybridization was performed under low-stringency conditions as described previously (15).
RT-PCR analysis and primer extension.
Total RNA was extracted by using a High Pure RNA isolation kit (Roche, Mannheim, Germany) as described by the manufacturer. Additionally, purified RNA was incubated with 300 U of DNase I (Roche) per ml at 37°C for 60 min and then repurified by using an RNeasy Mini kit (Qiagen, Hilden, Germany). Reverse transcription (RT)-PCRs were performed with a OneStep RT-PCR kit (Qiagen) used according to the instructions of the manufacturer with gene-specific primers (Table 1). The RT reaction was performed at 50°C for 30 min with 100 ng of total RNA. PCR amplification was performed in the same tube after an initial activation step at 95°C for 15 min with each primer at a concentration of 0.6 μM, each deoxynucleoside triphosphate at a concentration of 400 μM, 5× OneStep RT-PCR buffer containing 12.5 mM MgCl2, and 2 μl of OneStep RT-PCR enzyme mixture in a 50-μl (total volume) reaction mixture. Initial denaturation was performed at 95°C for 15 min (activation step), and final extension was performed at 72°C for 10 min. The cycling conditions were 94°C for 1 min, 50 to 52°C for 1 min (Table 1), and 72°C for 1 min for 30 cycles with a Biometra T3 thermocycler (Biometra). The purified RNA was analyzed for genomic DNA contamination by performing PCR with primers specific for the flaA gene as described above, except for the RT step (30 min at 50°C). In control experiments, the amounts of RT-PCR products were analyzed each third round of amplification by electrophoresis, starting with cycle 18 of 36 cycles, and the results showed that the yield of amplification products depended on the quantity of RNA present in the sample.
TABLE 1.
Primers used for RT-PCR
| Gene | Forward primer | Reverse primer | Temp for PCR (°C)a | Length (bp)b |
|---|---|---|---|---|
| flaA | CATGATGCAAACATCGATCGA | CTGCTACTTCTGTTCCTGTTG | 52 | 480 |
| fliA | AACGCATTGCACATCATCTGC | ATAAGACATCATCGGTTACTC | 50 | 400 |
| fleN | TAGCCATAGCGTTATCTCAAC | GTAATCTAACTGCACATCCAG | 52 | 540 |
| fleQ | GTGACAAGCTTCGGACTATTT | GAAAGAGAATGTATATTGCGA | 50 | 490 |
| rpoN | TGGTCAACATCTCACGTTAAC | CCTAGCAACTCAATGTCTTCA | 51 | 600 |
| fliM | GAGATCGATGCATTACTGGAT | TAATAATCGACCAAGTCATACACA | 51 | 450 |
| icmR | ATACTGATGACAGTGCACGAA | GATGATAATTTGAAACCACGTTC | 50 | 350 |
| flesR | AAGTATATCGATATGGAGTTT | AACTATTAAAACATGACTCAT | 45 | 480 |
Temperature used for the amplification step in the RT-PCR.
Length of amplified template.
Primer extension analysis was carried out with IRD-labeled primer fleQPE (5′-AAATAGTCCGAAGCTTGTCAC-3′) by using an automated DNA sequencer (LI-COR-DNA 4000; MWG-Biotech). The primer (4 pmol) was annealed to 20 μg of total RNA, and the RT reaction was performed as described recently (19). The sequencing reaction was performed by using the primer that was used for the primer extension analysis.
SDS-PAGE and immunoblotting.
Total cell extracts of L. pneumophila strains were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. SDS-PAGE was performed as described by Laemmli (27). Legionella was grown on BCYE agar plates for 24 to 72 h at 30°C unless indicated otherwise, harvested, and suspended in distilled water, and the optical density at 600 nm was adjusted to 1. Three hundred microliters was centrifuged, and the cells were then suspended in 50 μl of Laemmli buffer and loaded onto an SDS-13% polyacrylamide gel. Western blotting was carried out by using polyclonal antibodies specific for L. pneumophila Corby flagellin and P. aeruginosa FleQ protein. The anti-FlaA antiserum was generated as described recently (19) by using purified flagella of L. pneumophila Corby.
Intracellular replication assays.
U937 cells were cultured in RPMI 1640 (PAA) containing 2 mM l-glutamine and 10% fetal calf serum at 37°C with 5% CO2. U937 cells (106 cells per well) were differentiated in 24-well plates with 10 ng of phorbol 12-myristate 13-acetate (Sigma) per ml for 48 h before use. Adherent cells were washed with RPMI 1640 prior to infection.
The ability of L. pneumophila strains to grow in macrophage-like U937 cells was determined in coculture assays. Bacterial strains were cultivated on BCYE agar plates for 3 days at 37°C. Differentiated U937 cells were infected with a bacterial suspension in RPMI 1640 (multiplicity of infection, 0.01), and the plates were centrifuged at 800 × g for 3 min and incubated at 37°C up to 4 days. The initial time was defined as 2 h postinfection. Due to the low multiplicity of infection, no washing or gentamicin treatment was performed. Macrophages were lysed daily with cold H2O and combined with the culture supernatant. Serial dilutions were spread on BCYE agar plates to determine the number of CFU. All assays were performed independently in triplicate.
Electron microscopy.
Bacteria were grown for 4 days on BCYE agar plates at 30°C. Then bacteria were suspended in distilled water, and 1 drop of the suspension was applied to Pioloform (Merck)-coated copper grids. After sedimentation of the bacteria and removal of the remaining fluid, the samples were each stained with 1 drop of 1% phosphotungstic acid (Sigma) (pH 6.5) or shadowed with platinum-palladium and examined with a transmission electron microscope (EM10; Zeiss) at 60 kV.
Nucleotide sequence accession number.
The sequences reported here have been deposited in the GenBank database under accession numbers AJ566390 (fleQ) and AJ580316 (rpoN).
RESULTS
Cloning of the rpoN and fleQ genes of L. pneumophila Corby.
Recently, we identified putative σ54 promoter elements upstream of most of the major flagellar operons (21). Therefore, we searched for the presence of an RpoN homolog and for the presence of proteins with a σ54 interaction domain in the deduced protein sequences of the genome of L. pneumophila (htp://genome3.cpmc.columbia.edu/∼legion). A homolog of RpoN and homologs of FleQ, FleR, and PilR proteins were identified. Primers specific for the putative rpoN (Rpon-F [5′-ATCTTACGTTGCATCACAATAACT-3′] and RpoN-R [5′-CAGTGAATGCTCTTAGTGCAGGAG-3′]) and fleQ (FleQ-F [5′-CCGTTATAATGATTACCGAGTGGA-3′ ] and FleQ-R [5′-TCCCAGTTACAGCGAATCCGTGAT-3′]) homologs were generated, and the corresponding genes of L. pneumophila Corby were amplified, cloned, and analyzed further. The genetic maps of the cloned fleQ and rpoN regions are shown in Fig. 1.
Nucleotide and protein sequence analysis of fleQ and FleQ.
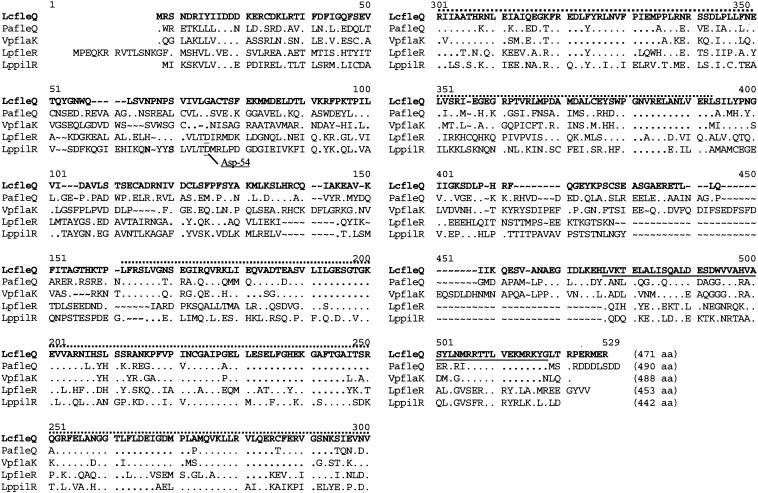
The putative fleQ gene encompasses 1,413 bp and encodes a protein with a calculated molecular mass of 53 kDa (Fig. 2). A P. aeruginosa-specific anti-FleQ antibody cross-reacted in Western blot analysis with the FleQ protein of L. pneumophila, confirming the presence of a FleQ-like protein and that the molecular mass was approximately 53 kDa (Fig. 3A, lanes 1 and 3). Computer analysis revealed the presence of a Pfam-σ54 interaction domain, an AAA domain (ATP binding site), and a C-terminal Pfam-HTH_8 domain (DNA binding site). The FleQ protein of L. pneumophila Corby is 99, 55, 54, and 54% identical to FleQ of L. pneumophila Philadelphia, FleQ of P. aeruginosa, the σ54-dependent transcriptional activator of Vibrio cholerae, and FlaK of Vibrio parahaemolyticus, respectively. Downstream of fleQ, we identified two putative open reading frames encoding a putative serine protease and a hypothetical 93-amino-acid protein (ORF93) that exhibited no significant homology to any protein described so far (Fig. 1A).
FIG. 2.
Comparison of the amino acid sequences of L. pneumophila Corby FleQ (LcfleQ), FleQ of P. aeruginosa (PafleQ), FlaK of V. parahaemolyticus (VpflaK), FleR of L. pneumophila Philadelphia (LpfleR), and PilR of L. pneumophila Philadelphia (LppilR). The σ54 interaction domain is indicated by a dotted line, and the C-terminal HTH_8 domain is underlined. Amino acids identical to the amino acids in L. pneumophila Corby FleQ are indicated by periods. The putative phosphor acceptor site of FleR and PilR at amino acid position 54 is indicated (Asp-54). ∼, gaps; aa, amino acids.
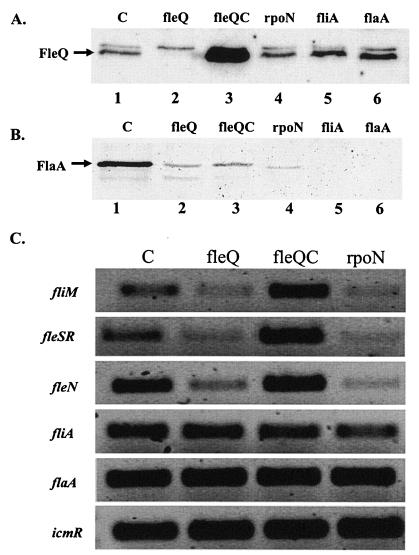
FIG. 3.
Western blot and RT-PCR analyses of L. pneumophila rpoN and fleQ mutant strains. (A and B) Western blot analysis performed with strains grown on BCYE agar at 30°C for 4 days and with an anti-FleQ antibody (kindly provided by Reuben Ramphal, Gainesville, Fla.) (A) or an anti-FlaA antibody (B). Equal amounts of whole-cell lysates were loaded onto the polyacrylamide gel. (C) RT-PCR performed with whole-cell RNA isolated from strains grown on BCYE agar plates at 30°C for 3 days. Abbreviations: C, L. pneumophila Corby (wild type); flaA, flaA mutant strain; fleQ, fleQ mutant strain; fleQC, complemented fleQ mutant strain; fliA, fliA mutant strain; rpoN, rpoN mutant strain.
As mentioned above, two other proteins containing a σ54 interaction domain were identified in L. pneumophila. These proteins exhibtied 38% (FleR) and 37% (PilR) identity to FleQ. The corresponding genes were downstream of genes that coded for the putative sensor kinase FleS or PilS. Upstream of fleS and pilS we identified typical putative σ54 promoters (Table 2). Furthermore, FleR and PilR also had a C-terminal HTH_8 domain and an Asp-54 residue that represented a putative phosphor acceptor site of an N-terminal sensor interaction domain (Fig. 2). An Asp-54 residue was not found in the FleQ proteins of L. pneumophila and P. aeruginosa. Furthermore, up- and downstream of fleQ no putative sensor kinase gene was identified. When the amino acid sequences of these five proteins were compared, it was obvious that the σ54 interaction domain is the most conserved region (Fig. 2).
TABLE 2.
Putative promoter sequences of various flagellar genes
| Region or gene | Sequence | Reference |
|---|---|---|
| σ70 consensus | TTGACA--N17--TATAATa | |
| fleQ t1 | GTCACA--N17--CATTAT--N46--ATGa | This study |
| fleQ t2 | ATAAAT--N17--AGTTAT--N51--ATGa | This study |
| σ28 consensus | TAAA---N15--GCCGATAAa | |
| flaA | TAAA---N15--TCCGATAA--N94--ATGa | 15 |
| fliD | TATA---N15--TCCGATAA--N153-ATGa | 21 |
| σ54 consensus | TGGCAC--N5--TTGCAb | |
| fliM | TGGCAC--N5--TTGCA---N113---ATGb | 21 |
| fleS | TGGCCT--N5--TTGCT---N35---ATGb | 21 |
| fleN | TGGATG--N6--TTGCA---N88---ATGb | 21 |
| pilS | TGGTTC--N5--ATGCG---N137---ATGb | This study |
The region before N17 or N15 is the −35 region, and the region after N17 or N15 is the −10 region.
The region before N5 or N6 is the −24 region, and the region after N5 or N6 is the −12 region.
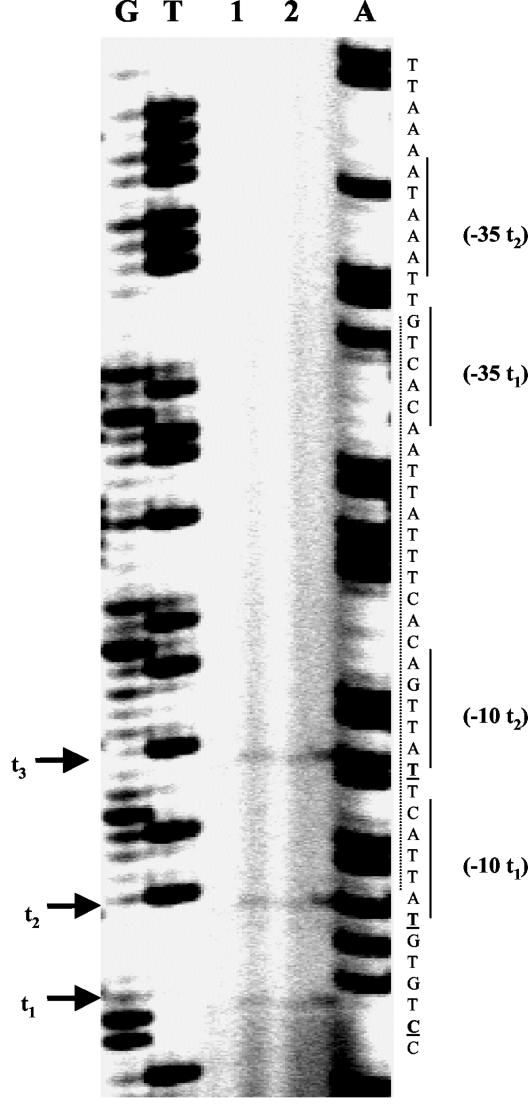
Five nucleotides upstream of the start codon (ATG) of FleQ, there is a ribosome binding site (AGGATA). Two putative σ70-like promoter elements were identified 46 and 51 bp upstream of the start codon by primer extension analysis (Fig. 4 and Table 2). However, another transcription initiation site of fleQ was found, but upstream of the transcriptional start site t3 no putative promoter element was identified (Fig. 4). Furthermore, a putative Vfr (homolog of the E. coli cyclic AMP receptor protein) binding site, containing an upstream activation sequence-like element (TGT-N12-ACA), was observed overlapping the fleQ promoter element (Fig. 4).
FIG. 4.
Primer extension experiments to map the transcriptional start site of the L. pneumophila fleQ gene. Total RNA was isolated from L. pneumophila cultures grown on BCYE agar plates at 30°C for 3 days (see Materials and Methods). The results of two independent experiments are shown (lanes 1 and 2). Transcriptional start sites are indicated by arrows (t1 to t3). Lanes G, T, and A contained DNA sequencing ladders. The positions of putative promoter elements (−10, −35) are indicated, and the position of a putative Vfr (putative E. coli cyclic AMP receptor protein homolog) binding site is indicated by the dotted line.
Nucleotide and protein sequence analysis of rpoN and RpoN.
The L. pneumophila RpoN homolog with a theoretical molecular mass of 52.9 kDa is encoded by a 1,392-bp open reading frame (Fig. 1B). Computer analysis revealed the presence of a σ54 family signature and the Pfam_σ54-activator interaction domain (AID), Pfam_σ54-core binding domain (CBD), and Pfam_σ54-DNA binding domain (DBD) commonly found in σ54 factors. RpoN of L. pneumophila is 53.9 and 49.2% identical to RpoN of V. cholerae and P. aeruginosa, respectively. The genetic map is shown in Fig. 1B. Five nucleotides upstream of the start codon, there is a conserved ribosome binding site (AGAGGA), but no typical promoter sequences were identified. However, a putative σ70-like −10 sequence (GATAAT) is present. Two genes encoding the putative 50S ribosomal proteins L28 and L33 are located upstream of rpoN (Fig. 1B). L28 and L33 of L. pneumophila Corby are 71.4 and 60.4% identical to L28 of P. aeruginosa and L33 of Yersinia pestis, respectively. In the genome of L. pneumophila Philadelphia genes for a putative σ54 modulation protein and a putative phosphocarrier (HPr) were identified downstream of rpoN (data not shown). Identical arrangements of these three genes have been described for E. coli, P. aeruginosa, and V. cholerae (23, 24, 26).
Analysis of the rpoN and fleQ mutant strains of L. pneumophila Corby.
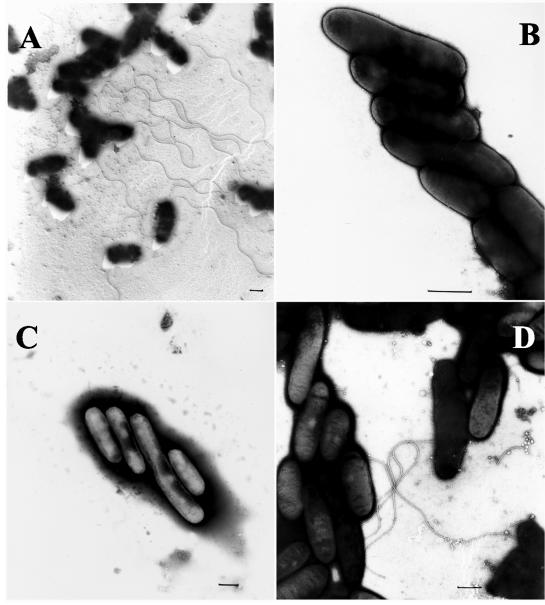
After growth for 4 days on BCYE agar plates at 30°C, the fleQ mutant expressed smaller amounts of FlaA protein than the wild type expressed, as determined by Western blot analysis with an anti-FlaA antiserum (Fig. 3B, lanes 1 and 2). As expected, the fleQ mutant also did not exhibit any detectable FleQ protein (Fig. 3A, lane 2). The complemented strain did not express the flagellin as well as the wild-type strain expressed it (Fig. 3B, lane 3), but this was probably due to the overexpression of fleQ (Fig. 3A, lane 3). After three more days of incubation, no FlaA protein was detected in the fleQ mutant, whereas large amounts of FlaA protein were still detectable in the wild-type strain (data not shown). A similar behavior was observed for bacterial strains grown in supplemented YEB medium (data not shown). Agar-grown bacteria were examined by electron microscopy for the presence of flagella. The fleQ strain was nonflagellated at any time tested (Fig. 5B), whereas the wild type was flagellated after 4 days of incubation on agar plates (Fig. 5A). Electron microscopy of the complemented strain revealed the presence of flagella, but again the flagellation was not fully comparable to the wild-type flagellation (Fig. 5D). From these data we concluded that FleQ is required for full expression of flaA and for assembly of the flagellum in L. pneumophila.
FIG. 5.
Electron microscopy of L. pneumophila strains. Bacteria were grown on BCYE agar plates at 30°C for 4 days (see Materials and Methods). Bars = 0.5 μm. (A) L. pneumophila Corby (wild type); (B) fleQ mutant strain; (C) rpoN mutant strain; (D) complemented fleQ mutant strain.
The rpoN mutant was tested accordingly. Similar to the results obtained with the fleQ mutant, only very small amounts of the FlaA protein were detected in the rpoN mutant (Fig. 3B, lane 4), and expression of FlaA at the wild-type level could not be complemented by plasmid-encoded rpoN (data not shown). Furthermore, the rpoN mutant appeared to be nonflagellated in an electron microscopy analysis (Fig. 5C). As the rpoN mutant contained amounts of FleQ comparable to the amounts in the wild-type strain (Fig. 3A, lanes 1 and 4), we concluded that the phenotypes observed for the rpoN mutant were not based on reduced fleQ expression in this strain and that flagellar expression in L. pneumophila depends on the presence of an active σ54 factor for direct expression of flagellar genes or for expression of an unknown additional factor.
To further analyze the abilities of the two mutants to express the flagellin but to be nonflagellated, we performed RT-PCR experiments (Fig. 3C) using RNA isolated from the L. pneumophila wild-type strain and fleQ, rpoN, and complemented fleQ mutant strains (see Materials and Methods) and gene-specific primer pairs (Table 1). Compared to the amounts in the wild-type strain, only small amounts of fliM transcripts were detectable in the rpoN and fleQ mutant strains, suggesting that in both of these mutants this gene is positively regulated by RpoN and FleQ. The fleN gene, which had a putative σ54-like promoter element, was also positively regulated by RpoN and FleQ (Fig. 3C). In the fleQ mutant these phenotypes were successfully complemented (Fig. 3C). It is likely that the identified σ54 promoter elements of fliM and fleN are not recognized by the RNA polymerase when RpoN or FleQ is not present. The results of RT-PCR experiments suggest that transcription of the fleSR operon also is positively regulated by FleQ and RpoN (Fig. 3C). On the other hand, comparable amounts of flaA transcripts were identified in both mutant strains and the wild type (Fig. 3C). This confirmed the finding mentioned above obtained by Western blot analysis with the FlaA-specific antiserum that FlaA is produced in the mutants (Fig. 3B). Besides flaA transcripts, we detected in both mutants amounts of fliA transcripts that were comparable to the amounts in the wild type (Fig. 3C). The presence of FliA, a positive regulator of flaA, in the wild type and mutants may explain why the mutants are still able to express FlaA. However, as fliA seems to be expressed even though RpoN and FleQ are not present, it is not surprising that both mutants expressed the flagellin. RT-PCR also revealed that RpoN and FleQ seemed not to be involved in expression of icmR (Fig. 3C), which encodes a subunit of the type IV secretion system of L. pneumophila. This suggests that neither RpoN nor FleQ is involved in regulation of this virulence factor.
Intracellular replication of the rpoN and fleQ mutants in host cells and distribution of rpoN and fleQ in legionellae.
The rpoN mutant and the fleQ mutant were tested for the ability to replicate intracellularly in the macrophage-like cell line U937. Compared to the replication of the wild-type strain, both mutants were still able to replicate (data not shown).
Southern blot analysis revealed that both rpoN and fleQ are conserved in L. pneumophila strains (data not shown). However, they seemed not to be as conserved in legionellae as the flagellin gene, because most of the non-L. pneumophila strains tested (see Materials and Methods) did not cross-hybridize with the fleQ-specific DNA probe (data not shown), whereas an flaA-specific probe was able to bind to the DNA of all flagellated strains tested (15). With the rpoN-specific probe, weak hybridization signals were obtained only with L. bozemanii, L. dumoffii, L. feelii S1, L. gormanii, and L. longbeachae S1.
DISCUSSION
Over 50 genes are required for assembly and functioning of the bacterial flagellum, and it has been shown that translational and posttranslational regulation also plays an important role in flagellar assembly. Furthermore, different promoter classes and regulators of various fla regulon hierarchies have been described (for a review see reference 1).
In this paper we describe cloning and characterization of rpoN and fleQ homologs of L. pneumophila Corby. The rpoN gene encodes an alternative σ54 factor. σ54 factors can be regarded as defective holoenzymes, because they initiate transcription only in concert with an activator protein (30). Most of these enhancer proteins are controlled by their own signal transducing pathways, which allows the bacteria to respond to a wide range of environmental signals through one sigma factor (5). We were able to identify three of these putative activators (FleQ, FleR, and PilR) in the genome sequence of L. pneumophila Philadelphia, all of which exhibited σ54 interaction domains. DNA probes specific for the rpoN and fleQ genes hybridized with chromosomal DNA of all L. pneumophila strains but not with DNA of most of the non-L. pneumophila strains tested so far, suggesting that these factors are not very well conserved within the legionellae.
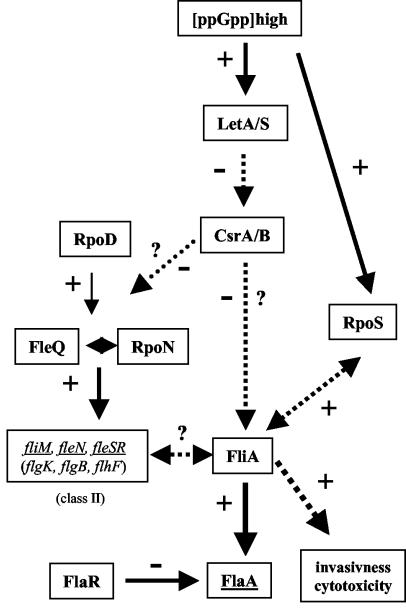
Analysis of the deduced amino acid sequence of rpoN revealed that RpoN has the σ54 factor domains (AID, CBD, and DBD) commonly found in σ54 factors (5). These domains are involved in activator interaction (AID), in interaction with the core RNA polymerase (CBD), and in DNA binding (DBD or RpoN box). The RpoN protein exhibited the highest identity (55%) to σ54 of V. cholerae. Inactivation of rpoN or fleQ in L. pneumophila led to nonflagellated mutant strains. The flagellar operon genes (class II) were found to contain putative σ54 promoter elements (Table 2) (21). Our results showed that flagellar expression depends on the presence of RpoN and its activator protein, FleQ. However, FlaA was still expressed at low levels in both mutants, but it was not assembled into a flagellum. It was shown recently that FliA directly regulates flaA expression (Fig. 3B, lane 5) (20). Here, we demonstrated by using RT-PCR that the fliA transcript is present in both mutant strains (Fig. 3C). This suggests that flaA is expressed in the fleQ and rpoN mutants, probably as a consequence of FliA expression. The flagellin may not be assembled into a flagellum because of the lack of expressed basal body genes. This hypothesis is supported by the reduced amounts of the transcript of fliM (an operon encoding several putative basal body genes) observed in the fleQ and rpoN mutants (Fig. 3C). These results also demonstrate that the fliM, fleSR, and fleN genes, all containing a putative σ54-dependent promoter, are positively regulated by RpoN and FleQ. Now it has to be shown if this is also true for other class II genes containing putative σ54-dependent promoters (Fig. 6) (21). We identified FleR of L. pneumophila as a putative σ54 interaction protein. FleS has been cloned recently, but the role of FleSR in flaA expression in L. pneumophila has not been determined yet (31). We started to generate an FleR mutant to analyze the role of FleR in the cascade of flagellar gene regulation. RT-PCR results suggest that fleSR expression is positively regulated by FleQ and RpoN (Fig. 6). In P. aeruginosa the FleSR two-component system is also involved in flagellin expression, in addition to FleQ and RpoN. Furthermore, we also identified PilR as a putative σ54 interaction protein, and experiments are under way to generate and analyze a pilR mutant strain of L. pneumophila Corby. It has to be determined if pilR is necessary for flagellation, for piliation, or for the virulence of L. pneumophila. A proposed cascade of flagellar regulation is shown in Fig. 6.
FIG. 6.
Proposed cascade of flaA gene regulation in L. pneumophila Corby. The dotted arrows indicate unknown modes of regulation (direct or indirect). Putative class II genes of the regulation cascade are indicated. The role of FlaR is not known yet. +, positive regulation; −, negative regulation; ?, proposed link; CsrA/B, carbon storage regulator; FlaA, flagellin; FlaR, transcriptional regulator (LysR family); fleSR, putative two-component system; FleQ, transcriptional regulator; FliA, alternative σ28 factor; LetA/S, two-component system; RpoN, alternative σ54 factor; RpoD, σ70 factor. (Modified from reference 21 with the permission of the publisher.)
In this paper, we show that RpoN and FleQ are involved in flagellar gene expression in L. pneumophila Corby. RpoN and FleQ are necessary for flagellar expression and assembly. It is likely that FleQ expression is RpoD dependent, because we were able to identify σ70-like promoter elements in front of the transcription initiation sites of fleQ by primer extension analysis (Fig. 4). We have to analyze if fleQ transcription is also Vfr dependent, as described for P. aeruginosa (7). A putative Vfr binding site was identified overlapping the fleQ promoter element, and a homolog of the Vfr gene is present in the genome sequence of L. pneumophila Philadelphia. Furthermore, FleQ expression is not dependent on the presence of RpoN or FliA, as shown by Western blot analysis (Fig. 3A). RT-PCR results suggest that RpoN and FleQ are not involved in the regulation of fliA and icmR gene expression (Fig. 3C). The icmR gene encodes a protein of the type IV secretion system needed for intracellular replication of L. pneumophila in this host. Furthermore, both mutants were able to replicate intracellularly in U937 cells, suggesting that both genes are not required for intracellular replication of L. pneumophila. A putative cascade of flagellar gene expression has been determined, and this cascade is similar to those described for Pseudomonas and Vibrio (1, 8). Experiments are under way to characterize the expression of fliA, because FliA is known to be involved in the virulence of Legionella (14, 20). The CsrA protein seems to be involved in fliA expression, but the activator of fliA expression has not been identified (11, 29). Further analysis of this cascade of gene regulation should help us understand the role of FliA in the link between virulence and flagellar expression in L. pneumophila.
Acknowledgments
We thank Reuben Ramphal (Gainesville, Fla.) for providing the anti-FleQ antibody and Bianca Hochhut for careful reading of the manuscript.
This work was supported by grants GRK 587/1-01 and HE2845/2-1 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5:160-165. [DOI] [PubMed] [Google Scholar]
- 2.Arora, S. K., B. W. Ritchings, E. C. Almira, S. Lory, and R. Ramphal. 1997. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J. Bacteriol. 179:5574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, L., M. Ott, A. Debes, U. Rdest, J. Heesemann, and J. Hacker. 1991. Distribution, expression, and long-range mapping of legiolysin gene (lly)-specific DNA sequences in legionellae. Infect. Immun. 59:3333-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosshardt, S. C., R. F. Benson, and B. S. Fields. 1997. Flagella are a positive predictor for virulence in Legionella. Microb. Pathog. 23:107-112. [DOI] [PubMed] [Google Scholar]
- 5.Buck, M., M.-T. Gallegos, D. J. Sudholme, Y. Guo, and J. D. Gralla. 2000. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182:4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta, N., E. P. Ferrell, K. J. Kanack, S. E. West, and R. Ramphal. 2002. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is σ70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J. Bacteriol. 184:5240-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich, C., K. Heuner, B. C. Brand, M. Steinert, and J. Hacker. 2001. Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect. Immun. 69:2116-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein, P. H. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fettes, P. S., V. Forsbach-Birk, D. Lynch, and R. Marre. 2001. Overexpresssion of a Legionella pneumophila homologue of the E. coli regulator csrA affects cell size, flagellation, and pigmentation. Int. J. Med. Microbiol. 291:353-360. [DOI] [PubMed] [Google Scholar]
- 12.Fields, B. S., J. M. Barbaree, G. N. Sanden, and W. E. Morrill. 1990. Virulence of a Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect. Immun. 58:3139-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 14.Hammer, B. K., E. S. Tateda, and M. S. Swanson. 2002. A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44:107-118. [DOI] [PubMed] [Google Scholar]
- 15.Heuner, K., L. Bender-Beck, B. C. Brand, P. C. Lück, K.-H. Mann, R. Marre, M. Ott, and J. Hacker. 1995. Cloning and genetic characterization of the flagellum subunit gene (flaA) of Legionella pneumophila serogroup 1. Infect. Immun. 63:2499-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuner, K., J. Hacker, and B. C. Brand. 1997. The alternative sigma factor σ28 of Legionella pneumophila restores flagellation and motility to an Escherichia coli fliA mutant. J. Bacteriol. 179:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuner, K., B. C. Brand, and J. Hacker. 1999. The expression of the flagellum of Legionella pneumophila is modulated by different environmental factors. FEMS Microbiol. Lett. 175:69-77. [DOI] [PubMed] [Google Scholar]
- 18.Heuner, K., C. Dietrich, M. Steinert, U. B. Göbel, and J. Hacker. 2000. Cloning and characterization of a Legionella pneumophila specific gene encoding a member of the LysR family of transcriptional regulators. Mol. Gen. Genet. 264:204-211. [DOI] [PubMed] [Google Scholar]
- 19.Heuner, K., U. Meltzer, B.-K. Choi, and U. B. Göbel. 2001. Outer sheath associated proteins of the oral spirochete T. maltophilum. FEMS Microbiol. Lett. 197:187-193. [DOI] [PubMed] [Google Scholar]
- 20.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative σ28 factor on virulence and flagellum expression of L. pneumophila. Infect. Immun. 70:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuner, K., and M. Steinert. 2003. The flagellum of Legionella pneumophila and its link to the expression of the virulent phenotype. Int. J. Med. Microbiol. 293:133-145. [DOI] [PubMed] [Google Scholar]
- 22.Jepras, R. I., R. B. Fitzgeorge, and A. Baskerville. 1985. A comparison of virulence of two strains of Legionella pneumophila based on experimental aerosol infection of guinea-pigs. J. Hyg. 95:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, S., K. Ishimoto, and S. Lory. 1994. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J. Bacteriol. 176:1316-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, D. H., F. C. Franklin, and C. M. Thomas. 1994. Molecular analysis of the operon which encodes the RNA polymerase sigma factor sigma 54 of Escherichia coli. Microbiology 140:1035-1043. [DOI] [PubMed] [Google Scholar]
- 25.Jyot, J., N. Dasgupta, and R. Ramphal. 2002. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J. Bacteriol. 184:5251-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klose, K. E., and J. J. Mekalanos. 1998. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28:501-520. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Merriam, J. J., R. Mathur, R. Maxfield-Boumil, and R. R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molofsky, A. B., and M. S. Swanson. 2003. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50:445-461. [DOI] [PubMed] [Google Scholar]
- 30.Morett, E., and L. Segovia. 1993. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J. Bacteriol. 175:6067-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polesky, A. H., J. T. Ross, S. Falkow, and L. S. Tompkins. 2001. Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect. Immun. 69:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruckler, J. M., R. F. Benson, M. Moyenuddin, W. T. Martin, and B. S. Fields. 1995. Association of flagellum expression and intracellular growth of Legionella pneumophila. Infect. Immun. 63:4928-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Saunders, N. A., N. Doshi, and T. G. Harrison. 1992. A second serogroup of Legionella erythra serologically indistinguishable from Legionella rubrilucens. J. Appl. Bacteriol. 72:262-265. [DOI] [PubMed] [Google Scholar]
- 35.Stone, B. J., and Y. Abu Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with the expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]