Abstract
Purpose
To review the literature on targeted therapy for orbital and periocular basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) and provide examples of patients recently treated with such therapy.
Methods
We reviewed the literature on clinical results of targeted therapy and the molecular basis for targeted therapy in orbital and periocular BCC and cutaneous SCC. We also present representative cases from our practice.
Results
Mutation in the patched gene (PTCH1) has been implicated in BCC, and overexpression of epidermal growth factor receptor (EGFR) has been shown in SCC. Vismodegib, an inhibitor of smoothened, which is activated upon binding of hedgehog to Ptc, has been shown to significantly decrease BCC tumor size or even produce complete resolution, especially in cases of basal cell nevus syndrome. Similarly, EGFR inhibitors have been shown to significantly decrease SCC tumor size in cases of locally advanced and metastatic disease. We describe successful outcomes after vismodegib treatment in a patient with basal cell nevus syndrome with numerous bulky lesions of the eyelid and periocular region and erlotinib (EGFR inhibitor) treatment in a patient with SCC who was deemed not to be a good surgical candidate because of advanced SCC of the orbit with metastasis to the regional lymph nodes, advanced age, and multiple medical comorbidities.
Conclusion
Targeted therapy using hedgehog pathway and EGFR inhibitors shows significant promise in treatment of orbital and periocular BCC and cutaneous SCC, respectively. Such targeted therapy may be appropriate for patients who are not good candidates for surgery.
INTRODUCTION
The mainstay of treatment for orbital and periocular cutaneous malignancies such as basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) is surgical excision, sometimes supplemented by adjuvant radiation therapy.1 However, locally advanced BCC or cutaneous SCC characterized by large tumor size, multiple lesions, or locally recurrent disease that may not be amenable to surgical management, and patients with advanced age or multiple medical comorbidities may not be good candidates for surgery. Also, patients with syndromes that are associated with numerous cutaneous lesions of the periocular region and face, such as basal cell nevus syndrome (Gorlin syndrome), may not be good candidates for surgical excision, and treatment of such patients has been challenging.2
The recent discovery of underlying genetic mutations of the hedgehog pathway in BCC and overexpression of the epidermal growth factor receptor (EGFR) in SCC have opened the door to consideration of targeted therapy in inoperable cases of advanced BCC or cutaneous SCC of the orbit and periocular region.3,4
We herein present a review of targeted therapy for BCC with hedgehog pathway inhibition and for SCC with EGFR inhibitors and present 2 representative cases of patients with orbital and periocular lesions treated at our institution using these new nonsurgical treatment modalities.
METHODS
Review articles such as this are exempt from institutional review board review at our institution. The manuscript was written in compliance with Declaration of Helsinki. The MEDLINE standard computer database (US National Library of Medicine, Bethesda, MD) and advanced database were searched for articles from 1946 to present. The following key words were used: Hedgehog, vismodegib, basal cell nevus syndrome, basal cell carcinoma, squamous cell carcinoma, cetuximab, erlotinib, gatifinib, Iressa, GDC0449, OSI774, CP358, IMC-C225, ZD1839. The clinicaltrial.gov registry was also searched with the same above listed key words for ongoing trials and published data.
RESULTS
Hedgehog Pathway and BCC
As early as 1992, genetic linkage analysis showed that 9q22-31 was the site of a gene associated with basal cell nevus syndrome.5 In 1996, two groups concurrently reported that mutation in the patched 1 gene (PTCH1), located at 9q22.3, was linked to this syndrome.6,7 Since then, mutation in PTCH1 (the human equivalent of Ptc) has been implicated in sporadic cases of BCC as well.8 Ptc is best known for its role in development of specific spatial patterns during Drosophila embryogenesis.9 Ptc is a transmembrane protein activated by hedgehog (Hh). All signaling through the hedgehog pathway requires a third protein, smoothened (Smo), which is activated upon binding of Hh to Ptc.10 In the absence of Hh binding, Ptc inhibits the action of Smo. A mutation resulting in constitutively activated Smo has also been implicated in sporadic cases of BCC.11 In vivo studies comparing BCC to normal skin have found that downstream transcription factors of the hedgehog pathway are upregulated in BCC.12 The result of an activated hedgehog pathway is cellular proliferation13 and angiogenesis14.
Hedgehog Pathway Inhibitor Vismodegib for BCC
Vismodegib (GDC-0449) was molecularly engineered to have a structure similar to that of benzimidazole,15 a known antagonist of the hedgehog pathway. Vismodegib was first studied in humans in 2008 and was approved by the US Food and Drug Administration for treatment of metastatic and locally advanced BCC in January 2012. Since the identification of vismodegib, multiple small molecule inhibitors of the hedgehog pathway have been developed,16,17 including a drug designed for topical application that is currently being studied in phase 1 clinical trials.18 However, the only Food and Drug Administration–approved hedgehog pathway inhibitor for treatment of metastatic and locally advanced BCC is vismodegib.
In an in vitro study, vismodegib downregulated GLI1, a transcription factor for hedgehog, by approximately 90% of compared to controls.15 In a clinical study, vismodegib downregulated GLI1 expression by more than 50% in 73.5% of patients as revealed by examination of skin biopsy specimens.19 The maximal plasma concentration of vismodegib was reached 48 hours after a single dose of 150 mg administered orally, and this maximal plasma concentration was maintained for 72 hours; these results established the dosage of 150 mg daily by mouth for subsequent trials.20 In 33 patients with locally advanced or metastatic BCC who were enrolled in a phase 1 trial of vismodegib in 68 patients with a variety of metastatic solid tumors, the overall rate of either partial or complete response was 58%19; the response rate was 50% in patients with metastatic BCC and 60% in patients with locally advanced BCC.21 Locally advanced BCC was defined as at least 1 lesion 10 mm or more in longest diameter and surgery considered inappropriate by a specialist for one of the following reasons: the patient had recurrence after 2 or more surgical procedures and curative resection was unlikely or surgery was expected to result in substantial morbidity or deformity. The most frequently reported side effects were muscle spasms, dysgeusia (altered sense of taste), fatigue, alopecia, and nausea. Overall, 20 (29.4%) of the 68 patients in the study experienced grade 3 adverse events, and 6 (8.8%) experienced grade 4 adverse events; hyponatremia was the most common adverse event in both categories.19
A phase 2 clinical trial of vismodegib22 in 104 patients was conducted in the United States, Europe, and Australia from February 2009 through November 2010. Of the 33 patients with metastatic BCC, 10 (30%) had a response as judged by independent review, and 15 (45%) had a response as judged by site investigators. In the 63 patients with locally advanced BCC for whom outcomes were analyzed, 27 (43%) had a response as judged by an independent reviewer, of whom 13 (21%) had a response (absence of residual disease on biopsy), and 38 (60%) had a response as judged by site investigators. Disease progression was noted in 6 (18%) of the patients with metastatic disease. All patients had at least 1 adverse event, and 26 (25%) of the patients had grade 3 or 4 events, with decrease in weight, muscle spasm, and fatigue being the most common. The most impressive responses to treatment with vismodegib were observed in patients with Gorlin syndrome.
An additional phase 2 trial in 41 patients with basal cell nevus syndrome, defined as the presence of at least 2 of the major criteria as defined by Kimonis et al23 (Table 1), were randomly assigned 2:1 to receive vismodegib or placebo. Vismodegib was associated with a decrease in the number of new and existing surgically eligible BCCs,24 defined as BCCs with a diameter of 3 mm or greater on the nose or periorbital skin, 5 mm or greater elsewhere on the face, or 9 mm or greater on the trunk or limbs. New, surgically eligible BCCs occurred at a rate of 2 per year in the vismodegib group compared to 29 per year in the control group (p<0.001). There was also a significantly greater decrease in the size of existing surgically eligible BCCs in patients receiving vismodegib (65% in the vismodegib group vs 11% in the control group; p=0.003). Histologically, upon examination of specimens randomly collected from patients receiving vismodegib, microscopic BCCs were detected in 88% of specimens collected at 1 month and 46% of specimens collected at 3 months. As expected patients experienced recurrence of BCC after discontinuation of vismodegib at the original sites; however, the rate of new surgically eligible BCC was significantly less than those in the placebo arm.
Table 1.
Major criteria for basal cell nevus syndrome
| 1. | More than 2 basal cell carcinomas or 1 diagnosed before the age of 20 years |
| 2. | Odontogenic keratocyst of the jaw proven by histologic examination |
| 3. | Three or more palmar or plantar pits |
| 4. | Bilamellar calcification of the falx cerebri |
| 5. | Bifid, fused, or markedly splayed ribs |
| 6. | First-degree relative with basal cell nevus syndrome |
Case Report: Treatment of BCC with Vismodegib
The following case demonstrates the remarkable effect of vismodegib in a patient with basal cell nevus syndrome with numerous periocular lesions.
A 30-year-old man with a history of basal cell nevus syndrome diagnosed at age 7 years presented to The University of Texas MD Anderson Cancer Center in November 2006 with a large lesion on the left lower eyelid that had been present for 1 year and was increasing in size. The patient had previously undergone cryotherapy and excision of hundreds of BCCs throughout his body and was using imiquimod (Aldara) for treatment of his nonocular lesions and tolerating it well. Examination of the ocular adnexae showed a full-thickness nodular lesion with telangiectasia involving more than two-thirds of the left lower eyelid (Figure 1A) and multiple smaller BCCs on the right and left upper and lower eyelids as well as many throughout the face (Figure 1B). The patient underwent wide local excision of the left lower eyelid lesion and repair with a tarsoconjunctival flap, which produced a good functional and cosmetic result (Figure 1C). The remaining periocular lesions were observed until August 2008, when one of the medial canthal BCCs showed significant enlargement interfering with the patient's vision (Figure 1D). This lesion was excised, and the resulting defect was repaired with a glabellar flap and an advancement flap from the lateral left lower eyelid (Figure 1E).
Fig 1A, 1B.


External photograph of a patient with basal cell nevus syndrome and multiple basal cell carcinomas (BCC) on the face including a large BCC on the left lower eyelid that was surgically removed.
Fig 1C.
Postoperative photo in the same patient demonstrates a nice outcome in the left lower eyelid but another large BCC has developed and is enlarging in the left medial canthus, which was subsequently excised.
Fig 1D.
The same patient after 16 weeks of treatment with vismodegib. He experienced significant resolution of all his facial and periorbital BCCs.
In November 2009, the patient was enrolled in a phase II trial of vismodegib 150 mg per day. At examination 4 weeks after the start of vismodegib, there was decreased thickness and diameter of 5 target lesions located at the left temple, right sideburn, right medial canthus, left medial canthus, and right shoulder. The patient noted that many lesions had “flaked off,” which indicated loss of the exophytic component. At examination 12 weeks after the start of vismodegib, the lesions on both canthi showed resolution of tumor with residual erythema or plaque. By 16 weeks after the start of vismodegib, all 5 target lesions had mostly resolved with residual scar (Figure 1F). Punch biopsies of the target lesions at 24 weeks after the start of vismodegib showed scar without carcinoma in 4 lesions and BCC at the peripheral edge in the right sideburn lesion. At 80 weeks after the start of vismodegib, all skin lesions showed no evidence of carcinoma. Adverse events included dyspepsia, dysgeusia (altered sense of taste), weight loss, leg cramps, alopecia, and low back pain. Of note, by 48 weeks after the start of vismodegib, the patient's sense of taste had begun to improve. The other adverse events persisted but were tolerable.
EGFR and Cutaneous SCC
EGFR, also known as HER1 or ErbB1, is a transmembrane receptor protein in the ErbB family of receptor kinases. EGFR has multiple ligands, including EGF and transforming growth factor-α). Upon binding by these ligands, EGFR forms either a homodimer or heterodimer with another member of the ErbB family of receptors.4,25 The results of EGFR activation in keratinocytes are cellular proliferation; induction or blockage of differentiation; cellular migration; and increased survival and resistance to apoptosis.26 Overexpression of EGFR has been implicated in mucosal and cutaneous carcinoma of the head and neck, glioma, breast carcinoma, bladder cancer, prostate cancer, kidney cancer, and non-small cell lung carcinoma (NSCLC).25,27
In a study of 13 patients with metastatic cutaneous SCC, EGFR was strongly expressed in all cases at various layers of the skin irrespective of the level of differentiation, whereas adjacent normal skin showed expression only at the basal layer of epidermis.28 Moreover, Ch'ng et al found that the degree of overexpression of EGFR correlated with the aggressiveness of disease: in 64 patients with head and neck cutaneous SCC, 29 with metastasis detected either at the time of diagnosis of the primary tumor or after diagnosis of the primary tumor and 25 patients without metastasis, strong EGFR overexpression was found in 79% of cases with metastasis compared to 36% of cases without metastasis.29 Shimizu et al found that in 5 cases of cutaneous SCC with metastasis, the metastatic lesion showed stronger EGFR expression than the primary tumor in 4 of the 5 cases, and in the fifth case neither the primary tumor nor the metastasis showed EGFR expression.30 A multivariate analysis by Ch'ng et al found that EGFR expression was an independent predictor of metastasis even when stage and degree of differentiation were taken into consideration (p=0.05).29 EGFR overexpression has also been demonstrated in studies of conjunctival SCC previously published by our group.31 All 5 conjunctival SCC specimens included in the report from MD Anderson had moderate to strong expression of EGFR in both the invasive and in situ components.31
EGFR Inhibitors for Cutaneous SCC
Inhibitors of EGFR have been investigated for treatment of various cancers, first in 1999 when gefitinib was investigated for treatment of NSCLC. Subsequently, gefitinib was investigated for treatment of solid tumors, including those involving the pancreas, lung, brain, bladder, breast, and head and neck. The first clinical trial investigating the use of EGFR inhibitors for cutaneous SCC was a 2006 clinical trial of erlotinib in conjunction with radiation. EGFR inhibitors can be divided into small molecule tyrosine kinase inhibitors e.g., gefitinib and erlotinib) and monoclonal antibodies to EGFR (e.g., cetuximab), which have greater specificity but require intravenous infusion.26
Cetuximab is a mouse-human chimeric monoclonal antibody that prevents binding of EGF to EGFR and inhibits downstream kinase activation.32 Cetuximab is currently approved by the US Food and Drug Administration for use in certain types of colorectal cancer in conjunction with chemotherapy and for use in certain types of head and neck cancer in conjunction with radiation therapy. A phase 2 clinical trial33 of cetuximab as single-agent therapy for cutaneous SCC included 36 patients with metastatic or surgically unresectable SCC. 26 patients (72%) had lesions with high EGFR expression. The cetuximab dosage was a 400 mg/m2 loading dose followed by weekly infusions of 250 mg/m2. The rate of disease control (partial or complete response or stable disease) was 69% on the basis of intent-to-treat analysis and 81% based on actual treatment received. The most frequent side effect was an acne-like rash, which occurred in 87% of the patients (Figure 2A); mean time to appearance of a rash was 14 days.
Fig 2A.
External photograph of patient with recurrent squamous cell carcinoma of eyelid with extension into the orbit demonstrates moderate proptosis, hyperglobus, periorbital erythema, and chemosis of the left globe.
Gefitinib (Iressa; ZD1839) is a tyrosine kinase inhibitor created with the goal of attenuating the activity of EGFR tyrosine kinase in the hope of decreasing toxicity instead of completely blocking EGFR tyrosine kinase. Gefitinib was approved in 2003 as monotherapy for NSCLC, but in 2005 the approval was limited to patients in whom gefitinib had previously been shown to be effective because of a lack of significant survival benefit, especially compared to survival with erlotinib treatment. Gefitinib is a potent selective inhibitor of EGF-stimulated tumor cell growth and blocks autophosporylation in tumor cell lines in vitro.34 This drug is now being examined for treatment of cutaneous SCC of the head and neck. In a study of 22 patients with recurrent or aggressive disease—defined as lesions that were 2 cm or larger in greatest diameter, invaded deep tissue, or were associated with perineural invasion and/or metastasis to lymph nodes—neoadjuvant treatment with gefitinib 250 mg daily produced an overall response rate of 45.5%.35 Even more impressive is that 3 of the 4 patients with complete response had no histologic evidence of carcinoma at the time of surgery. The most common side effects were fatigue, diarrhea, acneiform rash, and nausea, and 59.1% of patients experienced grade 2 or 3 side effects. No grade 4 side effects or treatment-related deaths were reported.
Erlotinib (Tarceva) was approved by the US Food and Drug Administration in 2004 for treatment of locally advanced or metastatic NSCLC after failure of 1 chemotherapy regimen and for treatment of pancreatic cancer in combination with gemcitabine. Erlotinib is a tyrosine kinase inhibitor through competitive inhibition with ATP.36 Like gefitinib, erlotinib inhibits tumor cell proliferation and autophosphorylation.36 In the global phase 4 study of erlotinib for NSCLC, adverse events were noted in 54% of patients. The most prevalent side effects were rash and diarrhea37; others included mucositis, headache, and hyperbilirubinemia.38 Ocular side effects included trichomegaly leading to corneal ulcer,39,40 conjunctivitis, and ectropion with epiphora.41
Clinical trials of erlotinib for cutaneous SCC have not yet been completed; however, case reports have indicated favorable responses in metastatic cutaneous SCC42,43 and locally advanced cutaneous SCC.44 Recently, our group reported a patient with advanced recurrent SCC of the eyelid with extension into the orbit who had a significant response to oral erlotinib.44 To date, our group has used EGFR inhibitors to treat 3 patients with recurrent, surgically unresectable SCC of the orbit and periocular region. All three patients have had durable response of the orbital SCC with in a followup period ranging from 6 months to 18 months (median = 12 months).
Case Report: Treatment of Cutaneous SCC with Erlotinib
The following case report, a brief summary of a previously reported case45, demonstrates the potential impact of EGFR inhibitors in patients with SCC of the orbit and periocular region.
A 90-year-old woman presented to MD Anderson Cancer Center in January 2011 after a biopsy of a left orbital mass revealed invasive SCC. The woman had had 2 prior episodes of left visual decrease and diplopia and subsequently was noted by her family to have left proptosis (Figure 2A). Diagnostic magnetic resonance imaging revealed extraconal masses in the left orbit with involvement of the left temporal fossa (Figure 2B) as well as involvement of the left preauricular node, which was later confirmed by biopsy. No cranial nerve involvement was noted at the time of presentation to MD Anderson. Two years before this presentation, in 2008, the patient had a left lateral canthus carcinoma excised by her dermatologist. She also had a remote history of breast carcinoma treated with bilateral mastectomy without adjuvant chemotherapy or radiation.
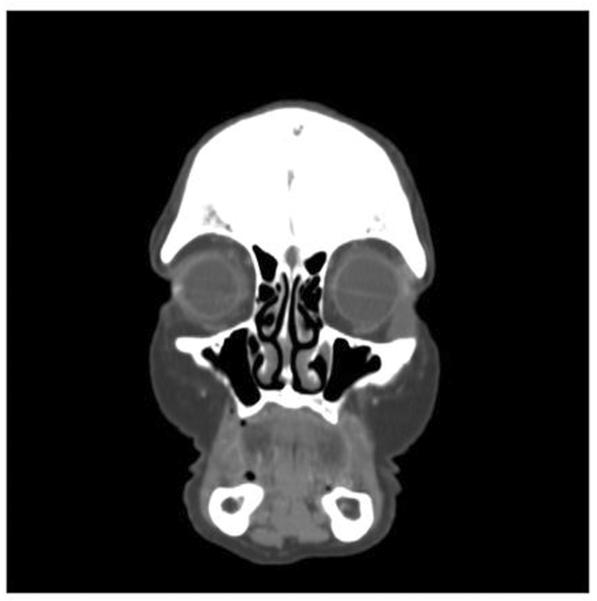
Fig 2B.
Coronal computed tomography scan in the same patient at presentation shows a bilobed mass in the left inferior orbit and temporalis fossa.
Definitive management would have entailed surgical management with left orbital exenteration, parotidectomy, and neck dissection followed by free flap reconstruction and adjuvant radiation therapy. Because of her advanced age and history of 2 myocardial infarction status post 2 stent placement, the patient elected noninvasive treatment. She was entered into a clinical trial of erlotinib therapy in February 2011. Her visual acuity on the left improved from light perception to 20/400 after 3 months of erlotinib therapy. By June 2012, both proptosis and ocular motility had improved significantly, although some residual tumor remained (Figure 2C,D).
Fig 2C.
Photograph of same patient at last contact, after 16 months of treatment with erlotinib, demonstrates resolution of proptosis, erythema, and chemosis of the left eye. The patient's orbital pain was also resolved.
Fig 2D.
Coronal computed tomography scan of the patient following at last contact demonstrates significant reduction in tumor size.
DISCUSSION
With increased understanding of the molecular mechanism underlying BCC and cutaneous SCC, the development of targeted therapy against the aberrant activation of cellular pathways became possible. Although surgical excision remains the mainstay of treatment for BCC and cutaneous SCC in the orbit and periocular region, targeted therapy has shown promise in cases of locally advanced or recurrent disease and cases in which patient factors preclude surgery. Systemic chemotherapy results in significant cytotoxic affects, whereas targeted therapy is associated with fewer side effects and is better tolerated by patients. Targeted therapy, still in its early phase of use, will likely prove to be an important addition to our armamentarium for managing orbital and periocular cutaneous carcinomas. On the basis of our review of the literature and our experience to date, the indications for considering targeted therapy for BCC or cutaneous SCC of the orbit or periocular region include disease that is unresectable because of multiple lesions, involvement of the orbit or skull base, and the combination of advanced age and multiple medical comorbidities.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
The authors have no commercial associations or financial disclosures that might pose a conflict of interest with information presented in this manuscript.
REFERENCES
- 1.Slutsky JB, Jones EC. Periocular cutaneous malignancies: A review of the literature. Dermatol Surg. 2012;38:552–69. doi: 10.1111/j.1524-4725.2012.02367.x. [DOI] [PubMed] [Google Scholar]
- 2.Southwick GJ, Schwartz RA. The basal cell nevus syndrome: disaster occurring among a series of 36 patients. Cancer. 1979;44:2294–305. doi: 10.1002/1097-0142(197912)44:6<2294::aid-cncr2820440644>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Tang JY, Marghoob AA. Emerging treatments and signaling pathway inhibitors. Semin Cutan Med Surg. 2011;30:S14–8. doi: 10.1016/j.sder.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Farndon PA, Del Mastro RG, Kilpatrick MW, Evans DRG. Location of gene for Gorlin syndrome. Lancet. 1992;339:581–2. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- 6.Hahn H, Wicking C, Zaphiropoulos PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RL, Rothman AL, Xie J, et al. Human homolog of `patched', a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 8.Barnes EA, Heidtman KJ, Donoghue DJ. Constitutive activation of the shh-ptc1 pathway by a patched1 mutation identified in BCC. Oncogene. 2005;24:902–15. doi: 10.1038/sj.onc.1208240. [DOI] [PubMed] [Google Scholar]
- 9.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Murone M, Luoh SM, et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 12.Bonifas JM, Pennypacker S, Chuang PT, et al. Activation of expression of hedgehog target gene in basal cell carcinomas. J Invest Dermatol. 2001;116:739–42. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 13.Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 14.Pola R, Ling LE, Silver M, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 15.Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449: A potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–81. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 16.Low JA, de Sauvage FJ. Clinical experience with hedgehog pathway inhibitors. J Clin Oncol. 2010;28:5321–6. doi: 10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 17.Stanton BZ, Peng LF. Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst. 2010;6:44–54. doi: 10.1039/b910196a. [DOI] [PubMed] [Google Scholar]
- 18.Tang T, Tang JY, Li D, et al. Targeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of smoothened. Clin Cancer Res. 2011;17:3378–87. doi: 10.1158/1078-0432.CCR-10-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LoRusso PM, Jimeno A, Dy G, et al. Pharmacokinetic dose-scheduling study for hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:5774–82. doi: 10.1158/1078-0432.CCR-11-0972. [DOI] [PubMed] [Google Scholar]
- 21.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 22.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestation in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299–308. [PubMed] [Google Scholar]
- 24.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–8. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 26.Ivan D, Prieto VG, Esmaeli B, et al. Epidermal growth factor receptor (EGFR) expression in periocular and extraocular sebaceous carcinoma. J Cutan Pathol. 2010;37:231–6. doi: 10.1111/j.1600-0560.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 27.Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Path Res Prac. 2011;207:337–342. doi: 10.1016/j.prp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Maubec E, Duvillard P, Velasco V, et al. Immunohistohemical analysis of EGFR and HER-2 in patients with metastatic squamous cell carcinoma of the skin. Anticancer Res. 2005;25:1205–1210. [PubMed] [Google Scholar]
- 29.Ch'ng S, Low I, Ng D, et al. Epidermal growth factor receptor: a novel biomarker for aggressive head and neck cutaneous squamous cell carcinoma. Hum Pathol. 2008;39:344–9. doi: 10.1016/j.humpath.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu T, Izumi H, Oga A, et al. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology. 2001;202:203–6. doi: 10.1159/000051637. [DOI] [PubMed] [Google Scholar]
- 31.Shepler TR, Prieto VG, Diba R, et al. Expression of the epidermal growth factor receptor in conjunctival squamous cell carcinoma. Ophthal Plast Reconstr Surg. 2006;22:113–5. doi: 10.1097/01.iop.0000202609.92772.c3. [DOI] [PubMed] [Google Scholar]
- 32.Fan Z, Masui H, Altas I, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–8. [PubMed] [Google Scholar]
- 33.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–26. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 34.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 35.Lewis CM, Glisson BS, Feng L, et al. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:1435–46. doi: 10.1158/1078-0432.CCR-11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 37.Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva lung cancer survival treatment study. J Thorac Oncol. 2010;5:1616–22. doi: 10.1097/JTO.0b013e3181f1c7b0. [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase 1 and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 39.Lane K, Goldstein SM. Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg. 2007;23:65–6. doi: 10.1097/IOP.0b013e31802d9802. [DOI] [PubMed] [Google Scholar]
- 40.Marquez G, Herrera-Acosta E, Vidal I, et al. A case of trichomegaly of the eyelashes and facial hypertrichosis induced by erlotinib (Tarceva) Int J Dermatol. 2009;48:97–8. doi: 10.1111/j.1365-4632.2009.03752.x. [DOI] [PubMed] [Google Scholar]
- 41.Methvin AB, Gausas RE. Newly recognized ocular side effects of erlotinib. Ophthal Plast Reconstr Surg. 2007;23:63–5. doi: 10.1097/IOP.0b013e31802d97f0. [DOI] [PubMed] [Google Scholar]
- 42.Engelhardt C, Curiel-Lewandrowski C, Warneke J, Cranmer L. Metastatic cutaneous squamous cell carcinoma responding to erlotinib therapy. J Am Acad Dermatol. 2011;65:237–8. doi: 10.1016/j.jaad.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 43.Read WL. Squamous carcinoma of the skin responding to erlotinib: Three cases. J Clin Oncol. 2007;25:16519. [Google Scholar]
- 44.El-Sawy T, Sabichi AL, Myers JN, et al. EGFR Inhibitors for Treatment of Orbital Squamous Cell Carcinoma. Arch Ophthalmol. doi: 10.1001/archophthalmol.2012.2515. in press. [DOI] [PubMed] [Google Scholar]