Abstract
Previously, we compared molecular profiles of one population of wild-type (WT) mouse facial motoneurons (FMNs) surviving with FMNs undergoing significant cell death after axotomy. Regardless of their ultimate fate, injured FMNs respond with a vigorous pro-survival/regenerative molecular response. In contrast, the neuropil surrounding the two different injured FMN populations contained distinct molecular differences that support a causative role for glial and/or immune-derived molecules in directing contrasting responses of the same cell types to the same injury. In the current investigation, we utilized the facial nerve axotomy model and a presymptomatic amyotrophic lateral sclerosis (ALS) mouse (SOD1) model to experimentally mimic the axonal die-back process observed in ALS pathogenesis without the confounding variable of disease onset. Pre-symptomatic SOD1 mice had a significant decrease in FMN survival compared with WT, which suggests an increased susceptibility to axotomy. Laser microdissection was used to accurately collect uninjured and axotomized facial motor nuclei of WT and presymptomatic SOD1 mice for mRNA expression pattern analyses of pro-survival/pro-regeneration genes, neuropil-specific genes, and genes involved in or responsive to the interaction of FMNs and non-neuronal cells. Axotomized pre-symptomatic SOD1 FMNs displayed a dynamic pro-survival/regenerative response to axotomy, similar to WT, despite increased cell death. However, significant differences were revealed when the axotomy-induced gene expression response of presymptomatic SOD1 neuropil was compared with WT. We propose that the increased susceptibility of presymptomatic SOD1 FMNs to axotomy-induced cell death and, by extrapolation, disease progression, is not intrinsic to the motoneuron, but rather involves a dysregulated response by non-neuronal cells in the surrounding neuropil.
INDEXING TERMS: motoneuron, neuropil, facial nerve axotomy, SOD1, ALS
Facial nerve axotomy is a well-established injury paradigm (Moran and Graeber, 2004) for the exploration of motoneuron (MN) survival and peripheral nerve regeneration in wild-type (WT) and transgenic mice (Jones et al., 2005; Moran and Graeber, 2004). In WT mice, facial nerve axotomy results in the emergence of one facial motoneuron (FMN) population in the ventromedial (VM) facial subnuclear region, where all FMNs survive, and another in the ventrolateral (VL) region, where significant cell loss is observed (Canh et al., 2006). In a recent study, we tested the hypothesis that differential FMN molecular responses to axotomy would accompany contrasting neuronal survival levels in the facial subnuclear regions (Mesnard et al., 2010). However, we unexpectedly found that the mRNA expression responses for FMN-specific genes in the VM and VL facial subnuclear regions were similar, despite significant differences in FMN survival levels after injury (Mesnard et al., 2010).
Furthermore, the FMNs in the axotomized VL exhibited increased FMN-specific mRNA expression levels compared with the VM, which we interpret to be a compensatory mechanism by the surviving VL FMNs. Interestingly, analysis of the molecular response for neuropil-specific genes established that non-neuronal cells surrounding the injured FMNs showed altered mRNA expression patterns in the VL compared with the VM. We hypothesize from those findings that MN survival following injury is directly dependent on the neuropil response, and furthermore, that a dysregulated neuropil surrounding the MN may play a role in promoting amyotrophic lateral sclerosis (ALS) pathogenesis.
ALS is a fatal MN degenerative disease affecting both the upper and lower MNs with muscle weakness, atrophy, and paralysis. Although the majority of patient cases result from a sporadic onset, 10% of ALS cases are due to a familial form of the disease, including inherited mutations in the Cu/Zn superoxide dismutase-1 (SOD1) gene. Transgenic mice overexpressing the human mutant SOD1 gene develop a similar pathology to ALS patients, involving muscle weakness, inflammation, axonal die-back, and MN degeneration (Deng et al., 1993; Gurney et al., 1994; Rosen et al., 1993; Rowland and Shneider, 2001; Shibata, 2001).
Cell-type-specific expression studies of the mutant SOD1 gene have implicated the involvement of non-neuronal cells in initiating disease onset and promoting disease progression (Beers et al., 2006; Dobrowolny et al., 2008; Pramatarova et al., 2001; Wang et al., 2009; Wong and Martin, 2010; Yamanaka et al., 2008). In addition, central/upper MNs undergo degeneration in SOD1 mice (Haenggeli and Kato, 2002; Nimchinsky et al., 2000; Zang and Cheema, 2002), and the loss of presynaptic afferent innervation to lower MNs has been observed in ALS mouse models and patient spinal cords (Ikemoto et al., 2002; Miyazaki et al., 2009; Zang et al., 2005). Following facial nerve axotomy in WT mice, presynaptic stripping surrounding the injured FMN cell bodies is a reactive process by which microglia and astrocytes interact with and shield the injured FMN from afferent input (Graeber et al., 1988; Jones et al., 1997b, 1999a; Kreutzberg, 1996a,b). Therefore, the central MN degeneration observed in SOD1 mice, resulting in the loss of synaptic input onto lower MNs, potentially initiates a local glial response in the perineuronal microenvironment devoid of presynaptic input.
An axonal pathology has been observed in SOD1 mice in which motor axons withdraw from neuromuscular junctions, producing an axonal die-back response that precedes functional neuromuscular junction and MN degeneration (Carrasco et al., 2010; Fischer et al., 2004; Hegedus et al., 2007; Kennel et al., 1996; Park and Vincent, 2008). The response to peripheral nerve injury resembles the axonal die-back response, as both processes result in axonal disconnection from neuromuscular junctions in target muscle and afferent presynaptic stripping surrounding the MN centrally. Thus, facial nerve axotomy, a common experimental peripheral nerve injury model, may be used as an investigative tool to delineate the underlying molecular mechanisms that result in MN degeneration in presymptomatic SOD1 mice.
In addition, in vivo time lapse imaging has revealed that disease progression in the SOD1 mouse is associated with the development of two MN populations, one that is degenerative, in which axonal branches die back, and one that is compensatory, in which thin, sprouting axonal branches reinnervate previously denervated neuromuscular junctions (Schaefer et al., 2005). In our previous study (Mesnard et al., 2010), we utilized laser micro-dissection to accurately collect uninjured and axotomized facial subnuclei in order to examine gene expression profile patterns in differing subnuclear populations of axotomized FMNs, and also between axotomized FMNs and their surrounding microenvironment. Specific genes were selected based on their functionality and cellular localization, and our previous study further established the localization for some of the genes as generated by MN or neuropil. The axotomy-induced mRNA expression changes that were assessed included pro-survival/pro-regeneration genes (βII tubulin, growth-associated protein of 43 kilodaltons [GAP-43], hemopoietic- and neurologic-expressed sequence-1 [Hn1], and brain-derived neurotrophic factor [BDNF]), neuropil-specific genes (glial fibrillary acidic protein [GFAP], interferon γ [IFNγ], and tumor necrosis factor α [TNFα]), and genes involved in or responsive to the interaction of MNs and non-neuronal cells (fractalkine [CX3CL1], pituitary adenylate cyclase-activating polypeptide [PACAP], and caspase 8).
βII tubulin, GAP-43, Hn1, and PACAP are MN-specific, whereas BDNF is produced by MNs and non-neuronal cells, and all function by supporting MN survival and/or promoting MN regeneration (Armstrong et al., 2008; Jones and Oblinger, 1994; Kobayashi et al., 1996; Tetzlaff et al., 1991; Zujovic et al., 2005). CX3CL1 is an MN-specific chemokine involved in the interaction between MN and microglia (Harrison et al., 1998). Caspase 8 becomes activated downstream of death receptor activation in MNs and non-neuronal cells, and activates effector caspases for apoptotic degeneration (Friedlander, 2003). IFNγ and TNFα are pro-inflammatory cytokines specific to non-neuronal cells in the surrounding neuropil of MNs (Mesnard et al., 2010; Raivich et al., 1998). GFAP is elevated in reactive astrocytes undergoing cytoskeletal rearrangement in the neuropil surrounding injured MNs (Graeber and Kreutzberg, 1988; Jones et al., 1997c; Laskawi and Wolff, 1996).
Our recent study provided the foundation to investigate the response of presymptomatic SOD1 FMNs and neuropil to axotomy. We hypothesize that increased SOD1 FMN susceptibility to axotomy-induced cell death (Mariotti et al., 2002a) is due to a dysregulated microenvironment surrounding the injured, diseased FMN, and is similar to the gene expression changes with injury that we discovered in the subnuclear regions of the facial nucleus in WT mice (Mesnard et al., 2010). Facial nerve axotomy was used as an investigative tool during the pre-symptomatic stage to examine the injury-induced die-back response of SOD1 FMNs and non-neuronal cells, without the confounding variable of disease onset. In adult WT rodents, the response to facial nerve axotomy by MNs is inherently regenerative, with injured MNs increasing gene expression required for survival and axonal elongation, whereas the non-neuronal cell response provides an environment supporting MN survival and regeneration (Aldskogius and Kozlova, 1998; Fu and Gordon, 1997; Jones et al., 2005; Lieberman, 1971; Moran and Graeber, 2004; Raivich et al., 1998; Schiefer et al., 1999). The current investigation examined whether pre-symptomatic SOD1 FMNs and the surrounding microenvironment are capable of initiating a regenerative molecular response similar to that observed in adult WT rodents. The data presented in this investigation suggest that the increased susceptibility of presymptomatic SOD1 FMNs to axotomy-induced cell death and, by extrapolation, disease progression, is not intrinsic to the MN, but rather involves a dysregulated response by the non-neuronal cells in the surrounding neuropil.
MATERIALS AND METHODS
Animals and surgical procedures
For all of the experiments in this study, female mice were obtained from Jackson Laboratory (Bar Harbor, ME) at 7 weeks of age, permitted 1 week to acclimate to their environment before manipulation, and used at 8 weeks of age in all experiments. Specifically, the three separate groups of mice utilized were C57BL/6 (#000664) wild-type control mice, B6SJLF1 (#100012) hybrid wild-type control mice, and B6SJL transgenic SOD1G93A (B6SJL-Tg(SOD1-G93A)1Gur; #002726) mice. C57Bl/6 wild-type mice are the most widely used inbred strain and are wild-type at all loci in their genome. B6SJL WT mice are a hybrid cross of a C57BL/6 female and an SJL male as the F1 generation, and are heterozygous for C57Bl/6 and SJL alleles at all loci in their genome (Jackson Laboratory). The transgenic SOD1 mice are also on a mixed hybrid B6SJL background, and specifically are bred from a B6SJLF1 female and a B6SJL male that is a transgenic SOD1 hemizygous carrier (Jackson Laboratory). According to Jackson Laboratory, the B6SJL transgenic SOD1 mice are maintained on the mixed B6SJL background with the offspring being a cross between an F1 and a F2 generation, in order to prevent the segregation of alleles and genetic drifting. Hereafter, the C57BL/6 wild-type mice are referred to WT, B6SJLF1 wild-type mice as B6SJL WT, and B6SJL transgenic SOD1G93A mice as SOD1. The presymptomatic SOD1 response to facial nerve axotomy was compared with the well-established axotomy-induced response that occurs in WT mice involving FMN survival and mRNA expression changes.
The mice were provided autoclaved pellets and water ad libitum, and were housed under a 12-hour light/dark cycle in microisolater cages contained within a laminar flow system to maintain a pathogen-free environment. All surgical procedures were completed in accordance with National Institutes of Health guidelines on the care and use of laboratory animals for research purposes. The mice were anesthetized with 3% isoflurane prior to and during all surgical procedures. Using aseptic techniques, the right facial nerve was exposed at its exit from the stylomastoid foramen and completely transected (Jones and LaVelle, 1985; Serpe et al., 1999). The proximal and distal facial nerve stumps were manually pushed away from each other in order to prevent reconnection. The left facial nerve remained intact, leaving the left facial nucleus to serve as an internal control for comparison purposes.
Facial motoneuron (FMN) counts
WT (n = 6), B6SJL WT (n = 5), and SOD1 (n = 6) mice received a right facial nerve axotomy at 8 weeks of age and survived for 28 days post axotomy. Because the SOD1 mice received the axotomy at 56 days of age (8 weeks) and were sacrificed at 84 days of age, the SOD1 mice were considered presymptomatic throughout the experimental time interval (Alexianu et al., 2001; Kennel et al., 1996; Warita et al., 1999; Yoshihara et al., 2002), and did not display any disease symptoms upon behavioral observation conducted at the time of sacrifice. All mice were euthanized by CO2 asphyxiation, and the brains were rapidly removed and frozen in a −30°C biphasic solution containing n-butyl bromide (62.5%) and 2-methyl butane (37.5%). Cryostat sections were collected at a thickness of 25 μm throughout the rostrocaudal extent of the facial motor nucleus. To determine the relative number of FMNs following a facial nerve axotomy, cell profile counts were performed on every 25-μm section as previously described (Canh et al., 2006; Serpe et al., 1999). Specifically, the sections were fixed in 4% paraformaldehyde for 15 minutes, washed twice in PBS for 5 minutes each, stained with 1X thionin (pH 3.5), washed in nanopure water for 5 minutes, and dehydrated through a graded ethanol series (50%, 70%, 95%, 100%) for 30 seconds each. Then, the sections were cleared in Hemo-de overnight, coverslipped with Permount, and laid flat to air-dry for 2–3 days.
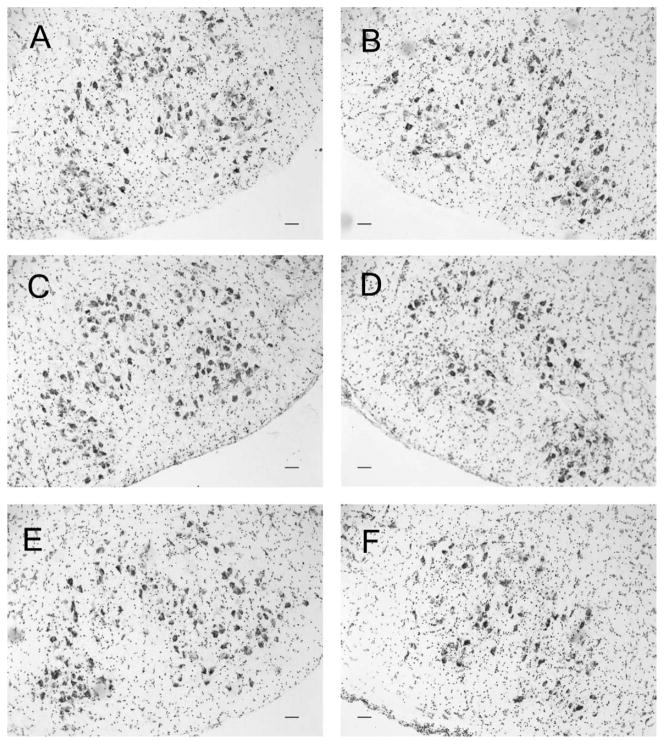
The 1X working thionin solution used in this experiment was prepared by using 38 ml of 1 M thionin acetate (Sigma, St. Louis, MO, #T3387), plus the addition of 560 ml of nanopure water and 200 ml of a sodium acetate/glacial acetic acid working buffer. The sodium acetate/glacial acetic acid working buffer is comprised of 20 ml of 1 M socium acetate (Sigma #71183) and 180 ml of 1 M glacial acetic acid (Sigma #A9967), and then 1-ml additions of the 1 M glacial acetic acid were added to reach a pH of 3.5. The sections were matched so that the rostral and caudal ends of the uninjured control facial nuclei and the axotomized facial nuclei were aligned for each mouse (Serpe et al., 1999). Animal groups were coded by one investigator and subsequently analyzed under blind conditions by a second investigator, who was unaware of the group divisions. Representative photomicrographs of thionin-stained control and axotomized facial motor nuclei (Fig. 1) were obtained with an Olympus microscope by using a QImaging camera (Retiga 2000R) and Image-Pro software (version 6.3). The images were converted to black and white in Image-Pro, and then transferred into Adobe illustrator for figure development; only the size of the images was manipulated and no other modifications were made to the contrast or brightness.
Figure 1.
Representative photomicrographs of thionin-stained control and axotomized facial motor nuclei 28 days after facial nerve axotomy. Control (A,C,E) and axotomized (B,D,F) facial motor nuclei of wild-type (WT; A,B), B6SJL WT (C,D), and presymptomatic SOD1 (E,F) mice. Scale bar = 100 μm in a–F.
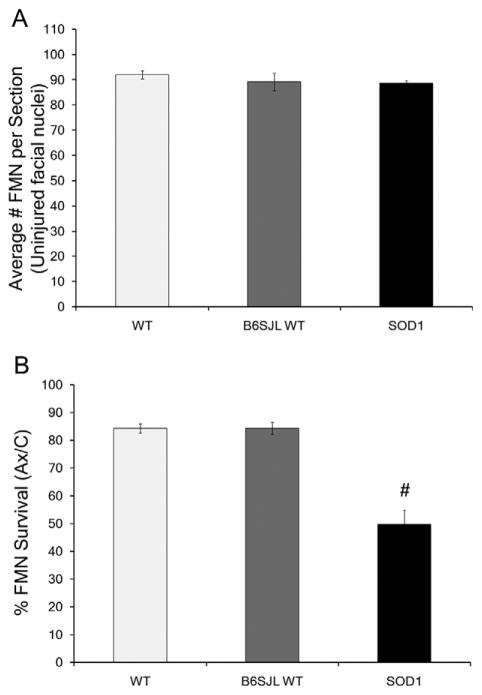
To calculate the average number of FMNs per section, the total number of FMNs in the uninjured control nucleus was divided by the total number of sections counted for that nucleus (Fig. 2A). To calculate the mean percent of FMN survival, the total number of FMNs in the axotomized nucleus was divided by the total number of FMNs in the uninjured control nucleus, and multiplied by 100% (Fig. 2B). The FMN counts were presented as the average number of FMNs per section ± the standard error of the mean (Fig. 2A), or as the average percent of FMN survival ± the standard error of the mean (Fig. 2B). The Abercrombie correction factor [N = n × T/(T + D)], where N is the actual number of cells, n is the number of nuclear profiles, T is the section thickness (25 μm), and D is the average diameter of nuclei (Coggeshall, 1992), was used to compensate for overcounting of large cells that may extend throughout sequential sections. The average diameter of nuclei (D) was determined from a minimum of 20 FMN nuclei for each mouse in each group (ImagePro software), and measured in WT mice as 18.8 μm for control and 18.5 μm for axotomized FMN nuclei, in B6SJL WT mice as 18.5 μm for control and 19.2 μm for axotomized FMN nuclei, and in SOD1 mice as 18.3 μm for control and 18.6 μm for axotomized FMN nuclei. FMN profiles were counted based on their morphology containing visible nuclei and nucleoli (n). Statistical analysis was accomplished by using a one-way ANOVA, followed by the Student-Newman-Keuls post hoc multiple comparison test, with significance at P < 0.05 (GB-Stat School Pak).
Figure 2.
Assessment of facial motoneuron (FMN) levels in WT, B6SJL WT, and presymptomatic SOD1 facial motor nuclei 28 days after facial nerve axotomy. A: Average number of facial motoneurons (FMN) per section ± SEM in the uninjured control facial nuclei of WT, B6SJL WT, and SOD1 mice, following axotomy of the contralateral facial nerve. B: Average percent survival ± SEM of FMN from axotomized facial nuclei of WT, B6SJL WT, and SOD1 mice, relative to the uninjured control nuclei. One-way ANOVA with Student-Newman-Keuls multiple comparison post hoc test: # represents a significant difference compared with wild-type (WT), at P < 0.05.
Laser microdissection
All mice were euthanized by CO22 asphyxiation, and the brains were rapidly removed and frozen in a −30°C biphasic solution containing n-butyl bromide (62.5%) and 2-methyl butane (26.5%). WT and SOD1 mice were sacrificed without injury (n = 3), and at 3, 7, 14, or 28 days post axotomy (n = 6–9/time point/group). Two separate groups of B6SJL WT mice were sacrificed at 7 (n = 3) and 28 (n = 4) days post axotomy for strain comparison with WT mice. Because the SOD1 mice received a right facial nerve axotomy at 8 weeks of age (56 days of age) and were sacrificed before or at 4 weeks following axotomy (28 days post axotomy and 84 days of age), the SOD1 mice were still in the presymptomatic stage (Alexianu et al., 2001; Kennel et al., 1996; Warita et al., 1999; Yoshihara et al., 2002). Coronal cryosections were collected at a thickness of 25 μm throughout the rostrocaudal extent of the facial motor nucleus. The tissue sections were thaw-mounted onto Leica glass polyethylene (PEN) foil membrane-slides (Nuhsbaum, McHenry, IL), and stored at −80°C. The frozen tissue sections were allowed to acclimate to −20°C prior to laser microdissection.
Each slide, containing 8–10 tissue sections, was then fixed, stained, and laser microdissected to minimize RNA degradation. Specifically, the tissue underwent a quick fixation in 100% ethanol for 1 minute and was then washed twice in 0.1% DEPC-treated nanopure water for 15 seconds each, quickly stained for 30 seconds with 2X thionin, washed twice in 0.1% DEPC-treated nanopure water for 15 seconds each, and dehydrated in a three-step graded ethanol series for 30 seconds each (70%, 90%, and 100%, respectively) prior to air-drying in an unsealed, covered container for 3 minutes (Mesnard et al., 2010). The 2X working thionin solution (pH 3.5) used in this study was developed by using 76 ml thionin acetate stock (Sigma #T3387) in DEPC-treated nanopure water, plus the addition of 522 ml of DEPC-treated nanopure water and 200 ml of a sodium acetate/glacial acetic acid working buffer. The control and axotomized facial motor nuclei were laser microdissected by using a Leica AS laser microdissector (Nuhsbaum) controlled manually (Mesnard et al., 2010). All laser microdissected tissue samples were collected into 65 μl of extraction buffer (Arcturus PicoPure RNA Isolation Kit; Applied Biosystems, Foster City, CA) added to the collection tube caps. Thus, two samples were collected for each mouse, as all of the laser microdissected sections of control facial motor nuclei were pooled together separately from all of the laser microdissected sections of axotomized facial motor nuclei.
Semiquantitative real-time RT-PCR
From laser microdissected facial motor nucleus collected samples, total cellular RNA was isolated with the PicoPure RNA Isolation Kit (Arcturus #KIT0204), including a DNase treatment step (Qiagen, Chatsworth, CA, #79254). Complementary DNA was obtained by using Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, #11904-018) according to the manufacturer’s instructions. Semiquantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed by using the iCycler iQ detection system (BioRad, Hercules, CA) for 25-μl PCR reactions (Fargo et al., 2008; Jones et al., 2000; Mesnard et al., 2010; Sharma et al., 2009). RT-PCR cycle parameters included a 95°C initial denaturing step for 10 minutes, followed by 45 cycles of three repeated steps of denaturing at 95°C for 30 seconds, annealing for 30 seconds at the optimal annealing temperature (TA) for each primer set, and extension at 65°C for 30 seconds. PCR primers were designed from published mouse sequences, consisting of 15–24 nucleotides each and producing ~100 base-pair amplicons (Table 1). The TA for each primer set was determined experimentally, by using either normal, uninjured whole mouse brain cDNA or mouse spleen cDNA as the template, over a temperature gradient selected according to the designed primer’s estimated annealing temperature. To determine the specificity of the amplified product, a melt curve analysis was performed immediately after the amplification protocol and compared with the experimentally determined melt temperatures (Tm) for each gene investigated (Table 1).
TABLE 1.
RT-PCR Primers Designed From Published Murine Complementary DNA Sequences1
| Gene | Primer sequence | Accession no. | TA (°C) | TM (°C) | Amplicon length (bp) |
|---|---|---|---|---|---|
| βII Tubulin | F 5′-TGGCAACAAATATGTACCTC-3′ R 5′-GAATGGTCCCGACCTC-3′ |
NM009450 | 54 | 84 | 79 |
| GAP-43 | F 5′-CCTAAGGAAAGTGCCCGACAG-3′ R 5′-CAGGTGGGGGCAACGTGG-3′ |
BC028288 | 54 | 84 | 102 |
| Hn1 | F 5′-TTGGGAACTGCACAGCGTGAGC-3′ R 5′-GCAGGGAAAGACAGCGGTACAT-3′ |
U90123 | 55 | 80 | 90 |
| CX3CL1 | F 5′-GCCATGTTTGCTTACCAGA-3′ R 5′-TGGCACCAGGACGTATGAGTT-3′ |
BC006650 | 55 | 87 | 117 |
| BDNF | F 5′-CCATAAGGACGCGGACTTG-3′ R 5′-GACATGTTTGCGGCATCCA-3′ |
X55573 | 54 | 82 | 120 |
| GFAP | F 5′-AGAACAACCTGGCTGCGTAT-3′ R 5′-TCTCCTCCTCCAGCGATTC-3′ |
AF332062 | 55 | 84 | 93 |
| Caspase 8 | F 5′-CGGTGAAGAACTGCGTTTC-3′ R 5′-CGCCAGTCAGGATGCTAAG-3′ |
AF067834 | 53 | 81 | 123 |
| PACAP | F 5′-GGGGAATTCTGAGGTAATGAC-3′ R 5′-GACAATGCCCACTTGAGTTAG-3′ |
D14716 | 56 | 84 | 109 |
| TNFα | F 5′-ATTCACTGGAGCCTCGAATG-3′ R 5′-AGGAAGGCCTGAGATCTTATC-3′ |
X02611 | 53 | 82 | 102 |
| IFNγ | F 5′-GCCATCAGCAACAACATAAGC-3′ R 5′-GTTGACCTCAAACTTGGCAATA-3′ |
K00083 | 53 | 80 | 111 |
| GAPDH | F 5′-GAACATCATCCCTGCATCCA-3′ R 5′-CCAGTGAGCTTCCCGTTCA-3′ |
M32599 | 50-57 | 81 | 78 |
The optimal annealing temperatures (TA) and the peak melt curve temperatures (TM) were determined experimentally.
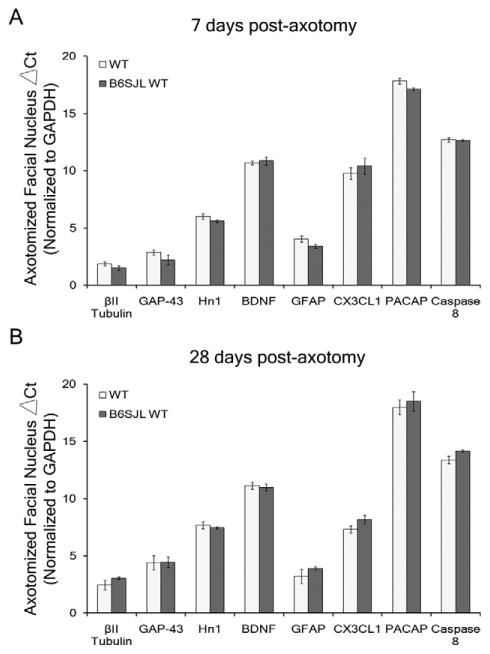
Relative mRNA expression levels acquired during the PCR amplification were analyzed by using the ΔΔCT method against the well-established axotomized facial nucleus internal standard, glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Ando et al., 2003; Raivich et al., 1998). The ΔCt is the difference between the threshold cycle level for the gene of interest and the threshold cycle level for GAPDH within each sample, which is calculated for the control and axotomized nuclei separately. Then, the difference between the axotomized ΔCt and the control ΔCt equals the ΔΔCt value. The mRNA expression levels were presented as the average ΔCt levels of mRNA expression ± the standard error of the mean for the uninjured control nuclei (Table 2) and for the axotomized nuclei (Fig. 3). As previously published by our laboratory (Fargo et al., 2008; Mesnard et al., 2010), the change in mRNA expression is given as the percent change, by (2−ΔΔCt − 1) × 100, of the axotomized samples relative to the control samples. The mRNA expression changes are presented as the average percent change ± the standard error of the mean (Figs. 4–6). The mRNA expression baseline levels or axotomy-induced changes were statistically compared by using a two-way ANOVA (group × time, for each gene individually), followed by the Student-Newman-Keuls multiple comparison post hoc test, with significance at P < 0.05 (GB-Stat School Pak).
TABLE 2.
Average ΔCt levels ± SEM for Baseline mRNA Expression1
| Gene | 7 days post axotomy
|
28 days post axotomy
|
||||
|---|---|---|---|---|---|---|
| WT | B6SJL WT | SOD1 | WT | B6SJL WT | SOD1 | |
| βII tubulin | 3.21 ± 0.12 | 3.10 ± 0.23 | 3.10 ± 0.08 | 3.40 ± 0.24 | 3.50 ± 0.21 | 3.44 ± 0.28 |
| GAP-43 | 6.34 ± 0.15 | 6.73 ± 0.52 | 6.58 ± 0.11 | 6.78 ± 0.17 | 6.50 ± 0.25 | 6.54 ± 0.23 |
| Hn1 | 8.58 ± 0.31 | 8.37 ± 0.48 | 8.30 ± 0.07 | 7.90 ± 0.21 | 8.43 ± 0.15 | 7.60 ± 0.30 |
| BDNF | 12.29 ± 0.21 | 11.73 ± 0.41 | 12.24 ± 0.32 | 12.46 ± 0.29 | 12.03 ± 0.37 | 12.72 ± 0.42 |
| GFAP | 5.40 ± 0.30 | 5.97 ± 0.07 | 5.60 ± 0.51 | 5.54 ± 0.34 | 5.97 ± 0.44 | 5.00 ± 0.62 |
| CX3CL1 | 7.61 ± 0.19 | 6.93 ± 0.27 | 7.12 ± 0.30 | 6.70 ± 0.21 | 7.13 ± 0.07 | 7.28 ± 0.26 |
| PACAP | 20.41 ± 0.19 | 20.57 ± 0.13 | 19.67 ± 0.63 | 19.82 ± 0.24 | 20.07 ± 0.28 | 19.76 ± 0.24 |
| Caspase 8 | 14.45 ± 0.25 | 15.03 ± 0.18 | 14.28 ± 0.37 | 15.26 ± 0.27 | 14.70 ± 0.21 | 14.60 ± 0.30 |
In the uninjured control (left) facial nucleus of wild-type (WT), B6SJL WT, and SOD1 mice at 7 and 28 days post axotomy of the right facial nucleus.
Figure 3.
Axotomy-induced mRNA expression levels in the axotomized nucleus of WT and B6SJL WT mice. A,B: Average ΔCt levels of mRNA expression ± SEM in WT and B6SJL WT axotomized facial nuclei for βII tubulin, GAP-43, Hn1, BDNF, GFAP, CX3CL1, PACAP, and Caspase 8 standardized to GAPDH at 7 (A) and 28 (B) days post axotomy. ΔCt is the difference between the threshold cycle level for the gene of interest and the threshold cycle level for GAPDH, the internal standard, within each sample. One-way ANOVA with Student-Newman-Keuls multiple comparison post hoc test: # represents a significant difference compared with wild-type (WT), at P < 0.05.
Figure 4.
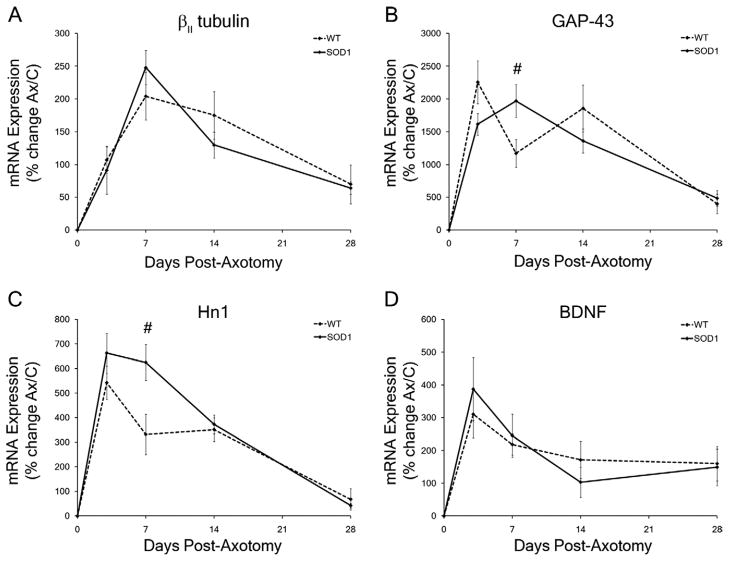
Pro-survival and pro-regeneration mRNA expression levels in WT and presymptomatic SOD1 facial motor nuclei in response to facial nerve axotomy. A–D: Average percent of mRNA expression ± SEM in the axotomized facial nuclei relative to the uninjured control nuclei in wild-type (WT) and SOD1 mice. Time-course of mRNA expression includes no injury (0), 3, 7, 14, and 28 days post axotomy for βII tubulin (A), GAP-43 (B), Hn1 (C), and BDNF (D). Two-way ANOVA (group × time, for each gene individually) with Student-Newman-Keuls multiple comparison post hoc test: # represents a significant difference compared with wild-type (WT), at P < 0.05.
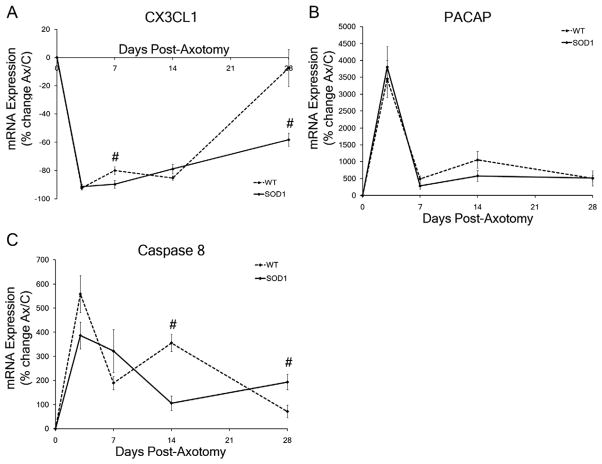
Figure 6.
Motoneuron-neuropil interaction mRNA expression levels in wild-type (WT) and presymptomatic SOD1 facial motor nuclei in response to facial nerve axotomy. A–C: Average percent of mRNA expression ± SEM in the axotomized facial nuclei relative to the uninjured control nuclei in WT and SOD1 mice. Time-course of mRNA expression includes no injury (0), 3, 7, 14, and 28 days post axotomy for CX3CL1 (A), PACAP (B), and Caspase 8 (C). Two-way ANOVA (group × time, for each gene individually) with Student-Newman-Keuls multiple comparison post hoc test: # represents a significant difference compared with WT, at P < 0.05.
Gel electrophoresis
Gel electrophoresis was accomplished by using RT-PCR products for genes induced by axotomy, with below detectable levels of mRNA expression in the uninjured control nuclei, as previously described (Mesnard et al., 2010). Electrophoresis was performed by using Criterion precast 10% TBE gels (BioRad #345-0052) in a Criterion cell vertical electrophoresis system (BioRad) for 90 minutes at 100 V, using 1X TBE running buffer (10X TBE nucleic acid electrophoresis buffer; BioRad #161-0770) which contained 100 μl SYBR Green I nucleic acid gel stain (Invitrogen #S7567). PCR-amplified products were collected from the associated wells within 96-well PCR plates and diluted 4:1 with nucleic acid sample loading buffer (BioRad #161-0767), resulting in 12-μl sample and 3 μl buffer. Each gel contained a well loaded with a 100 base-pair (bp) molecular marker (BioRad #170-8361), and the remaining wells contained PCR products for GAPDH (positive control), PCR products for the gene of interest, and non-reverse-transcribed PCR products (negative control) from each sample. The gels were scanned on a STORM 860 PhosphorImager (Molecular Dynamics, Sunnyvale, CA) by using ImageQuant software for visualization.
RESULTS
The current investigation examined the hypothesis that the axotomized SOD1 FMNs would elicit a differential FMN survival and regeneration-associated gene expression response compared with that occurring after axotomy in nontransgenic, WT mice. In order to assess this hypothesis experimentally, and utilizing previously published results of a “typical” axotomy response, we first determined whether the numbers of FMNs, the FMN survival response, the baseline levels of mRNA expression, and the axotomy-induced mRNA expression response of hybrid B6SJL WT were similar to the well-established WT response. We then used the more commonly recognized and investigated WT mouse strain for comparison purposes with the SOD1 cellular and molecular axotomy-induced responses.
Baseline levels of FMN numbers and gene expression
To assess the numbers of FMNs prior to injury, the average number of FMNs per section ± SEM in uninjured control WT and B6SJL WT facial motor nuclei were compared. The photomicrographs in Figure 1 are representative sections from uninjured control and axotomized facial nuclei of WT, B6SJL WT, and SOD1 mice. No statistically significant differences were observed between the average number of FMNs per section in uninjured control (left) facial nuclei of WT and B6SJL WT mice (Fig. 1A and C, respectively), with an average of 92 ± 2 and 89 ± 3 FMNs per section, respectively, at 28 days post axotomy of the right facial nerve (Fig. 2A). These results demonstrate that both mouse strains used in the present study had similar FMN numbers without injury.
To determine whether FMN survival levels were altered by the hybrid B6SJL background, the average percent of FMN survival ± SEM was assessed and compared in WT and B6SJL WT facial nuclei. In agreement with our previous findings (Serpe et al., 2003), FMN survival levels in WT mice were 84 ± 1.7%, relative to the uninjured control nucleus at 28 days post axotomy (Figs. 1B, 2B). In B6SJL WT mice, FMN survival levels were similar to WT at 84 ± 2.2% relative to the uninjured control nucleus at 28 days post axotomy (Figs. 1D, 2B). These results indicate that both mouse strains used in this study demonstrated similar FMN survival levels after axotomy.
To confirm that a mixed B6SJL background strain does not alter the baseline gene expression levels, the mRNA expression levels in the uninjured control and axotomized facial nuclei of WT and B6SJL WT mice were compared, and the results are represented as the average ΔCt levels ± SEM standardized to GAPDH. In accordance with the FMN count data demonstrating no difference in FMN numbers without injury, no statistically significant differences in the average ΔCt levels (baseline levels) of mRNA expression for βII tubulin, GAP-43, Hn1, BDNF, GFAP, CX3CL1, PACAP, and caspase 8 were observed between B6SJL WT and WT uninjured control (left) facial nuclei at 7 or 28 days after axotomy of the right facial nerve (Table 2). Furthermore, no statistically significant differences in the average ΔCt levels of mRNA expression for βII tubulin, GAP-43, Hn1, BDNF, GFAP, CX3CL1, PACAP, and caspase 8 were observed between B6SJL WT and WT axotomized facial nuclei at 7 or 28 days post axotomy (Fig. 3A and B, respectively). These results suggest that the genetic backgrounds of the mice used in this study had similar baseline and axotomy-induced mRNA expression levels for the constitutively expressed genes investigated.
No effect of the SOD1 mutation or disease on FMN numbers or baseline gene expression
To determine whether the SOD1 mutation or disease had an effect on the number of FMNs prior to facial nerve axotomy, we assessed the average number of FMNs per section ± SEM in the uninjured control facial nuclei of SOD1 mice compared with both WT and B6SJL WT mice. No significant difference was revealed when comparing the average number of FMNs per section in the uninjured control facial nucleus of SOD1 mice (89 ± 1) with either WT or B6SJL WT uninjured control facial nuclei (Figs. 1A,C,E, 2A). Thus, the SOD1 mutation or disease had no impact on the number of FMNs without injury.
To assess whether the SOD1 mutation or disease influenced the baseline gene expression levels in the facial motor nucleus, the average ΔCt mRNA expression levels ± SEM in the SOD1 uninjured control facial nuclei were compared with WT and B6SJL WT. No statistically significant differences were detected in the average ΔCt levels (baseline levels) of mRNA expression for βII tubulin, GAP-43, Hn1, BDNF, GFAP, CX3CL1, PACAP, and caspase 8 in the SOD1 uninjured control (left) nuclei at 7 or 28 days after axotomy of the right facial nucleus compared with WT and B6SJL WT (Table 2). In addition, no statistically significant differences were observed in the average ΔCt levels of mRNA expression for βII tubulin, GAP-43, Hn1, BDNF, GFAP, CX3CL1, PACAP, and caspase 8 between the uninjured control (left) nuclei of SOD1 mice at 7 or 28 days after axotomy of the right facial nucleus, which refers to 63 and 84 days of age, respectively (Table 2). These results suggest that neither the SOD1 mutation nor disease onset had any effect on the baseline mRNA expression levels in the SOD1 facial nuclei at the time-points and for the constitutively expressed genes investigated. The molecular expression quantification and FMN counts without injury further confirmed that at the age of the SOD1 mice used in the current study, mice were still presymptomatic.
Presymptomatic SOD1 FMN are more susceptible to axotomy-induced death
To examine whether peripheral nerve injury impacts SOD1 FMN survival, presymptomatic SOD1 mice received a right facial nerve axotomy, and FMN survival levels ± SEM were assessed and compared with WT and B6SJL WT mice. In contrast to the FMN survival levels in WT and B6SJL WT mice (Figs. 1B,D, 2B), presymptomatic SOD1 mice exhibited a significant decrease in FMN survival, with only 50 ± 5% of the FMNs remaining in the axotomized nucleus, relative to the uninjured control nucleus at 28 days post axotomy (Figs. 1F, 2B; P < 0.05). Therefore, presymptomatic SOD1 FMN were more susceptible to axotomy-induced cell death compared with WT FMN.
Axotomy-induced pro-survival and pro-regeneration gene expression in WT vs. SOD1 facial nuclei
To establish whether pro-survival and/or pro-regenerative gene expression in SOD1 mice differed from WT, the average percent change of mRNA expression ± SEM for βII tubulin, GAP-43, Hn1, and BDNF was examined in WT and presymptomatic SOD1 facial nuclei following facial nerve axotomy. βII tubulin is involved in initiating injured neuronal regeneration through restructuring of the neuronal cytoskeleton (Jones and Oblinger, 1994; Jones et al., 1999b; Sharma et al., 2009). An upregulation in βII tubulin mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 4A; F4,55 = 21.75; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in βII tubulin mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (107 ± 20%, 204 ± 36%, 175 ± 36%, and 69 ± 29% respectively; P p0.05), relative to the uninjured control nucleus (Fig. 4A). In the axotomized SOD1 facial nucleus, a significant increase also occurred in βII tubulin mRNA expression at 3, 7, 14, and 28 days post axotomy (91 ± 37%, 248 ± 26%, 130 ± 20%, and 64 ± 10%, respectively; P < 0.05), relative to the uninjured control nucleus (Fig. 4A). No statistically significant differences were observed in βII tubulin mRNA expression between the axotomized WT and SOD1 facial nuclei (Fig. 4A; F1,55 = 0.07899; P = 0.7797).
GAP-43 has been associated with growth cone development, compensatory sprouting, and regenerating neuronal axons (Akazawa et al., 2004; Jones et al., 1997a; Palacios et al., 1994; Tetzlaff et al., 1991). An upregulation in GAP-43 mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 4B; F4,52 = 32.958; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in GAP-43 mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (2,257 ± 328%, 1,170 ± 214%, 1,856 ± 358%, and 398 ± 146%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 4B). In the axotomized SOD1 facial nucleus, a significant increase also occurred in GAP-43 mRNA expression at 3, 7, 14, and 28 days post axotomy (1,614 ± 167%, 1,968 ± 250%, 1,360 ± 182%, and 482 ± 118%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 4B). GAP-43 mRNA expression was found to be significantly increased in the axotomized SOD1 facial nucleus at 7 days post axotomy compared with the axotomized WT response (Fig. 4B; F = 9.9328; P < 0.01).
Although the specific function of Hn1 is unknown in the central nervous system, Hn1 is produced by developing and regenerating MNs, and therefore has been designated as a regeneration-associated gene (Zujovic et al., 2005). An upregulation in Hn1 mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 4C; F4,53 = 43.2246; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in Hn1 mRNA expression was observed at 3, 7, and 14 days post axotomy (543 ± 69%, 332 ± 82%, and 351 ± 48%, respectively; P < 0.01), relative to the uninjured control nucleus, whereas Hn1 mRNA expression was not affected by axotomy at 28 days post axotomy (68 ± 43%; Fig. 4C). In the axotomized SOD1 facial nucleus, a significant increase also occurred in Hn1 mRNA expression at 3, 7, 14, and 28 days post axotomy (663 ± 82%, 625 ± 74%, 372 ± 39%, and 42 ± 9%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 4C). Hn1 mRNA expression was found to be significantly increased in the axotomized SOD1 facial nucleus at 7 days post axotomy compared with the axotomized WT response (Fig. 4C; F = 15.0785; P < 0.01).
BDNF is secreted by MNs and non-neuronal cells, and supports MN survival during development and after axonal injury (Kobayashi et al., 1996; Meyer et al., 1992; Serpe et al., 2005; Widenfalk et al., 2001; Xin et al., 2008). An upregulation in BDNF mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 4D; F4,54 = 12.0931; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in BDNF mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (310 ± 72%, 219 ± 33%, 171 ± 57%, and 160 ± 53%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 4D). In the axotomized SOD1 facial nucleus, a significant increase also occurred in BDNF mRNA expression at 3, 7, 14, and 28 days post axotomy (388 ± 97%, 245 ± 66%, 103 ± 47%, and 149 ± 56%, respectively; P < 0.05), relative to the uninjured control nucleus (Fig. 4D). No statistically significant differences were observed in BDNF mRNA expression between the axotomized WT and SOD1 facial nuclei (Fig. 4D; F1,54 = 0.02114; P = 0.8849).
Axotomy-induced neuropil-specific gene expression in WT vs. SOD1 facial nuclei
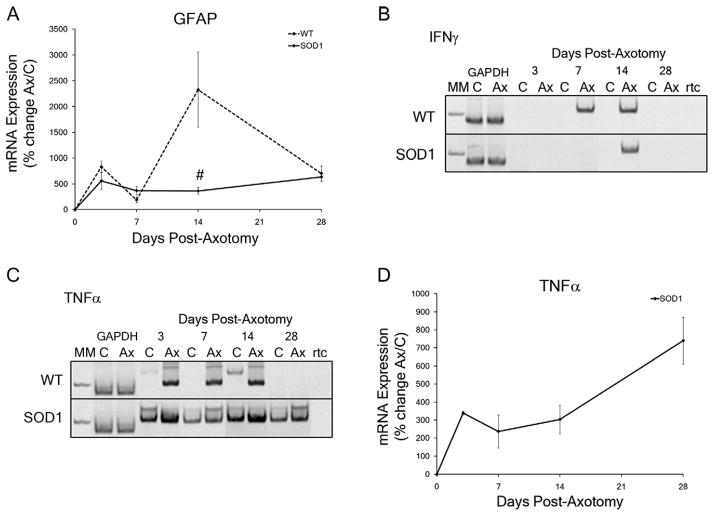
To determine whether neuropil-specific gene expression in SOD1 mice differed from WT mice after axotomy, changes in mRNA expression for GFAP, IFNγ, and TNFα were analyzed in WT and presymptomatic SOD1 facial nuclei after facial nerve axotomy. Astrocyte cytoarchitec-ture is composed of GFAP, and it is elevated in hypertrophic astrocytes to support the rearrangement of structural and contractile components (Bignami et al., 1972; Graeber and Kreutzberg, 1988). An upregulation in the average percent change of GFAP mRNA expression ± SEM in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 5A; F1,54 = 9.43325; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in GFAP mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (833 ± 115%, 190 ± 49%, 2,329 ± 728%, and 704 ± 150%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 5A). In the axotomized SOD1 facial nucleus, a significant increase also occurred in GFAP mRNA expression at 3, 7, 14, and 28 days post axotomy (560 ± 165%, 369 ± 79%, 363 ± 71%, and 637 ± 92%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 5A). GFAP mRNA expression in the axotomized SOD1 facial nucleus was significantly decreased compared with WT at 14 days post axotomy (Fig. 5A; F = 33.91221; P < 0.01), with a main effect between the WT and SOD1 groups (F1,54 = 8.2542; P = 0.0058).
Figure 5.
Neuropil-specific mRNA expression levels in wild-type (WT) and presymptomatic SOD1 facial motor nuclei in response to facial nerve axotomy. A: Average percent of mRNA expression ± SEM in the axotomized facial nuclei relative to the uninjured control nuclei in WT and SOD1 mice. Time-course of mRNA expression includes no injury (0), 3, 7, 14, and 28 days post axotomy for GFAP. B,C: Electrophoresis of RT-PCR products from control and axotomized WT and SOD1 facial nuclei at 3, 7, 14, and 28 days post axotomy for IFNγ (B; 111 base-pair amplicon) and TNFα (C; 102 base-pair amplicon), along with the molecular weight marker (MM) 100 base-pair band, the internal standard (GAPDH), and a no reverse-transcriptase control (rtc). D: Average percent of mRNA expression ± SEM in the axotomized SOD1 facial nuclei relative to the uninjured control nuclei. Time-course of mRNA expression includes no injury (0), 3, 7, 14, and 28 days post axotomy for TNFα. Two-way ANOVA (group × time, for each gene individually) with Student-Newman-Keuls multiple comparison post hoc test: # represents a significant difference compared with WT, at P < 0.05.
IFNγ is detected only in the neuropil of the facial nucleus after axotomy, and is secreted by proinflammatory immune cells (Mesnard et al., 2010; Raivich et al., 1998; Schoenborn and Wilson, 2007; Xin et al., 2008). IFNγ mRNA expression was below detectable levels in either the WT or SOD1 control facial motor nucleus, and therefore, a percent change in expression cannot be calculated (Fig. 5B, upper and lower panels). IFNγ mRNA expression was detectable in WT and SOD1 axotomized facial motor nuclei at specific time-points following axotomy with a base-pair amplicon length resembling the theoretical 111 base-pair amplicon, relative to the 100 base-pair molecular weight band (Fig. 5B, upper and lower panels). In the axotomized WT facial nucleus, IFNγ mRNA expression was induced by 7 days post axotomy, was maintained through 14 days post axotomy, and was below detectable levels at 28 days post axotomy (Fig. 5B, upper panel). In contrast, in the axotomized SOD1 facial nucleus, IFNγ mRNA expression was not induced until 14 days post axotomy and was below detectable levels at 28 days post axotomy (Fig. 5B, lower panel).
TNFα is a proinflammatory cytokine secreted by non-neuronal cells surrounding injured FMNs and is capable of directly inducing apoptosis upon binding to tumor necrosis factor receptor 1 (Bigini and Mennini, 2004; Hanisch, 2002; Mesnard et al., 2010). TNFα mRNA expression was below detectable levels in the WT uninjured control facial nuclei at all time-points investigated, and therefore, a percent change in expression cannot be calculated (Fig. 5C, upper panel). TNFα mRNA expression was detectable in WT axotomized facial motor nuclei at specific time-points following axotomy with a base-pair amplicon length resembling the theoretical 102 base-pair amplicon, relative to the 100 base-pair molecular weight band (Fig. 5C, upper panel). In the axotomized WT facial nucleus, TNFα mRNA expression was induced by 3 days post axotomy, was maintained until 14 days post axotomy, and was below detectable levels at 28 days post axotomy (Fig. 5C, upper panel). In contrast to WT, TNFα mRNA expression was detectable in the SOD1 uninjured control facial nuclei at all time-points investigated (Fig. 5C, lower panel). Additionally, in the axotomized SOD1 facial nucleus, TNFα mRNA expression was significantly upregulated at 3, 7, 14, and 28 days post axotomy (339 ± 10%, 237 ± 91%, 304 ± 79%, and 740 ± 130%, respectively; P < 0.05), relative to the uninjured control nucleus (Fig. 5D).
Axotomy-induced FMN-neuropil interaction gene expression in WT vs. SOD1 facial nuclei
To determine whether SOD1 mRNA expression for genes regulated by or regulating the interaction of FMNs with the surrounding neuropil differed from WT, the average percent change of mRNA expression ± SEM for CX3CL1, PACAP, and caspase 8 in WT and presymptomatic SOD1 facial nuclei in response to facial nerve axotomy was analyzed. CX3CL1 is an MN-specific chemokine cleaved/secreted rapidly after facial nerve axotomy; it binds to its cognate receptor, CX3CR1, on surrounding microglia cells (Harrison et al., 1998; Verge et al., 2004; Zhuang et al., 2007). A downregulation in CX3CL1 mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 6A; F4,54 = 201.5223; P < 0.0001). In the axotomized WT facial nucleus, a significant downregulation in CX3CL1 mRNA expression was observed at 3, 7, and 14 days post axotomy (−94 ± 1%, −80 ± 3%, and −84 ± 1%, respectively; P < 0.01), relative to the uninjured control nucleus, whereas CX3CL1 mRNA expression was not affected by axotomy at 28 days post axotomy (−8 ± 20%; Fig. 6A). In the axotomized SOD1 facial nucleus, a significant decrease also occurred in CX3CL1 mRNA at 3, 7, and 14 days post axotomy (−90 ± 2%, −90 ± 3%, and −72 ± 3%, respectively; P < 0.01) relative to the uninjured control nucleus (Fig. 6A). In contrast to the WT response, CX3CL1 mRNA expression in the SOD1 axotomized facial nucleus at 28 days post axotomy was significantly downregulated also at 28 days post axotomy (−57 ± 6%, respectively; P < 0.01), relative to the uninjured control nucleus (Fig. 6A). CX3CL1 mRNA expression was found to be significantly decreased in the axotomized SOD1 facial nucleus at 7 and 28 days post axotomy, compared with the axotomized WT response (Fig. 6A; P < 0.05), with a main effect between WT and SOD1 groups (F1,54 = 17.6361; P = 0.0001).
PACAP, expressed specifically by MNs and in the facial motor nucleus, mediates neuroprotection through functioning as a neurotransmitter, neurotrophic factor, and neuroimmune modulator (Armstrong et al., 2008; Mesnard et al., 2010; Shioda et al., 2006). An upregulation in PACAP mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 6B; F4,45 = 30.14798; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in PACAP mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (3453 ± 1268%, 490 ± 94%, 1,797 ± 352%, and 508 ± 34%, respectively; P < 0.01) relative to the uninjured control nucleus (Fig. 6B). In the axotomized SOD1 facial nucleus, a significant upregulation in PACAP mRNA expression was also observed at 3, 7, 14, and 28 days post axotomy (4,022 ± 932%, 239 ± 86%, 503 ± 228%, and 536 ± 161%, respectively; P < 0.01) relative to the uninjured control nucleus (Fig. 6B). No statistically significant differences were observed in PACAP mRNA expression between the axotomized WT and SOD1 facial nuclei (Fig. 6B; F1,45 = 0.07531; P = 0.785).
Caspase 8 is activated upon death receptor activation as an initiator caspase in apoptotic signaling cascades within MNs and non-neuronal cells undergoing apoptotic cell death (Friedlander, 2003; Martin et al., 2005; Thorburn, 2004). An upregulation in caspase 8 mRNA expression in response to axotomy occurred in both the WT and SOD1 facial motor nuclei with a main effect of postoperative time (Fig. 6C; F4,49 = 29.63785; P < 0.0001). In the axotomized WT facial nucleus, a significant upregulation in caspase 8 mRNA expression was observed at 3, 7, 14, and 28 days post axotomy (405 ± 177%, 190 ± 27%, 350 ± 21%, and 52 ± 24%, respectively; P < 0.01) relative to the uninjured control nucleus (Fig. 6C). In the axotomized SOD1 facial nucleus, a significant upregulation in caspase 8 mRNA expression was also observed at 3, 7, 14, and 28 days post axotomy (375 ± 87%, 285 ± 84%, 70 ± 38%, and 164 ± 42%, respectively; P < 0.01) relative to the uninjured control nucleus (Fig. 6C). Caspase 8 mRNA expression was found to be significantly decreased in the axotomized SOD1 facial nucleus at 14 days post axotomy (F = 28.8269; P < 0.01) and significantly increased at 28 days post axotomy (F = 8.3884; P < 0.05) compared with the axotomized WT response (Fig. 6C), with a statistical interaction determined between WT and SOD1 groups and postoperative time (F4,49 = 7.25568; P = 0.0001).
DISCUSSION
Previously, we characterized the phenotypic molecular response of facial motoneurons (FMNs) and the surrounding neuropil in WT mice in two distinct subregions of the facial motor nucleus with contrasting degrees of axotomy-induced cell death, and revealed two unexpected findings (Mesnard et al., 2010). Although virtually all the axotomized FMNs in the ventromedial (VM) subnucleus survive axotomy and significant axotomy-induced FMN cell death occurs in the ventrolateral (VL) subnucleus, the FMNs in both subregions of the facial nucleus present a molecular expression profile that is both pro-survival and pro-axonal regeneration. Furthermore, the non-neuronal cells in the microenvironment (neuropil) surrounding axotomized VL FMN cell bodies exhibit substantially different regional specific molecular expression profiles that could be causative in the subnuclear distinctions in FMN survival levels subsequent to axotomy. Therefore, we hypothesize that the cellular and molecular determinants underlying FMN survival after injury may not be directly dependent on intrinsic phenotypic responses of motoneurons (MN) to injury, but rather on environmental cues that impact neuronal signaling pathways associated with survival or cell death.
The current investigation examined the hypothesis that the presymptomatic SOD1 FMNs would elicit an “atypical” gene expression response following facial nerve transection axotomy compared with that occurring in nontransgenic, WT mice. In order to assess this hypothesis, we utilized previously published results of a “typical” axotomy response to select specific genes (Mesnard et al., 2010). The genes selected for analysis in this study were chosen because their cellular function and localization in response to facial nerve axotomy have been well described by us and others and, as such, were utilized as a cohort to define a “typical” profile response. The molecular response of presymptomatic SOD1 FMN and surrounding non-neuronal cells to facial nerve axotomy was compared with the well-documented “typical” axotomy-induced response in WT mice. Our results indicate that, in agreement with the literature (Mariotti et al., 2002a), presymptomatic SOD1 FMNs are considerably more susceptible to axotomy-induced cell death than WT, yet, from a molecular perspective, they display the “typical” phenotypic MN molecular response to injury. Analysis of the SOD1 non-neuronal response to peripheral axotomy revealed a distinct molecular profile compared with WT, and lends support to our hypothesis that MN survival after injury/disease is directly dependent upon molecular cues derived in the surrounding neuropil, whereas the injured/diseased MN are intrinsically preparing the molecular machinery for regeneration.
βII tubulin, GAP-43, and Hn1 are three MN-specific genes associated with regeneration or axonal elongation and are upregulated following injury (Harrison et al., 1998; Hoffman, 1989; Jones et al., 1997a; Jones and Oblinger, 1994; Skene, 1989; Zujovic et al., 2005). In response to facial nerve axotomy in WT mice, βII tubulin, GAP-43, and Hn1 mRNA expression levels were upregulated in the VM and VL facial subnuclear regions (Mesnard et al., 2010), and similar expression patterns were observed in the present study. Despite heightened cell death, βII tubulin, GAP-43, and Hn1 mRNA upregulation expression profiles were similar between WT and SOD1 mice. GAP-43 expression is elevated in ALS mouse models and patients during disease progression as well (Kage et al., 1998; Parhad et al., 1992). WT and SOD1 facial nuclei exhibit equivalent mRNA expression patterns for BDNF, a neurotrophic factor known to support MN survival and secreted by MN and non-neuronal cells (Dougherty et al., 2000; Kobayashi et al., 1996; Serpe et al., 2005), in response to facial nerve axotomy. Therefore, comparable to the similar mRNA profiles we previously observed in the axotomized WT VM and VL facial subnuclear regions (Mesnard et al., 2010), presymptomatic SOD1 mice responded to facial nerve axotomy with pro-survival/pro-regeneration gene expression levels that resemble the WT response, despite exaggerated FMN cell death.
Non-neuronal cells, including microglia, astrocytes, and infiltrating T cells, are activated in the neuropil surrounding FMNs following axotomy (Byram et al., 2004; Graeber and Kreutzberg, 1988; Graeber et al., 1988; Raivich et al., 1998). IFNγ, a cytokine secreted primarily by proinflammatory immune cells (Schoenborn and Wilson, 2007), mRNA induction occurs in the WT facial nucleus neuropil after facial nerve axotomy (Mesnard et al., 2010; Raivich et al., 1998). The present results indicated an induction of IFNγ mRNA in the axotomized WT and presymptomatic SOD1 facial nuclei, in accordance with the temporal infiltration pattern of CD3+ T cells (Raivich et al., 1998). However, IFNγ mRNA expression was only detected at one time-point in the axotomized presymptomatic SOD1 facial nuclei. The difference in IFNγ mRNA induction between the presymptomatic SOD1 and WT neuropil suggests a potential for an irregular SOD1 peripheral immune cell recruitment response to axotomy, contrasting to the WT response.
Astrocytes become hypertrophic following facial nerve axotomy with an upregulation of GFAP mRNA and protein prior to ensheathing the injured FMN with extension of their processes within two weeks after injury (Graeber and Kreutzberg, 1988; Jones et al., 1997c; Kreutzberg, 1996b; Laskawi and Wolff, 1996). GFAP mRNA expression was upregulated in the axotomized WT facial nucleus with a peak expression that occurred within two weeks after axotomy, demonstrating a similar expression pattern previously determined in WT VM and VL facial subnuclear regions (Mesnard et al., 2010). In the present study, the presymptomatic SOD1 facial nucleus following axotomy exhibited GFAP mRNA upregulation levels that were substantially lower and distinct from the high peak expression in WT. In an ALS rat model and in ALS patient spinal cords, the astrocyte response adjacent to dynamically changing MNs is abnormal, displaying weak activation, poor GFAP immunoreactivity, and a decrease in astrocytic processes surrounding MN somata (O’Reilly et al., 1995; Rafalowska et al., 2009; Rafalowska and Podlecka, 1998). The present results are consistent with the aforementioned studies and confirm a diminished, or dysregulated, astrocyte reaction flanking injured/diseased MNs in presymptomatic SOD1 mice.
TNFα, a potent proinflammatory cytokine capable of inducing apoptosis, is induced in the WT facial nucleus neuropil following a facial nerve axotomy (Mesnard et al., 2010; Raivich et al., 1998), and mRNA induction was confirmed in the present study in WT mice. TNFα predominantly localizes to activated microglia (Sawada et al., 2007), and is increased during disease progression in ALS patients and in SOD1 mice (Elliott, 2001; Hensley et al., 2003; Poloni et al., 2000; Sanagi et al., 2010). In contrast to the WT induction response, analysis of the TNFα mRNA levels in the presymptomatic SOD1 mice unexpectedly revealed constitutive mRNA expression prior to axotomy, in uninjured control facial nuclei where there is no FMN cell loss, and it was also significantly up-regulated in response to axotomy.
These results correlate with previous observations that reactive microglia have been observed in the uninjured SOD1 facial motor nucleus (Mariotti and Bentivoglio, 2000). In addition, microglial and macrophage activation in presymptomatic SOD1 peripheral nerves and spinal cords display phagocytic features (Graber et al., 2010; Sanagi et al., 2010). The current findings indicate that microglial cells are activated presymptomatically in SOD1 mice expressing TNFα mRNA, which is normally not expressed, and may develop a phagocytic phenotype instead of one that supports MN survival after axotomy. Therefore, in contrast to similar pre-survival/pro-regenerative expression patterns following axotomy by the FMN, the molecular expression profiles of presymptomatic SOD1 astrocytes, microglia, and/or peripheral immune cells deviated significantly from the normal WT response, and possibly are contributing to the pathogenesis that results in MN degeneration.
Fractalkine (CX3CL1) is a chemokine localized to neurons and is involved in microglial-neuronal interactions (Chapman et al., 2000; Harrison et al., 1998; Verge et al., 2004); gene expression is downregulated initially after axotomy (Harrison et al., 1998). As previously observed in the WT VM and VL facial subnuclear regions following axotomy (Mesnard et al., 2010), the current results confirmed a similar downregulated CX3CL1 mRNA expression pattern in the axotomized WT facial nucleus prior to returning to baseline levels. However, CX3CL1 mRNA expression downregulation in the SOD1 facial motor nucleus after axotomy was maintained throughout the time-course investigated. The results suggest either that the ability of SOD1 microglia to respond to, and interact with, injured SOD1 FMNs presymptomatically may be disrupted, or that the inability of injured SOD1 FMNs to return CX3CL1 mRNA expression levels back to baseline may be due to the increased FMN cell loss.
PACAP mRNA expression has been localized to FMNs following facial nerve axotomy (Armstrong et al., 2003; Mesnard et al., 2010), and this expression requires a functional adaptive immune system (Armstrong et al., 2004a,b, 2006). Furthermore, PACAP is a neuroprotective peptide capable of inhibiting proinflammatory cytokine production, such as TNFα, in vitro (Delgado et al., 2003; Kim et al., 2000); a deficiency of PACAP in vivo results in delayed axonal regeneration of axotomized FMN (Armstrong et al., 2008). In the present study, PACAP mRNA was significantly upregulated in the axotomized facial motor nuclei of WT and presymptomatic SOD1 mice early after axotomy, and although at decreased levels, remained elevated throughout the time-course. The results further confirmed that the pre-symptomatic SOD1 FMN response to axotomy is similar to WT.
Caspase 8 is a cysteine protease activated during apoptotic signaling cascades following the activation of TNF receptor 1 (TNFR1) or Fas death receptors by TNFα or FasL, respectively (Friedlander, 2003; Thorburn, 2004). Previous work established that caspase 8 mRNA is upregulated in the facial nucleus following axotomy (Mesnard et al., 2010). In the current investigation, caspase 8 mRNA was significantly upregulated in the WT facial nucleus early after axotomy, and a similar pattern of upregulation occurred in the axotomized presymptomatic SOD1 facial nuclei. However, significant differences in presymptomatic SOD1 caspase 8 mRNA expression levels were elicited over the time-course following axotomy compared with WT. Further examination of the cellular source for caspase 8 mRNA expression will establish whether the altered mRNA response in the presymptomatic SOD1 facial nuclei after axotomy is due to an irregular microglial activation and proliferation response compared with WT, in which the microglia are not responding to apoptotic signals. Collectively, the present results suggest that presymptomatic SOD1 non-neuronal cells exhibited a dysregulated gene expression response to axotomy, unlike the WT response, that could contribute to abnormal neuronal-neuropil cellular interactions and either promote MN death or fail to support MN survival.
In summary, neuronal-immune, glial-immune, and neuronal-glial interactions that occur in response to nerve injury or disease are highly dynamic and complex. The results presented in the current investigation suggest that the peripheral MN molecular response to injury is inherently geared toward survival and axonal regeneration, regardless of MN survival levels after axotomy. In contrast, the state of the microenvironment present in the facial nucleus at the time of injury may be a key element in directing the fate of MNs disconnected from their target, as is the case with either peripheral nerve transection or the axonal die-back pathology well documented for ALS (Carrasco et al., 2010; Fischer et al., 2004; Hegedus et al., 2007; Kennel et al., 1996; Park and Vincent, 2008). The present results further suggest an aberrant non-neuronal, rather than neuronal, response that either promotes MN cell death or fails to support MN survival in the SOD1 mouse. We hypothesize that this dysregulated response involves astrocytes, microglia, and peripheral immune cells. Peripheral inflammation prior to axonal damage alters the susceptibility of MNs to cell death (Dupuis and Loeffler, 2009). Thus, proinflammatory factors secreted centrally and/or peripherally during the presymptomatic ALS stage could influence that ability of MN to survive and regenerate once target disconnection has occurred.
We have also defined a physiologically relevant, endogenous mechanism of immune-mediated neuroprotection involving peripheral adaptive immune system interaction with centrally located microglia and/or astroctyes to support MN survival after injury in the WT mouse (Jones et al., 2005; Wainwright et al., 2009; Xin et al., 2008, 2010). In the future, extrapolation of that endogenous mechanism of immune-mediated neuroprotection to the ALS disease situation will open a novel avenue into the investigation of neural-immune dysfunction in the etiology of ALS.
Acknowledgments
Grant sponsor: the Les Turner Amyotrophic Lateral Sclerosis Foundation (to N.A.M. and K.J.J.); National Institutes of Health; Grant number: NS40433 (to K.J.J. and V.M.S.).
The authors thank Tom Alexander for his assistance with real-time RT-PCR and RT-PCR primer design.
LITERATURE CITED
- Akazawa C, Tsuzuki H, Nakamura Y, Sasaki Y, Ohsaki K, Nakamura S, Arakawa Y, Kohsaka S. The upregulated expression of sonic hedgehog in motor neurons after rat facial nerve axotomy. J Neurosci. 2004;24:7923–7930. doi: 10.1523/JNEUROSCI.1784-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Ando Y, Liang Y, Ishigaki S, Niwa J, Jiang Y, Kobayashi Y, Yamamoto M, Doyu M, Sobue G. Caspase-1 and -3 mRNAs are differentially upregulated in motor neurons and glial cells in mutant SOD1 transgenic mouse spinal cord: a study using laser microdissection and real-time RT-PCR. Neurochem Res. 2003;28:839–846. doi: 10.1023/a:1023258923002. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Rodriguez W, Cheung-Lau G, Trinh V, Waschek JA. Restoration of axotomy-induced PACAP gene induction in SCID mice with CD4+ T-lymphocytes. Neuroreport. 2004a;15:2647–2650. doi: 10.1097/00001756-200412030-00018. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004b;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Coute AC, Ngo DH, Waschek JA. Impairment of axotomy-induced pituitary adenylyl cyclase-activating peptide gene expression in T helper 2 lymphocyte-deficient mice. Neuroreport. 2006;17:309–312. doi: 10.1097/01.wnr.0000199465.54907.74. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigini P, Mennini T. Immunohistochemical localization of TNFalpha and its receptors in the rodent central nervous system. Methods Mol Med. 2004;98:73–80. doi: 10.1385/1-59259-771-8:073. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh MY, Serpe CJ, Sanders V, Jones KJ. CD4(+) T cell-mediated facial motoneuron survival after injury: distribution pattern of cell death and rescue throughout the extent of the facial motor nucleus. J Neuroimmunol. 2006;181:93–99. doi: 10.1016/j.jneuroim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, Bichler EK, Seburn KL, Pinter MJ. Nerve terminal degeneration is independent of muscle fiber genotype in SOD1 mice. PLoS One. 2010;5:e9802. doi: 10.1371/journal.pone.0009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol. 2003;73:155–164. doi: 10.1189/jlb.0702372. [DOI] [PubMed] [Google Scholar]
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Curr Opin Pharmacol. 2009;9:341–346. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Elliott JL. Cytokine upregulation in a murine model of familial amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2001;95:172–178. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. J Neurotrauma. 2008;25:561–566. doi: 10.1089/neu.2007.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Graber DJ, Hickey WF, Harris BT. Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:8. doi: 10.1186/1742-2094-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Kreutzberg GW. Delayed astrocyte reaction following facial nerve axotomy. J Neurocytol. 1988;17:209–220. doi: 10.1007/BF01674208. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Tetzlaff W, Streit WJ, Kreutzberg GW. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988;85:317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haenggeli C, Kato AC. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci Lett. 2002;335:39–43. doi: 10.1016/s0304-3940(02)01140-0. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, Mou S, Pye QN, Stewart C, West M, West S, Williamson KS. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Hoffman PN. Expression of GAP-43, a rapidly transported growth-associated protein, and class II beta tubulin, a slowly transported cytoskeletal protein, are coordinated in regenerating neurons. J Neurosci. 1989;9:893–897. doi: 10.1523/JNEUROSCI.09-03-00893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto A, Nakamura S, Akiguchi I, Hirano A. Differential expression between synaptic vesicle proteins and pre-synaptic plasma membrane proteins in the anterior horn of amyotrophic lateral sclerosis. Acta Neuropathol. 2002;103:179–187. doi: 10.1007/s004010100449. [DOI] [PubMed] [Google Scholar]
- Jones KJ, LaVelle A. Changes in nuclear envelope invaginations in axotomized immature and mature hamster facial motoneurons. Brain Res. 1985;353:241–249. doi: 10.1016/0165-3806(85)90212-3. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Oblinger MM. Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J Neurosci. 1994;14:3620–3627. doi: 10.1523/JNEUROSCI.14-06-03620.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KJ, Drengler SM, Oblinger MM. Gonadal steroid regulation of growth-associated protein GAP-43 mRNA expression in axotomized hamster facial motor neurons. Neurochem Res. 1997a;22:1367–1374. doi: 10.1023/a:1022071123255. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Durica TE, Jacob SK. Gonadal steroid preservation of central synaptic input to hamster facial motoneurons following peripheral axotomy. J Neurocytol. 1997b;26:257–266. doi: 10.1023/a:1018596316465. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Kinderman NB, Oblinger MM. Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: effects of axotomy and testosterone. Neurochem Res. 1997c;22:1359–1366. doi: 10.1023/a:1022019106417. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Coers S, Storer PD, Tanzer L, Kinderman NB. Androgenic regulation of the central glia response following nerve damage. J Neurobiol. 1999a;40:560–573. doi: 10.1002/(sici)1097-4695(19990915)40:4<560::aid-neu11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Storer PD, Drengler SM, Oblinger MM. Differential regulation of cytoskeletal gene expression in hamster facial motoneurons: effects of axotomy and testosterone treatment. J Neurosci Res. 1999b;57:817–823. [PubMed] [Google Scholar]
- Jones KJ, Alexander TD, Brown TJ, Tanzer L. Gonadal steroid enhancement of facial nerve regeneration: role of heat shock protein 70. J Neurocytol. 2000;29:341–349. doi: 10.1023/a:1007157105835. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Serpe CJ, Byram SC, Deboy CA, Sanders VM. Role of the immune system in the maintenance of mouse facial motoneuron viability after nerve injury. Brain Behav Immun. 2005;19:12–19. doi: 10.1016/j.bbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Kage M, Ikemoto A, Akiguchi I, Kimura J, Matsumoto S, Kimura H, Tooyama I. Primary structure of GAP-43 mRNA expressed in the spinal cord of ALS patients. Neuroreport. 1998;9:1403–1406. doi: 10.1097/00001756-199805110-00028. [DOI] [PubMed] [Google Scholar]
- Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20:3622–3630. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi NR, Bedard AM, Hincke MT, Tetzlaff W. Increased expression of BDNF and trkB mRNA in rat facial motoneurons after axotomy. Eur J Neurosci. 1996;8:1018–1029. doi: 10.1111/j.1460-9568.1996.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996a;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Principles of neuronal regeneration. Acta Neurochir Suppl. 1996b;66:103–106. doi: 10.1007/978-3-7091-9465-2_18. [DOI] [PubMed] [Google Scholar]
- Laskawi R, Wolff JR. Changes in glial fibrillary acidic protein immunoreactivity in the rat facial nucleus following various types of nerve lesions. Eur Arch Otorhinolaryngol. 1996;253:475–480. doi: 10.1007/BF00179953. [DOI] [PubMed] [Google Scholar]
- Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Mariotti R, Bentivoglio M. Activation and response to axotomy of microglia in the facial motor nuclei of G93A superoxide dismutase transgenic mice. Neurosci Lett. 2000;285:87–90. doi: 10.1016/s0304-3940(00)01034-x. [DOI] [PubMed] [Google Scholar]
- Mariotti R, Cristino L, Bressan C, Boscolo S, Bentivoglio M. Altered reaction of facial motoneurons to axonal damage in the presymptomatic phase of a murine model of familial amyotrophic lateral sclerosis. Neuroscience. 2002a;115:331–335. doi: 10.1016/s0306-4522(02)00448-7. [DOI] [PubMed] [Google Scholar]
- Mariotti R, Tongiorgi E, Bressan C, Kristensson K, Bentivoglio M. Priming by muscle inflammation alters the response and vulnerability to axotomy-induced damage of the rat facial motor nucleus. Exp Neurol. 2002b;176:133–142. doi: 10.1006/exnr.2002.7908. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnard NA, Alexander TD, Sanders VM, Jones KJ. Use of laser microdissection in the investigation of facial motoneuron and neuropil molecular phenotypes after peripheral axotomy. Exp Neurol. 2010;225:94–103. doi: 10.1016/j.expneurol.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Abe K. Spinal anterior horn has the capacity to self-regenerate in amyotrophic lateral sclerosis model mice. J Neurosci Res. 2009;87:3639–3648. doi: 10.1002/jnr.22156. [DOI] [PubMed] [Google Scholar]
- Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Young WG, Yeung G, Shah RA, Gordon JW, Bloom FE, Morrison JH, Hof PR. Differential vulnerability of oculomotor, facial, and hypoglossal nuclei in G86R superoxide dismutase transgenic mice. J Comp Neurol. 2000;416:112–125. doi: 10.1002/(sici)1096-9861(20000103)416:1<112::aid-cne9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- O’Reilly SA, Roedica J, Nagy D, Hallewell RA, Alderson K, Marklund SL, Kuby J, Kushner PD. Motor neuron-astrocyte interactions and levels of Cu,Zn superoxide dismutase in sporadic amyotrophic lateral sclerosis. Exp Neurol. 1995;131:203–210. doi: 10.1016/0014-4886(95)90042-x. [DOI] [PubMed] [Google Scholar]
- Palacios G, Mengod G, Sarasa M, Baudier J, Palacios JM. De novo synthesis of GAP-43: in situ hybridization histochemistry and light and electron microscopy immunocytochemical studies in regenerating motor neurons of cranial nerve nuclei in the rat brain. Brain Res Mol Brain Res. 1994;24:107–117. doi: 10.1016/0169-328x(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Parhad IM, Oishi R, Clark AW. GAP-43 gene expression is increased in anterior horn cells of amyotrophic lateral sclerosis. Ann Neurol. 1992;31:593–597. doi: 10.1002/ana.410310605. [DOI] [PubMed] [Google Scholar]
- Park KH, Vincent I. Presymptomatic biochemical changes in hindlimb muscle of G93A human Cu/Zn superoxide dismutase 1 transgenic mouse model of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2008;1782:462–468. doi: 10.1016/j.bbadis.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, Mora G, Camana C, Mazzini L, Bachetti T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalowska J, Podlecka A. Does the pathological factor in amyotrophic lateral sclerosis (ALS) damage also astrocytes? Folia Neuropathol. 1998;36:87–93. [PubMed] [Google Scholar]
- Rafalowska J, Dziewulska D, Gadamski R, Chrzanowska H, Modrzewska-Lewczuk M, Grieb P. Is the spinal cord motoneuron exclusively a target in ALS? Comparison between astroglial reactivity in a rat model of familial ALS and in human sporadic ALS cases. Neurol Res. 2009 doi: 10.1179/174313209X414542. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Sanagi T, Yuasa S, Nakamura Y, Suzuki E, Aoki M, Warita H, Itoyama Y, Uchino S, Kohsaka S, Ohsawa K. Appearance of phagocytic microglia adjacent to motoneurons in spinal cord tissue from a presymptomatic transgenic rat model of amyotrophic lateral sclerosis. J Neurosci Res. 2010;88:2736–2746. doi: 10.1002/jnr.22424. [DOI] [PubMed] [Google Scholar]
- Sawada T, Sano M, Omura T, Omura K, Hasegawa T, Funahashi S, Nagano A. Spatiotemporal quantification of tumor necrosis factor-alpha and interleukin-10 after crush injury in rat sciatic nerve utilizing immunohistochemistry. Neurosci Lett. 2007;417:55–60. doi: 10.1016/j.neulet.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Schiefer J, Kampe K, Dodt HU, Zieglgansberger W, Kreutzberg GW. Microglial motility in the rat facial nucleus following peripheral axotomy. J Neurocytol. 1999;28:439–453. doi: 10.1023/a:1007048903862. [DOI] [PubMed] [Google Scholar]
- Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Byram SC, Sanders VM, Jones KJ. Brain-derived neurotrophic factor supports facial motoneuron survival after facial nerve transection in immunodeficient mice. Brain Behav Immun. 2005;19:173–180. doi: 10.1016/j.bbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2009;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Shibata N. Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology. 2001;21:82–92. doi: 10.1046/j.1440-1789.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, Kitamura Y. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–560. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Mesnard NA, Beahrs TR, Politis CM, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve axotomy in CCR3-deficient mice. ASN Neuro. 2009;1:00024. doi: 10.1042/AN20090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sharma K, Grisotti G, Roos RP. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol Dis. 2009;35:234–240. doi: 10.1016/j.nbd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warita H, Itoyama Y, Abe K. Selective impairment of fast anterograde axonal transport in the peripheral nerves of asymptomatic transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1999;819:120–131. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19:2284–2302. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2008;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Mesnard NA, Serpe CJ, Sanders VM, Jones KJ. IL-10 within the CNS is necessary for CD4(+) T cells to mediate neuroprotection. Brain Behav Immun. 2010;25:820–829. doi: 10.1016/j.bbi.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci U S A. 2008;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J, Takeuchi H, Doyu M, Sobue G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- Zang DW, Cheema SS. Degeneration of corticospinal and bulbospinal systems in the superoxide dismutase 1(G93A G1H) transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2002;332:99–102. doi: 10.1016/s0304-3940(02)00944-8. [DOI] [PubMed] [Google Scholar]
- Zang DW, Lopes EC, Cheema SS. Loss of synaptophy-sin-positive boutons on lumbar motor neurons innervating the medial gastrocnemius muscle of the SOD1G93A G1H transgenic mouse model of ALS. J Neurosci Res. 2005;79:694–699. doi: 10.1002/jnr.20379. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujovic V, Luo D, Baker HV, Lopez MC, Miller KR, Streit WJ, Harrison JK. The facial motor nucleus transcriptional program in response to peripheral nerve injury identifies Hn1 as a regeneration-associated gene. J Neurosci Res. 2005;82:581–591. doi: 10.1002/jnr.20676. [DOI] [PubMed] [Google Scholar]