Abstract
Objectives
Evaluate the outcomes of preclinical diastolic dysfunction in diabetic patients
Background
Studies have reported a high prevalence of preclinical diastolic dysfunction among patients with diabetes mellitus.
Methods
We identified all diabetic patients with a tissue Doppler assessment of diastolic function in Olmsted County, MN from 2001 to 2007. Diastolic dysfunction was defined as a Doppler mitral E/e′ ratio >15. The main outcome was the development of heart failure (HF). Secondary outcomes were the development of atrial fibrillation and death.
Results
Overall, 1,760 diabetic patients with a tissue Doppler echocardiographic assessment of diastolic function were identified; 411 patients (23%) had diastolic dysfunction. Using multivariable Cox's proportional hazard modeling, we determined that for every 1 unit increase in the mitral E/e′ ratio, the hazard of HF increased by 3% (HR=1.03, 95% CI=1.01-1.06; p=0.006) and that diastolic dysfunction was associated with the subsequent development of HF after adjustment for age, sex, body mass index, hypertension, coronary disease and echocardiographic parameters (HR=1.61, 95% CI=1.17-2.20; p=0.003). The cumulative probability of the development of HF at 5 years for diabetic patients with diastolic dysfunction was 36.9% compared to 16.8% for patients without diastolic dysfunction (P<0.001). Furthermore, diabetic patients with diastolic dysfunction had a significantly higher mortality compared to those without diastolic dysfunction.
Conclusion
We demonstrated that an increase in the E/e′ ratio in diabetic patients is associated with the subsequent development of HF and increased mortality independent of hypertension, coronary disease or other echocardiographic parameters.
Keywords: Diastolic dysfunction, diabetes mellitus, heart failure, diabetic cardiomyopathy
Introduction
Diabetes mellitus (DM) is often complicated by concomitant hypertension and associated with increased cardiovascular complications, the most common of which are coronary artery diseases and the subsequent development of heart failure (HF). However, the existence of a primary myocardial disease in diabetic patients, “diabetic cardiomyopathy”, has been proposed (1-9). The existence of diabetic cardiomyopathy was first proposed by Rubler et al. in 1972 on the basis of postmortem findings (10,11). Subsequently, abnormalities in both systolic and diastolic performance in diabetic subjects have been demonstrated in animal and human studies. The pathogenesis of this left ventricular dysfunction in diabetic patients is not clearly understood. Microangiopathy, increased extracellular collagen deposition, or abnormalities in calcium transport alone or in combination are considered to be associated with this dysfunction (12-14). Furthermore, the evidence indicates that myocardial damage in diabetic patients affects diastolic function before systolic function (15). Unfortunately, there have been few population based studies to evaluate the outcomes of preclinical diastolic dysfunction in diabetics (16).
The combination of pulsed tissue Doppler velocity of the medial mitral annulus (e′) with the early passive transmitral inflow velocity (E) has been validated as a reliable index of left ventricular filling pressure (17). Studies have demonstrated a reduction of annular e′ in type 2 diabetes mellitus and that an increased E/e′ ratio was associated with left atrial enlargement and correlated independently with glycosylated hemoglobin (11). Thus E/e′ ratio may be used to detect and follow up the progression of diastolic dysfunction in diabetic patients (18).
The objective of our study was to determine if there is an association between preclinical diastolic dysfunction in DM patients and the subsequent development of HF. We used tissue Doppler indices of diastolic dysfunction as the measure of cardiac dysfunction because tissue Doppler indices correlate well with intra-cardiac pressure tracings of ventricular filling in patients with and without diabetes mellitus. We hypothesized that diastolic dysfunction in diabetes will be associated with the increased risk for the development of subsequent HF.
Methods
Study Setting
This study was conducted in the Olmsted County, Minnesota population. Health care providers in Olmsted County include the Mayo Clinic, Olmsted Medical Center, and a handful of private practitioners. Medical records for all providers are available for review via the comprehensive record-linkage system provided by the Rochester Epidemiology Project (REP). The REP allows for the indexing of all medical records of Olmsted County residents according to clinical and pathological diagnoses, surgical procedures, and billing information. Death certificates are also indexed according to cause of death. Records are quite complete as more than 90% of the population receives care at Mayo Clinic or Olmsted Medical Center and residents are seen on average every three years at the Mayo Clinic (19). The potential of this data source has been described elsewhere (19).
Diabetes and Echocardiography Correlation Cohort
After approval by the Mayo Clinic and the Olmsted County Medical Center Institutional Review Board, we retrospectively identified all diabetic patients with a tissue Doppler assessment of diastolic function within the Olmsted County, MN population. To avoid inclusion of patients in whom the diagnosis of heart failure (HF) was triggered by the results of echocardiography, we excluded patients who were diagnosed within 30 days after the echocardiogram. Furthermore, patients were excluded if the diagnosis of HF was made prior to the echocardiogram or if severe mitral or aortic valve regurgitation or stenosis was present. The main outcome was the development of HF.
We identified patients with a new diagnosis of diabetes mellitus in Olmsted County from 1996 through 2006 using the International Classification of Disease-9th Revision (ICD-9) code 250. This code has been validated in Olmsted County previously with an accuracy of over 98% (20). Among these patients, we identified those who subsequently developed heart failure by using ICD-9 code 428. This code has also been validated in Olmsted County: 90% of patients with this code have a physician diagnosis of HF and 82% of patients with this code meet Framingham Criteria for HF (21). All echocardiograms were performed from September 2001 through June 2007. Ejection fraction was evaluated by a modification of the method of Quinones et al (22). Doppler echocardiography was performed to determine the early mitral inflow velocity (E) and tissue Doppler evaluation was performed of the medial mitral annulus velocity (e′) as previously described (17). Diastolic dysfunction was defined as an E/e′ ratio > 15 as previously described (17). Left ventricular size and wall thickness were measured using the 2-dimensional image if available. Otherwise, the M-mode measurement was utilized.
Statistical Analysis
Categorical variables were summarized as percentages and continuous variables as mean ± standard deviation. Comparison between groups was based on two sample t-tests for continuous variables and Pearson's chi-square test for categorical variables. The major endpoint was the development of heart failure. Other endpoints analyzed included death and atrial fibrillation. Kaplan-Meier analysis was performed to estimate probabilities of events and the probabilities were compared between groups using the Log rank test statistic. Univariable and multivariable associations of clinical and echocardiographic variables with each endpoint were assessed with Cox's proportional hazard modeling using the event of interest and time from 30 days after the echocardiogram to the date of event or last follow-up as the outcome. In patients without an event, the date of latest follow-up was the time of the data collection (June 2007) or date of death. Patients with missing data were excluded from the multivariable analysis. However, fitting the model with missing indicators revealed similar results. Analyses were performed using JMP, version 6.0.0 (SAS Institute Inc., Cary, NC).
Results
Of the 12,014 Olmsted County residents with diabetes mellitus, 4,571 had echocardiographic data. From this group, we identified 2,770 diabetic patients with a tissue Doppler echocardiographic assessment of diastolic dysfunction. We excluded 845 patients with a diagnosis of heart failure (HF) prior to the echocardiogram, 130 patients with a diagnosis of HF made within 30 days after the echocardiogram, 1 patient with severe mitral or aortic valve regurgitation and 34 patients with severe aortic or mitral stenosis. Overall, 1,760 diabetic patients were included in this study and 411 patients (23%) had preclinical diastolic dysfunction defined as an E/e′ > 15 without a diagnosis of HF. Average time from echocardiogram to death or latest follow-up was 2.9 ± 1.8 years. Baseline characteristics are shown in Table 1. The DM patients with diastolic dysfunction were older, were more often female and had a higher prevalence of hypertension and coronary artery diseases as compared to the DM patients without diastolic dysfunction. Echographic findings of DM patients with diastolic dysfunction include greater left atrial volume and LV mass index.
Table 1. Baseline Characteristics.
| Diastolic Dysfunction | ||||
|---|---|---|---|---|
| Characteristic | Overall (n=1,760) | Present (n=411) | Absent (n=1,349) | P-value |
|
| ||||
| Age, years | 60±14 | 67±13 | 58±14 | <0.001 |
| Male Sex, No. (%) | 863 (49%) | 160 (39%) | 703 (52%) | <0.001 |
| Body Mass Index, kg/m2 * | 33±14 | 31±9 | 33±15 | 0.01 |
| Hypertension, No. (%) | 1507 (86%) | 374 (91%) | 1133 (84%) | 0.007 |
| Coronary Disease, No. (%) | 632 (36%) | 178 (43%) | 454 (34%) | 0.007 |
| Ejection Fraction, %† | 62±9 | 61±11 | 62±8 | 0.005 |
| E/e′ ratio | 13±6 | 21±6 | 11±3 | <0.001 |
| Left Atrial Volume ‡ | 63±24 | 73±25 | 60±23 | <0.001 |
| Deceleration Time ¥ | 217±50 | 216±69 | 217±46 | 0.49 |
| Left Ventricular Mass Index, g/m2 £ | 97±25 | 107±29 | 94±23 | <0.001 |
| Septal Thickness, mm ** | 10.9±1.9 | 11.4±2.1 | 10.8±1.8 | <0.001 |
| Posterior Wall Thickness, mm *** | 10.5±1.7 | 10.9±1.8 | 10.4±1.6 | <0.001 |
| Left Ventricular Size-Systole, mm†† | 31.2±5.9 | 31.4±7.0 | 31.1±5.5 | 0.40 |
| Left Ventricular Size-Diastole, mm††† | 48.8±5.6 | 50.0±5.9 | 48.7±5.5 | 0.47 |
Body mass index available in 1748 of 1760 (99.3%) patients.
Ejection fraction available in 1692 of 1760 (96.1%) patients.
Left atrial volume available in 1690 of 1760 (96.0%) patients.
Deceleration time available in 1631 of 1760 (92.7%) patients.
Septal wall thickness was available in 1646 of 1760 (93.5%) patients.
Posterior wall thickness was available in 1637 of 1760 (93.0%) patients.
Left ventricular size-systole was available in 1546 of 1760 (84.8%) patients.
Left ventricular size-diastole was available in 1707of 1760 (97.0%) patients.
Using Cox's proportional hazard modeling, we determined that the E/e′ ratio was independently associated with the subsequent development of HF after adjustment for age, gender, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time and left ventricular mass index. This analysis suggests that for every 1 unit increase in the mitral E/e′ ratio, the risk of HF increases by 3% (HR=1.03, 95% CI=1.01-1.06; p=0.006).
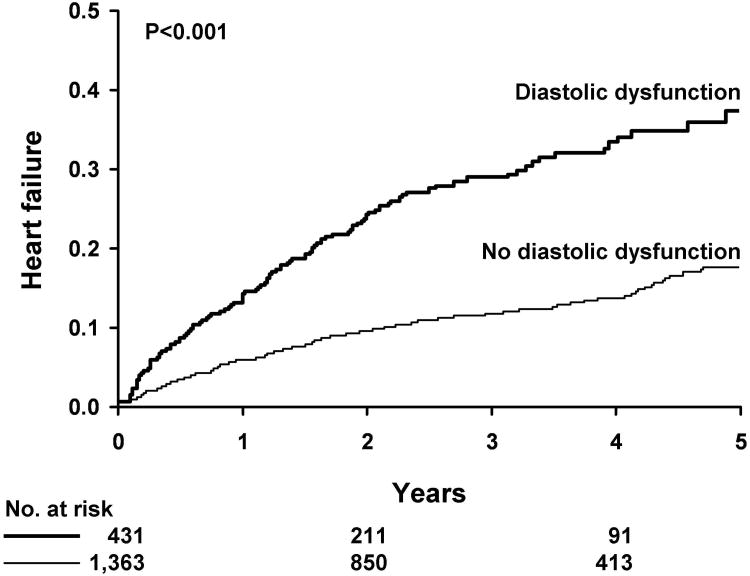
Diastolic dysfunction defined as an E/e′ > 15 was predictive of HF in Kaplan-Meier analysis (Figure 1). The cumulative probability of the development of HF for DM patients with diastolic dysfunction was 13.1% at 1 year and 36.9% at 5 years compared to 5.2% at 1 year and 16.8% at 5 years for DM patients without diastolic dysfunction (P<0.001). In multivariable Cox's proportional hazard regression analysis, we determined that diastolic dysfunction was associated with the subsequent development of HF after adjustment for age, sex, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time and left ventricular mass index (HR=1.61, 95% CI=1.17-2.20; p=0.003)(Table 2).
Figure 1. Kaplan-Meier Analysis of Diastolic Dysfunction and Subsequent Heart Failure in Diabetic Patients.
Diastolic dysfunction defined as an E/e′ > 15 is predictive of heart failure in Kaplan-Meier analysis. The cumulative probability of the development of heart failure for diabetic patients with diastolic dysfunction is 13.1% at 1 year and 36.9% at 5 years compared to 5.2% at 1 year and 16.8% at 5 years for diabetic patients without diastolic dysfunction.
Table 2. Multivariable Associations with Subsequent Heart Failure.
| Characteristic | Multivariable Analysis HR (95% CI) | P-Value |
|---|---|---|
|
| ||
| Age | 1.03 (1.02, 1.05) | <0.001 |
| Male Sex | 0.99 (0.72, 1.36) | 0.97 |
| Body Mass Index | 1.00 (0.99, 1.01) | 0.34 |
| Hypertension | 4.27 (1.92, 12.15) | <0.001 |
| Coronary Disease | 2.20 (1.62, 3.01) | <0.001 |
| Ejection Fraction | 0.96 (0.95, 0.98) | <0.001 |
| E/e′ > 15 (Diastolic Dysfunction) | 1.61 (1.17, 2.20) | 0.003 |
| Left Atrial Volume | 1.00 (1.00, 1.01) | 0.08 |
| Deceleration Time | 1.00 (1.00, 1.00) | 0.98 |
| Left Ventricular Mass Index | 1.01 (1.00, 1.01) | 0.01 |
HR=hazard ratio
Association of Diastolic Dysfunction and Death
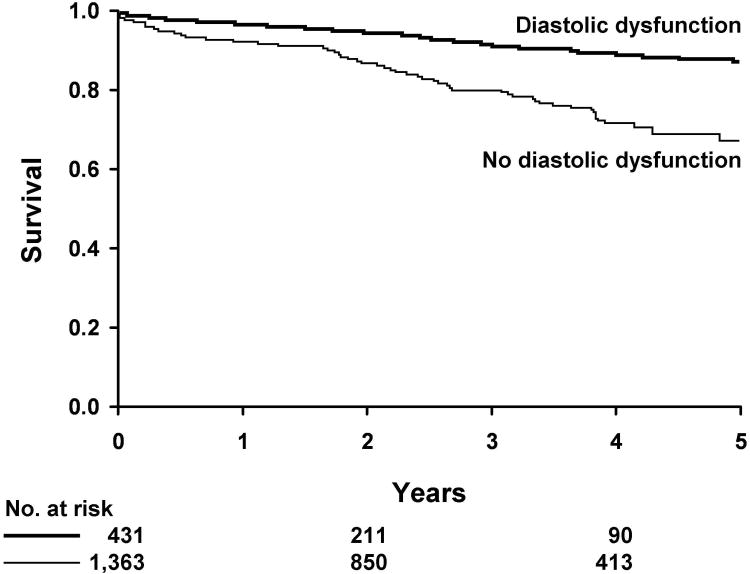
Diastolic dysfunction (defined as an E/e′ ratio > 15) was predictive of death in Kaplan-Meier analysis (Figure 2). The cumulative probability of death for diabetic patients with diastolic dysfunction was 6.9% at 1 year and 30.8% at 5 years compared to 3.1% at 1 year and 12.1% at 5 years for diabetic patients without diastolic dysfunction (P<0.001). In multivariable Cox's proportional hazard regression analysis, we determined that diastolic dysfunction was associated with death after adjustment for age, sex, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time and left ventricular mass index (HR=2.01, 95% CI=1.32-3.06; p=0.001).
Figure 2. Kaplan-Meier Analysis of Diastolic Dysfunction and Death in Diabetic Patients.
Diastolic dysfunction defined as an E/e′ ratio > 15 is predictive of death in Kaplan-Meier analysis. The cumulative probability of death for diabetic patients with diastolic dysfunction is 6.9% at 1 year and 30.8% at 5 years compared to 3.1% at 1 year and 12.1% at 5 years for diabetic patients without diastolic dysfunction.
Association of Diastolic Dysfunction and Atrial Fibrillation
In order to assess the association between diastolic dysfunction and the subsequent development of atrial fibrillation, we analyzed the 1,450 patients without a previous diagnosis of atrial fibrillation prior to the date of the echocardiogram. Among this sub-group, the cumulative probability of subsequent atrial fibrillation for diabetic patients with diastolic dysfunction was 6.8% at 1 year and 18.7% at 5 years compared to 3.1% at 1 year and 8.8% at 5 years for diabetic patients without diastolic dysfunction (P<0.001). In multivariable Cox's proportional hazard regression analysis, we determined that diastolic dysfunction was not significantly associated with the subsequent development of atrial fibrillation after adjustment for age, sex, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time and left ventricular mass index (HR=1.16, 95% CI=0.75-1.76; p=0.50).
Subgroup Analyses
In subgroup analysis of 1,356 diabetics with a diagnosis of hypertension prior to the date of echocardiography, diastolic dysfunction was independently predictive of subsequent heart failure (HR=1.67, 95% CI=1.20-2.33; p=0.003) and death (HR=2.14, 95% CI=1.36-3.36; p=0.001) after adjustment for age, sex, body mass index, coronary disease, ejection fraction, left atrial volume deceleration time and left ventricular mass index. However, after adjustment for the above variables, diastolic dysfunction was not independently associated with the subsequent development of atrial fibrillation (HR=1.01, 95% CI=0.62-1.63; p=0.95).
We used the criteria outlined by Lang and colleagues for the measurement of relative wall thickness (RWT) and the classification of left ventricle geometry. In 1,616 patients the left ventricle geometry could be defined. 554 (34%) had normal geometry, 524 (32%) had concentric remodeling, 343 (21%) had concentric hypertrophy and 195 (12%) had eccentric hypertrophy.
In multivariable analysis with adjustment for age, sex, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time, left ventricular mass index and RWT (as a continuos variable) diastolic dysfunction was independently associated with the subsequent development of heart failure (HR=1.63, 95% CI=1.20-2.21; p=002) and death (HR=2.13, 95% CI=1.43-3.15; p=002).
In multivariable analysis with adjustment for age, sex, body mass index, hypertension, coronary disease, ejection fraction, left atrial volume, deceleration time, and geometric pattern of the left ventricle (as a categorical variable) diastolic dysfunction was independently associated with the subsequent development of heart failure (HR=1.62, 95% CI=1.19-2.19; p=002) and death (HR=2.18, 95% CI=1.48-3.20; p<0.001).
Discussion
The present study is the first to use a community-based cohort of diabetic patients to determine if those with preclinical diastolic dysfunction are more likely to develop heart failure as compared to those without diastolic dysfunction. Our current findings demonstrate that preclinical diastolic dysfunction is common in patients with diabetes mellitus (DM) and confirms that preclinical diastolic dysfunction in those with DM is associated with an increased risk of the subsequent development of HF, mortality and atrial fibrillation.
Preclinical diastolic dysfunction in diabetes mellitus
Preclinical diastolic dysfunction has been broadly defined as diastolic dysfunction in patients with normal systolic function and no symptoms of heart failure (15,24). In diabetic patients, the existence of a preclinical diastolic dysfunction has been well defined and estimates of prevalence vary from 20% to 60% depending on the Doppler echocardiographic criteria that was used to define diastolic dysfunction (1-9). Several lines of evidence indicate that left ventricular (LV) diastolic dysfunction may precede LV systolic dysfunction in diabetic patients (15),(20). This early restrictive disease seen in diabetic patients is likely due to microangiopathy, interstitial fibrosis, extracellular collagen deposition, calcium transport abnormalities, and neurohormonal alterations, alone or in combination (12-14). Regan et al. carried out a cardiac catheterization study which demonstrated that normotensive, diabetic patients with normal ejection fraction without coronary artery disease and without clinical evidence of HF have an increased left-ventricular end-diastolic pressure and a decreased left ventricular end-diastolic volume (25). While systolic dysfunction, left ventricular (LV) hypertrophy, the presence of coronary disease and hypertension have all been shown to increase the risk for development of heart failure in DM patients, the prognostic impact of preclinical diastolic dysfunction in DM patients has not been well defined, but the association with the subsequent development of HF has been suspected (16).
We determined that the prevalence of preclinical LV diastolic dysfunction in our population was 23%. We also demonstrated that preclinical diastolic dysfunction in DM patients was associated with an increased risk for the subsequent development of HF and mortality after adjustment for multiple demographic, echocardiographic variables, hypertension and coronary disease. Though novel, these findings were hypothesized based on the known associations between diastolic dysfunction, HF, DM and mortality. Previously, Redfield and coworkers demonstrated that even mild diastolic dysfunction conferred a risk for increased mortality compared with subjects with normal diastolic function in the general population (24). Furthermore, DM is a well recognized risk factor for developing HF as demonstrated by the report from the Framingham Heart Study which showed that the frequency of HF was twice in DM men and five times in DM women compared to age-matched control subjects. Likewise, we have previously reported that the prevalence of DM in patients with HF is approximately 20% and DM is associated with increased mortally in patients with HF (26) while Tribouilloy and coworkers reported a markedly increased mortality in patients with HF and preserved ejection fraction among diabetics compared with non-diabetic subjects (27).
The above studies focus on the E to A ratio to diagnose diastolic dysfunction. The difference between the E to A ratio and the E/e′ ratio is physio-pathological: The E to A ratio is <1 when the relaxation is abnormal and LV filling pressure is still normal, but becomes > 1 when LV filling pressure rises. Indeed, there are limitations of a categorical variable such as the E to A ratio as shown in an analysis of The Strong Heart Study by Bella and colleagues where high values of the E to A ratio predict mortality compared to low values which do not (28). On the other hand, E/e′ ratio has a continuous behavior because its increase is always an expression of increased LV filling pressure. As we have shown with our cohort of diabetic patients, elevation of the E/e′ ratio is associated with increasing risk of subsequent heart failure.
Future directions
Despite the current medical therapy for HF and DM, our current study demonstrates that the prevalence of preclinical diastolic dysfunction is high in DM patients and associated with worse outcomes. Furthermore, we have recently reported that there is a direct correlation between the duration of diabetes mellitus and diastolic dysfunction and that significant diastolic dysfunction occurs 4 years after the onset of diabetes mellitus independent of coronary disease or hypertension (20). Hence, future studies should be carried out to test the hypothesis that screening and aggressive management of diabetic patients with preclinical diastolic dysfunction may delay the progression to HF with improved outcomes.
Study Limitations
The study is retrospective, we do not have data on medications or diabetic subtype and the study relies heavily on the ICD-9 coding variables to define heart failure. Though these codes have been validated as a diagnostic and research tool in Olmsted County, MN they do allow for potential bias. Furthermore, the patients were no recruited from the community, but were clinically referred for echocardiography by their primary physicians which may decrease the generalizability of the results. Finally, while the diversity of the Olmsted County population is increasing as shown by the 2000 census (29), characteristics of the Olmsted County population are similar to those of United States whites (19). Thus, these findings should be examined in different racial and ethnic groups.
Conclusion
This study confirms that preclinical diastolic dysfunction is prevalent in patients with DM. More importantly, we demonstrated that an increase in the E/e′ ratio in diabetic patients was associated with the subsequent HF and mortality independent of hypertension, coronary disease or other echocardiographic parameters.
Acknowledgments
Grant support: This study was funded by National Institutes of Health (HL 76611-04-P4) and made possible by the Rochester Epidemiology Project (grant #R01-AR30582 from the national Institute of Arthritis, Musculoskeletal and Skin Diseases).
Abreviations
- DM
diabetes mellitus
- E
passive transmitral left ventricular inflow velocity
- e′
tissue Doppler velocity of the medial mitral annulus during passive filling
- HF
heart failure
- HR
hazard ratio
- ICD-9
International Classification of Disease-9th Revision code
- CI
confidence interval
Footnotes
No conflicts of interest or financial disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bella JN, Devereux RB, Roman MJ, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study) American Journal of Cardiology. 2001;87:1260–5. doi: 10.1016/s0002-9149(01)01516-8. [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 3.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. Journal of the American College of Cardiology. 2006;48:1548–51. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) American Journal of Cardiology. 1991;68:85–9. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 5.Ilercil A, Devereux RB, Roman MJ, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. American Heart Journal. 2001;141:992–8. doi: 10.1067/mhj.2001.115302. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Gardin JM, Lynch JC, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. American Heart Journal. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. Journal of the American College of Cardiology. 2001;37:1943–9. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 8.Palmieri V, Bella JN, Arnett DK, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–7. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 9.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–54. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 10.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorf G, Rieger U, Koepp P. Cardiomyopathy in childhood diabetes mellitus: incidence, time of onset, and relation to metabolic control. Int J Cardiol. 1988;19:225–36. doi: 10.1016/0167-5273(88)90083-6. [DOI] [PubMed] [Google Scholar]
- 12.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 13.Spector KS. Diabetic cardiomyopathy. Clin Cardiol. 1998;21:885–7. doi: 10.1002/clc.4960211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tziakas DN, Chalikias GK, Kaski JC. Epidemiology of the diabetic heart. Coron Artery Dis. 2005;16(1):S3–S10. doi: 10.1097/00019501-200511001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. see comment. [DOI] [PubMed] [Google Scholar]
- 16.Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–51. doi: 10.2337/diacare.26.10.2949. comment. [DOI] [PubMed] [Google Scholar]
- 17.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 18.Di Bonito P, Moio N, Cavuto L, et al. Early detection of diabetic cardiomyopathy: usefulness of tissue Doppler imaging. Diabet Med. 2005;22:1720–5. doi: 10.1111/j.1464-5491.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol. 2009;103:1463–6. doi: 10.1016/j.amjcard.2009.01.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 22.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. see comment. [DOI] [PubMed] [Google Scholar]
- 25.Regan TJ, Lyons MM, Ahmed SS, et al. Evidence for cardiomyopathy in familial diabetes mellitus. Journal of Clinical Investigation. 1977;60:884–99. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–9. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction: a prospective five-year study. Heart. 2008;94:1450–5. doi: 10.1136/hrt.2007.128769. [DOI] [PubMed] [Google Scholar]
- 28.Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–33. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 29.Bureau USC. Census Data for Olmsted County. MN: United States Census; 2000. [Google Scholar]