Acute decompensated heart failure represents an enormous health care burden
Acute decompensated heart failure (ADHF) is the most common cause of hospital admission in persons over age 65, accounting for 1,000,000 admissions and over 6 million hospital days.1 The total cost of heart failure (HF) care in the United States is currently estimated at $21 billion and is projected to increase to $53 billion in 2030 with the majority of costs (80%) related to ADHF hospitalizations.2 The prognosis of patients admitted with ADHF is dismal, with a 20–30% readmission rate and a 20–30% mortality rate within six months after admission.3
Renal dysfunction is potently associated with adverse outcomes in ADHF
Studies have established the prognostic importance of renal function in patients with HF. Analysis from the second prospective randomized study of Ibopamine on mortality and efficacy (PRIME)4 was one of the first to demonstrate that estimated glomerular filtration rate (GFR) was the strongest predictor of mortality in ADHF. In the acute decompensated heart failure national registry (ADHERE), 63% of ADHF patients had moderate renal dysfunction (GFR < 60 ml/min m2) on admission and in-hospital mortality was strongly associated with the severity of renal dysfunction.5 A meta-analysis of 16 studies characterizing the association between renal impairment and mortality in HF patients indicated that one-year mortality increased incrementally across the range of renal function with a seven percent increase in risk for every 10 ml/min decrease in GFR.6 As recently reviewed,7 numerous more recent studies have confirmed the association of renal dysfunction with adverse short- and long-term outcomes in ADHF, an association which appears equally robust in ADHF patients with reduced (HFrEF) or preserved (HFpEF) ejection fraction (EF).
Acute kidney injury (AKI) during the ADHF hospitalization
In patients hospitalized with ADHF, several studies demonstrated that AKI during treatment of ADHF is common, with increases in creatinine ≥ 0.3 mg/dl occurring in 25–30% of patients. Regardless of baseline renal function, AKI is associated with poorer outcomes.7–9 In a recently proposed cardiorenal classification system, AKI during hospitalization for ADHF is termed “type 1” or “acute” cardiorenal syndrome.10 More recently, studies have demonstrated that the prognostic implications of AKI may be modified by its duration (transient versus persistent)11 and associated clinical factors (adequacy of decongestion).12, 13 Transient AKI or AKI occurring in the setting of more adequate decongestion were not associated with poorer outcomes whereas persistent AKI and AKI occurring in the presence of persistent congestion were. However, another large study found that persistent AKI was associated with poorer outcomes despite evidence of more adequate decongestion.14 While the factors mediating AKI or “type 1 cardiorenal syndrome” during an ADHF hospitalization are clearly multifactorial, the adverse prognostic implications of renal dysfunction and AKI during ADHF treatment provide strong rationale for development of therapeutic strategies that enhance decongestion while preserving renal function in ADHF.14
Pathophysiology of renal dysfunction in HF and type 1 cardiorenal syndrome
The etiologies of underlying renal dysfunction in HF and the mediators of type 1 cardiorenal syndrome are complex.15 The HF state leads to chronic renal hypo-perfusion due to impaired cardiac output 16, renal venous congestion17–19 and neurogenic and humorally mediated renal vasoconstriction. Renal dysfunction related to HF often occurs on the background of hypertensive, diabetic and atherosclerotic chronic kidney disease. This complex cardiorenal milieu may be further impacted by ADHF therapies and those used in the treatment of comorbid conditions in ADHF as well as hemodynamic alterations during the ADHF hospitalization.15
The concept of “Renal Adjuvant Therapy” for ADHF
While our understanding of the pathophysiology of renal dysfunction and type 1 cardiorenal syndrome is incomplete, existing knowledge provides several pathophysiologic “targets” that may inform design of therapies to preserve renal function and enhance the ability to attain adequate decongestion in ADHF. Inotropes or vasodilators are commonly used to increase cardiac output in ADHF and may enhance renal function via an effect on the heart and systemic vasculature. However, therapies that specifically target the kidney to enhance decongestion and preserve renal function can be considered “adjuvant” therapies for ADHF. A limited number of renal targeted therapies have been tested in ADHF.
Intra-renal adenosine receptors play a role in mediating “tubular glomerular feedback” wherein increased sodium delivery to the renal tubules with diuretic therapy results in renal vasoconstriction and proximal tubular sodium reabsorption as part of an intrinsic intra-renal homeostatic mechanism. To block this response, an adenosine 1-receptor antagonist (rolofylline) was developed and tested in a large, multicenter trial of patients with AHF and renal impairment. 20 While rolofylline showed promise in preliminary studies21, the pivotal trial showed no effect on symptom resolution, readmission rate, mortality or renal function when compared to placebo. 20, 22
Relaxin is an endogenous peptide that modulates cardiovascular adaptation to pregnancy and is a potent vasodilator which may also have beneficial effects on renal function. The Efficacy and Safety of Relaxin for the Treatment of Acute Heart Failure (RELAX-AHF) trial program tested recombinant human relaxin-2 (serelaxin) in ADHF patients with normal or increased (>125 mmHg) systolic blood pressure and included patients with preserved EF (26% of patients). Overall, relative to placebo, serelaxin improved dyspnea, had no effect on cardiovascular death or HF or renal failure hospitalization at 180 days, reduced 180 day all-cause mortality and had significant favorable effects on biomarkers reflective of congestion, renal function, hepatic function and myocardial necrosis.23,24 The degree to which renal specific effects vs systemic vasodilatation mediated the favorable effects of serelaxin in ADHF patients with normal or elevated blood pressure is unclear.
The clinical development of ularitide has paralleled that of nesiritide in many respects. Ularitide is a chemically synthesized form of urodilatin, a natriuretic peptide of renal origin which binds to the natriuretic peptide A receptor and produces hemodynamic and renal effects similar to those observed in preclinical and early clinical studies of nesiritide. In phase II dose finding studies, doses (7.5, 15 or 30 ng/kg/min) of ularitide were infused for 24 hours and produced substantial and dose related reductions in pulmonary capillary wedge pressure, systemic vascular resistance and systolic blood pressure without effects on urine output and with numerically greater but not statistically significant increases in creatinine25. Impairment in renal function was least evident with the 15 ng/kg/min dose and this dose is being tested in the ongoing Trial of Ularitide’s Efficacy and safety in patients with Acute Heart Failure (TRUE-AHF; NCT01661634) with a composite primary endpoint incorporating measures of clinical status.
There has been concern over the effects of diuretics on renal function in ADHF and particularly in ADHF patients who develop type 1 cardiorenal syndrome.26 Ultrafiltration represents a strategy to achieve decongestion without exposing the kidneys to diuretics and thus, a potential renal protective strategy. A small trial showed more effective decongestion without differential effects on renal function and a significant decrease in ADHF re-hospitalizations with ultrafiltration as compared to diuretics in ADHF.27 A recent study evaluated the effect of ultrafiltration on renal function and adequacy of decongestion in patients with type 1 cardiorenal syndrome.28 In this study, as compared to stepped pharmacologic care, ultrafiltration was associated with more severe AKI, no greater degree of decongestion and more adverse events. A larger trial is investigating the effect of ultrafiltration versus diuretics on time to HF events after ADHF hospitalization but is not specifically targeting patients with type 1 cardiorenal syndrome (NCT01474200). Thus, the potential to preserve renal function and enhance decongestion with ultrafiltration continues to be of interest in ADHF.
Rationale for the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE AHF) Trial: Low dose dopamine or nesiritide as renal adjuvant therapies
Clinically available agents have physiological properties that support their potential as renal adjuvant therapies to enhance decongestion and preserve renal function during diuretic therapy for ADHF. Two such therapies, low dose dopamine and low dose nesiritide will be independently tested against placebo in the ROSE AHF trial.
Renal dose dopamine as a renal adjuvant therapy in ADHF
Dopamine is an endogenous catecholamine with different pharmacologic effects on the systemic and renal vasculatures. Dopamine has been shown to exhibit sequential dose-dependent activation of dopaminergic, beta (β) adrenergic, and alpha (α) adrenergic receptors.29 At low doses, dopamine activates the dopamine receptors, resulting in vasodilatation of the renal, mesenteric, coronary, and cerebral vascular beds by stimulating dopamine A1 receptors and inhibition of norepinephrine release from sympathetic nerve endings by stimulating dopamine A2 receptors. At intermediate doses, dopamine activates β1-adrenergic receptors, providing inotropic and potentially, pro-arrhythmic effects. At high doses, dopamine activates α1- and α2-adrenergic receptors, providing systemic arterial vasoconstriction 30. Criteria for low (≤ 3 ug/kg/min), intermediate (4–5 ug/kg/min) and high (> 5 ug/kg/min) dose dopamine have been suggested, but individual responses may vary. In stable chronic HFrEF patients, Elkyam documented decreases in renal vascular resistance and increases in renal blood flow at dopamine doses of 2 ug/kg/ min and higher whereas cardiac output did not increase until the infusion rate reached 5 ug/kg/min even though systemic vascular resistance was lower than at baseline at all doses.31
Several studies have investigated the potential renal protective effects of low dose dopamine in patients considered at risk for development of renal failure. A systematic review and meta-analysis of these studies (61 trials; 3359 patients) indicated that most of the trials focused on patients undergoing surgery (40 trials) or receiving intravenous contrast (8 trials) with only one trial that included patients with ADHF. In this heterogeneous group of studies, dopamine did increase sodium excretion and decrease creatinine, but did not alter mortality nor the need for renal replacement therapy as compared to placebo. 32
Two small, single-center, open-label studies compared the renal effects of the combination of low dose dopamine in patients with ADHF receiving diuretic therapy. 33, 34 Both studies demonstrated preservation or improvement of renal function with low dose dopamine, whereas deterioration of renal function was observed with diuretic therapy alone.
The Dopamine in Acute Decompensated Heart Failure (DAD-HF) trial randomized 60 ADHF patients to receive high-dose IV furosemide (40 mg bolus followed by 20 mg/hour for eight hours), or low-dose IV furosemide (40 mg bolus followed by 5 mg/hour for eight hours) plus dopamine (5 μg/kg per min for eight hours).35 Urine volume was similar between the 2 groups while the incidence of AKI over the subsequent hospitalization course was higher in the high dose furosemide group (30%) than in the low dose furosemide plus dopamine group (6.7%). The Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) study was recently presented as an abstract at the late breaking trial session of the European Society of Cardiology Heart Failure 2013 Congress. The trial, which was conducted at four centers in Greece, randomized 161 ADHF patients to one of three treatment arms: High-dose furosemide (20 mg/hour); Low-dose furosemide (5 mg/hour) and Low-dose furosemide plus low-dose dopamine (5 ug/kg/min). The infusions were stopped after 8 hours, after which treatment was left to the discretion of the physician. While AKI was significantly more common in the high-dose group than in the 2 other groups at 24 hours (24% versus 7% to 11%, P<0.0001), this difference did not persist throughout the hospitalization. There were no between-group differences in the initial relief of symptoms of congestion as well as in clinical outcomes (including all-cause mortality, cardiovascular mortality, and heart failure rehospitalization) at 60 days or 1 year. The trial was stopped short of its enrollment goal of n=450 because an interim analysis showed that the primary outcome of all-cause mortality and HF rehospitalization at 60 days was unlikely to show a difference between the groups and because the group receiving dopamine had significantly higher heart rates, a finding which may suggest activation of β1-adrenergic receptors by the relatively high dose of dopamine used in the trial. (Giamouzis G, et al “The dopamine in acute decompensated heart failure II trial (DAD-HFII) Late Breaking Trials 2, Heart Failure 2013. abstract 285. http://clinicaltrials.gov/show/NCT01060293)
In summary, while the findings of the DAD-HF trials may lessen enthusiasm for testing the effect of low dose dopamine on renal function and decongestion efficacy in ADHF, we believe the ROSE AHF trial is better suited than the DAD-HF trials to test the effect of this therapy in ADHF for several reasons. The duration of dopamine therapy will be much longer (72 hours) in ROSE AHF providing sustained renal vasodilatation. The dose of dopamine used in ROSE AHF is more clinically relevant as it is the most commonly used “renal” dose and provides renal vasodilatation with less risk of β-adrenergic stimulation which may offset favorable benefits. Unlike the DAD-HF trials, the ROSE AHF trial tests only the effect of dopamine versus placebo when added to optimal diuretic dosing whereas in the DAD-HF trials, the effects of dopamine were confounded by different diuretic doses in those treated or not with dopamine, and diuretic doses were not calibrated to patient’s outpatient diuretic requirements.
Low dose nesiritide as a renal adjuvant therapy in ADHF
Brain natriuretic peptide (BNP) is a cardiac peptide with vasodilating, renin and aldosterone inhibiting, natriuretic and diuretic properties.36 Human recombinant BNP (nesiritide) was approved by the FDA for the management of ADHF owing to its acute hemodynamic effects to lower systemic and atrial pressures.37 The approved recommended dose of nesiritide is a bolus of 2 μg/kg followed by infusion of 0.01 μg/kg/min. In the trial which supported labeling of nesiritide for ADHF, this dose produced reduction in systolic blood pressure exceeding 13.5 mmHg in 25% of patients.37
While preclinical studies have demonstrated the renal enhancing effects of intravenous (IV) administration of BNP, most human studies have not shown evidence of favorable renal effects with approved dose of nesiritide. Wang et al administered approved dose of nesiritide or placebo for 24 hours each on consecutive days in a randomized, double blind, crossover study performed in patients with type 1 cardiorenal syndrome. Changes in renal blood flow, urinary volume and GFR were not different during the nesiritide and placebo study periods.38 A small, double blind, placebo controlled study randomized 75 patients with ADHF and renal dysfunction to a 48 hour infusion of the approved dose of nesiritide or placebo in addition to usual care. There was no significant difference in the incidence of type 1 cardiorenal syndrome between the nesiritide or placebo groups39
In a randomized, open label study, 71 ADHF patients with underlying renal dysfunction were randomized to a standardized diuretic regimen with or without the approved dose of nesiritide for 48 hours. Nesiritide patients had smaller increases in creatinine despite greater blood pressure reduction but nesiritide did not enhance diuretic responsiveness. 40 The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated HF (ASCEND-HF) randomized 7141 patients with ADHF to the approved dose of nesiritide or placebo and demonstrated that while there was minimal improvement in dyspnea in patients who received nesiritide, there was no change in renal function and mortality. Importantly, the incidence of hypotension in nesiritide treated patients was 26.6%, significantly greater than that observed in placebo treated patients.41 An ancillary analysis from ASCEND showed that full dose nesiritide was not associated with improved urine output after adjusting for diuretic dose, baseline blood pressure and baseline renal function but did not examine treatment blood pressure or investigator reported hypotension as covariates affecting renal response to natriuretic peptides42.
In experimental HF, favorable renal effects of natriuretic peptides are attenuated in the presence of reduced renal perfusion pressure and restored when renal perfusion pressure is kept constant.43 Intra-renal infusion of nesiritide to avoid systemic hypotension is associated with natriuresis, diuresis and increases in GFR. 44 Building on the concept of preserving renal protective effects of natriuretic peptides by limiting systemic hypotension, Riter et al compared intravenous administration of low dose nesiritide (0.0025 – 0.005 μg/kg/min without a bolus) to the approved dose or no nesiritide in a case control study of ADHF patients matched for severity of underlying renal dysfunction. As compared to patients not treated with nesiritide, standard dose nesiritide reduced systolic blood pressure (121 mmHg to 112 mmHg) where as low dose nesiritide did not (97 mmHg to 99 mmHg), despite lower blood pressure at baseline. Low dose nesiritide was associated with significant improvements in creatinine and BUN whereas these parameters did not improve in the standard dose nesiritide group. Lastly, low dose nesiritide therapy was associated with similar diuresis despite a diuretic dose that was < 50% of that used in patients who received full dose nesiritide.45
The NAPA study demonstrated that patients with LV systolic dysfunction undergoing coronary artery bypass grafting using cardiopulmonary bypass, had improvement in renal function and clinical outcomes when treated with nesiritide at 0.01 μg/kg/min without bolus. These effects were more pronounced in patients with reduced renal function at baseline 46. In a small, double blind, placebo controlled study of patients with renal insufficiency undergoing cardiopulmonary bypass surgery, as compared to placebo, low dose nesiritide (as used in ROSE AHF) preserved renal function in the perioperative period. 47
In summary, while studies of full dose nesiritide have not provided evidence of beneficial effects of nesiritide on renal function or diuretic responsiveness, full dose nesiritide is consistently associated with significant hypotension, a factor known to adversely affect renal function and blunt responsiveness to diuretics and natriuretic peptides. Preliminary studies using low dose nesiritide in ADHF and cardiac surgery patients have provided a consistent signal of benefit. Thus, the rationale for testing the effect of low dose nesiritide in ROSE AHF trial is strong.
ROSE AHF: Study objectives
The ROSE AHF study uses a novel study design to address two independent objectives. One primary objective is to test the hypothesis that as compared to placebo, addition of low dose dopamine to optimal diuretic dosing preserves or enhances renal function, as measured by change in serum cystatin-C and cumulative urinary volume over a 72 hour treatment period in patients with ADHF and renal dysfunction. The other primary objective of the ROSE AHF study is to test the hypothesis that as compared to placebo, addition of low dose nesiritide to optimal diuretic dosing preserves or enhances renal function as measured by change in serum cystatin-C and cumulative urinary volume over a 72 hour treatment period in patients with ADHF and renal dysfunction.
ROSE AHF: Study setting
The ROSE AHF study has been designed by and is being conducted within the National Heart, Lung, and Blood Institute (NHLBI) sponsored Heart Failure Clinical Research Network (HFN). The objective of the HFN is designed to conduct small to intermediate-sized randomized clinical trials that either provide “proof of concept” for novel therapies or provide an evidence base for therapies commonly used in ADHF based on “expert opinion”.
ROSE AHF: Study design
The ROSE AHF study (Clinicaltrials.gov NCT01132846) is a double blind, placebo controlled, multi-center randomized clinical trial. A total of 360 patients hospitalized for the treatment of ADHF who have renal dysfunction were enrolled within 24 hours of admission. Specific inclusion criteria are listed in Table 1. As biomarkers other than renal function (for example BNP assays) are inconsistently measured in clinical practice, entry level BNP was not an inclusion criterion as it may limit future generalizability of our results.
Table 1.
Inclusion Criteria
Inclusion Criteria
|
To enhance the efficiency of the trial, limit the number of patients who require a central line for dopamine administration and to test the two independent hypotheses posed in ROSE AHF, patients were initially randomized in an open, 1:1 allocation ratio to the nesiritide strategy or the dopamine strategy (Figure).
Figure. ROSE AHF Patient Flow Diagram.
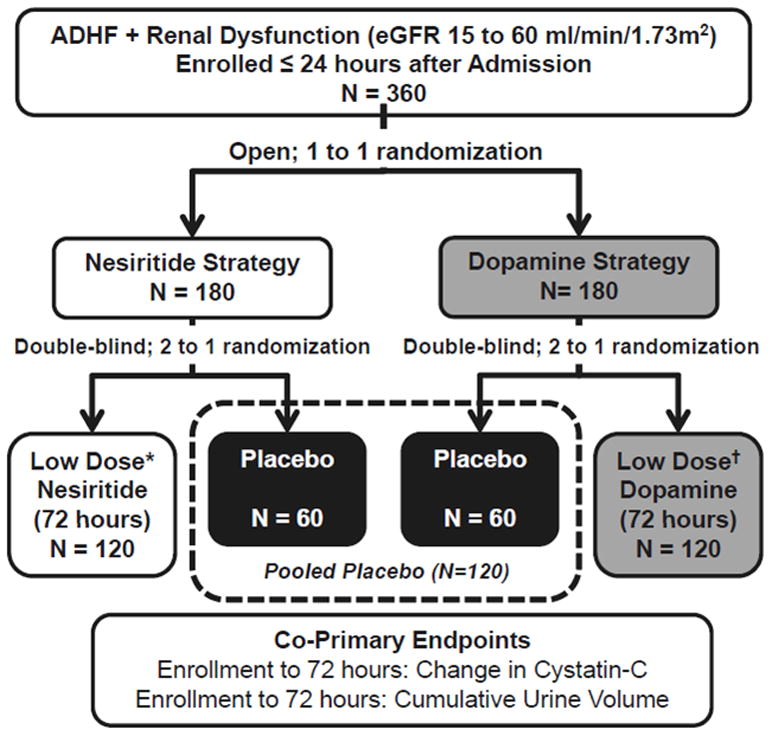
All patients receive open label, protocol specified optimal diuretic dosing. * Dose of nesiritide is 0.005 ug/kg/min administered for 72 hours without a starting bolus dose; †Dose of dopamine is 2 ug/kg/min administered for 72 hours; eGFR, estimated glomerular filtration rate.
Patients randomized to the nesiritide strategy were subsequently be randomized in a double blind, 2:1 ratio to low dose nesiritide (0.005 ug/kg/min administered via a peripheral intravenous (IV) catheter for 72 hours) or placebo. Patients randomized to the dopamine strategy underwent placement of appropriate vascular access for dopamine administration as stipulated by local practice guidelines and subsequently were randomized in a double blind, 2:1 ratio to low dose dopamine (2 ug/kg/min for 72 hours) or placebo. Randomization allocations were based on a permutated block randomization scheme stratified by clinical site and was performed using an automated web-based system.
To test the two primary hypotheses, the placebo patients will be pooled. Patients randomized to receive dopamine will be compared with the pooled placebo group. Similarly, patients randomized to receive nesiritide will be compared to the pooled placebo group.
ROSE AHF: Background therapy
All patients received optimal open-label diuretic treatment. Based on the results of the Diuretic Optimization Strategies Evaluation (DOSE) trial13, the protocol-specified optimal total daily IV diuretic dose of furosemide (or equivalent) is equal to 2.5 x the total daily oral outpatient furosemide (or equivalent) dose at 7 days prior to admission. One half of the total daily diuretic dose is administered twice daily as a bolus for at least 24 hours. The range of allowed daily furosemide doses is 80 to 600 mg. Patients naive to outpatient loop diuretics received 80 mg/day of IV furosemide. After 24 hours, diuretic dosing may be adjusted by the managing provider. All patients are placed on a 2000 mg sodium diet and 2000 cc fluid restriction. Use of other medications is at the discretion of the managing physician. After the primary endpoint assessment at 72 hours, study drug is discontinued and all treatment is at the treating provider’s discretion.
ROSE AHF: Study assessments
After consent, patients undergo baseline history and physical, measurement of vital signs, phlebotomy for biomarkers (including cystatin C; HFN Core Biomarker Laboratory, University of Vermont) and electrolytes (local laboratory) and patient global well-being and dyspnea visual analogue scale (VAS) measurements. These assessments and adverse event assessments are repeated at 24, 48 and 72 hours. A baseline urine sample is collected for urinary biomarkers and daily 24 hour urine collections for urinary volume, urinary sodium excretion and urinary biomarkers are performed for 72 hours. A more limited data set is collected at day seven or discharge, whichever occurs first. All patients have a telephone assessment of vital status and hospitalizations at 60 days and vital status at six months post-discharge.
ROSE AHF: Endpoints
The goal of renal adjuvant therapy in AHF is to enhance decongestion (efficacy) while preserving renal function (safety). Thus, for both of the hypotheses tested in ROSE AHF, 72 hour cumulative urinary volume as an index of decongestion efficacy and change in cystatin-C from randomization to 72 hours as a measure of renal function preservation will serve as the co-primary endpoints.
Cumulative urinary volume reflects diuretic responsiveness over the course of study drug administration and the potential for decongestion, is subject to fewer potential measurement errors than fluid balance, weight change or physical exam changes and is more clinically relevant than changes in biomarkers such as BNP. Symptom relief is affected by numerous factors and shown to be poorly correlated to adequacy of decongestion48. Thus, this endpoint, while novel, is well designed to reflect the postulated effect of the study intervention.
Cystatin-C is freely filtered by the glomerulus and (unlike creatinine) is not dependent on muscle mass and its determinants (age, nutritional status, ethnicity and gender).49 Cystatin-C and change in cystatin-C are potent prognostic markers in AHF23, 50 and change in cystatin-C was a secondary endpoint in an ADHF trial 28. Given the potential advantages of cystatin-C as a more specific marker of renal function, this novel endpoint is used as the primary safety endpoint in ROSE AHF, and change in creatinine is a secondary endpoint.
Secondary endpoints are listed in Table 2. Tertiary endpoints will include changes in primary and secondary endpoints at other time periods (24 and 48 hours), biomarkers reflective of HF severity and renal function, length of stay, readmissions and mortality at 60 days and mortality at six months.
Table 2.
Secondary Endpoints
Secondary Endpoints
|
Pre-specified subgroup analyses will be conducted to determine whether the treatment effect on the primary endpoints and selected secondary endpoints is modified by pertinent covariates including blood pressure, age, and blood urea nitrogen levels.
ROSE AHF: Statistical Considerations
Sample Size Justification
A difference of 0.3 mg/L in serum cystatin-C is considered to be clinically meaningful since a change of cystatin-C of ≥ 0.3 at 48 hours in patients hospitalized with AHF was associated with a statistically significant; two fold increase in 180 day mortality23. Based on prior HFN AHF trials, missing data for the change in serum cystatin-C from randomization to 72 hours is expected to be less than 15%. Assuming 15% missing data (leaving 102 subjects per treatment group) and a standard deviation (SD) for the change between randomization and 72 hours of 0.62 mg/L 40, the study would have 88% power to detect a clinically significant difference in the change in cystatin-C between low-dose dopamine vs. placebo (or low-dose nesiritide vs. placebo) at the two-sided 0.025 level of significance. A SD for the change in cystatin-C of 0.59 mg/L would provide 91% power and any SD of less than 0.68 mg/L would provide greater than 80% power to detect a clinically meaningful difference.
In a previous study in AHF40, the SD for the change in cumulative fluid balance from randomization to 72 hours was approximately 2900 mL. Based on prior HFN clinical trials, the amount of missing data is expected to be less than 10% for the cumulative urine volume at 72 hours. With a Type I error rate of 0.025 and a sample size of 108 evaluable subjects per treatment arm, the study would have 90% power to detect a treatment difference of > 1400 mL and 80% power to detect a difference of > 1224 mL.
Analysis of Primary Endpoints
The primary analysis will be conducted on an intention-to-treat basis. A general linear model with an indicator for the specific treatments being compared versus placebo, will be used to examine the treatment effect for the co-primary endpoints. For the primary comparisons, placebo subjects will be pooled across the nesiritide and dopamine strategy arms. Comparisons of low-dose dopamine versus placebo and low-dose nesiritide versus placebo will each be conducted using a Type I error rate of 0.025 for each co-primary endpoint. Comparison of change in cystatin-C (the difference in paired values of cystatin-C at baseline and 72 hours) will be adjusted for baseline cystatin-C. Sensitivity analyses will be performed using multiple imputations to account for missing data.
Analysis of Secondary and Tertiary Endpoints
General linear models and nonparametric approaches will be used to analyze the continuous outcomes. For binary outcomes, logistic regression analysis will be used to compare each treatment versus placebo and estimate the associated odds ratio and 95% confidence intervals. Time-to-event comparisons will be conducted using Kaplan-Meier curves and log-rank tests. For analyses of patient global well-being VAS and dyspnea VAS, the value of zero will be imputed for all measurements missing due to death. Sensitivity analyses, including the worst-rank score analysiswill be employed to assess the influence of informatively missing values on the results. For all secondary and tertiary endpoints, a p value < 0.05 will be considered statistically significant.
Exploratory Analyses
If both the low-dose dopamine and low-dose nesiritide treatment are statistically superior to placebo, an exploratory analysis will be conducted to compare the two active treatment arms.
ROSE AHF Ancillary Study
Reliable Evaluation of Dyspnea in the ROSE AHF Trial (RED-ROSE)
This objective of this prospective, ancillary study is to evaluate novel endpoints for assessing treatment effect in ADHF trials. Most ADHF trials with dyspnea relief as an endpoint have not demonstrated a benefit of therapies on dyspnea relief. This may indicate that dyspnea improves adequately with standard therapy and thus, is not a critical unmet need in the treatment of ADHF. Alternatively, inability to demonstrate improvement in dyspnea may reflect shortcomings in the tools used to assess dyspnea in hospitalized ADHF patients. While the dyspnea VAS score is accepted as a valid assessment of change in ADHF symptoms51, there is no standardization of conditions (oxygen supplementation, position, activity) at the time of the VAS assessment. Further, in up to 48% of patients with ADHF, other symptoms (body swelling or fatigue) are reported as the “most bothersome symptom”52 thus assessment of dyspnea may not reflect their clinical status or response to therapy.
The ADHF Syndromes International Working Group has proposed a more rigorous “provocative” Dyspnea Severity Score (pDSS) in which dyspnea is assessed under standardized and incrementally more strenuous conditions. 53 In the RED-ROSE study, patients enrolled in the ROSE AHF study undergo sequential assessment of dyspnea using a slight modification of the pDSS as well as assessment of their “most bothersome symptom” using symptom specific VAS tools. Changes in these metrics will be compared to those in the standard dyspnea and wellbeing VAS scores over the course of the hospitalization. Further, the association of changes in the pDSS and most bothersome symptom VAS scores with objective measures of decongestion (NT-proBNP, fluid and weight loss) will be assessed and compared to the standard dyspnea and well-being VAS scores.
Conclusion
There are currently no FDA approved renal adjuvant therapies for patients with ADHF and renal dysfunction. The ROSE AHF study will determine if low dose dopamine or low dose nesiritide, both of which are clinically available, enhance decongestion and preserve renal function in this high risk population. The ROSE AHF trial has several strengths, including the multicenter design, rigorous entry criteria and efficient use of NHLBI resources by pooling the placebo groups for comparison to each tested therapy, a strategy which reduces the number of patients needed to address the hypotheses by 25%. Furthermore, because of the different biological mechanisms of low dose dopamine and low dose nesiritide, this study will provide mechanistic insights into the pathophysiology of renal dysfunction in ADHF. Enrollment in ROSE AHF has been completed. The results of the ROSE AHF trial are expected to provide clinically relevant information regarding appropriate management of ADHF patients.
Acknowledgments
Sources of Funding
Supported by grants from the NHLBI: Coordinating Center: U10 HL084904; Regional Clinical Centers: U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01HL084890, U01 HL084891, U10 HL110342, U10 HL110262, U01 HL084931, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338. This work is also supported by the National Center for Advancing Translational Sciences (NCATS): UL1TR000454; and the National Institute on Minority Health and Health Disparities (NIMHD): 8 U54 MD007588
Footnotes
Disclosures
Potential conflicts of interest including financial disclosures are listed below:
HHC: Research Grant from NIH; Mayo Clinic and HHC have filed patents for chimeric natriuretic peptides; Mayo Clinic has licensed patents to Nile Therapeutics and Anexon with other patents pending at the U.S. patent office; Royalties from Nile Therapeutics, Anexon Inc and Up To Date; Co-founder of Zumbro Discovery Inc.
MMG: Scientific Advisory Board: Cardioxyl and Merck,
GMF: Research Grant from: NIH, Novartis, Amgen, Otsuka & Roche Diagnostics; Consulting: Novartis, Amgen & Roche Diagnostics
AFH: Consulting: Janssen <10K; Honorarium: Corthera <10K
EB: Research Grant from Duke Clinical Research Institute: Duke receives funds from the NHLBI for the Heart Failure Network and Dr. Braunwald has a subcontract with Duke for his responsibilities as Network Chair; Research Grant from Johnson & Johnson; Honoraria: Bayer for lectures at symposia
MMR: Research Grant from NIH; Royalties from Anexon Inc
The rest of the authors have no disclosures
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the united states: A policy statement from the american heart association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felker GM, Adams KF, Jr, Konstam MA, O’Connor CM, Gheorghiade M. The problem of decompensated heart failure: Nomenclature, classification, and risk stratification. Am Heart J. 2003;145:S18–25. doi: 10.1067/mhj.2003.150. [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 5.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the adhere database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 7.Brandimarte F, Vaduganathan M, Mureddu GF, Cacciatore G, Sabbah HN, Fonarow GC, Goldsmith SR, Butler J, Fedele F, Gheorghiade M. Prognostic implications of renal dysfunction in patients hospitalized with heart failure: Data from the last decade of clinical investigations. Heart failure reviews. 2013;18:167–176. doi: 10.1007/s10741-012-9317-z. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 9.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, Krumholz HM. Worsening renal function: What is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 10.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felker GMLK, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette, Braunwald E, O’Connor CM for the NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair JE, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Konstam MA, Gheorghiade M. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the everest trial. Eur Heart J. 2011;32:2563–2572. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 15.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: Pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 16.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 18.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine a1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 21.Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of kw-3902, an adenosine a1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551–1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O’Connor CM, Givertz MM. Effects of the adenosine a1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from protect (placebo-controlled randomized study of the selective adenosine a1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function) J Am Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (relax-ahf) development program: Correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (relax-ahf): A randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 25.Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, Kobalava Z, Nitsche K, Forssmann WG, Luss H, Meyer M. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Euro Heart J. 2006;27:2823–2832. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- 26.Bart BA, Goldsmith SR, Lee KL, Redfield MM, Felker GM, O’Connor CM, Chen HH, Rouleau JL, Givertz MM, Semigran MJ, Mann D, Deswal A, Bull DA, Lewinter MM, Braunwald E. Cardiorenal rescue study in acute decompensated heart failure: Rationale and design of carress-hf, for the heart failure clinical research network. J Card Fail. 2012;18:176–182. doi: 10.1016/j.cardfail.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 28.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg LI. Cardiovascular and renal actions of dopamine: Potential clinical applications. Pharmacol Rev. 1972;24:1–29. [PubMed] [Google Scholar]
- 30.Singer I, Epstein M. Potential of dopamine a-1 agonists in the management of acute renal failure. Am J of Kidney Dis. 1998;31:743–755. doi: 10.1016/s0272-6386(98)70043-5. [DOI] [PubMed] [Google Scholar]
- 31.Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A. Renal vasodilatory action of dopamine in patients with heart failure: Magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: Low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 33.Cotter G, Weissgarten J, Metzkor E, Moshkovitz Y, Litinski I, Tavori U, Perry C, Zaidenstein R, Golik A. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62:187–193. doi: 10.1016/S0009-9236(97)90067-9. [DOI] [PubMed] [Google Scholar]
- 34.Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: Does it protect renal function? Clin Cardiol. 1997;20:627–630. doi: 10.1002/clc.4960200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschope C, Triposkiadis F. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (dad-hf) trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 36.Chen HH, Burnett JC., Jr The natriuretic peptides in heart failure: Diagnostic and therapeutic potentials. Proc Assoc Am Physicians. 1999;111:406–416. doi: 10.1111/paa.1999.111.5.406. [DOI] [PubMed] [Google Scholar]
- 37.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 38.Wang DJ, Dowling TC, Meadows D, Ayala T, Marshall J, Minshall S, Greenberg N, Thattassery E, Fisher ML, Rao K, Gottlieb SS. Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine. Circulation. 2004;110:1620–1625. doi: 10.1161/01.CIR.0000141829.04031.25. [DOI] [PubMed] [Google Scholar]
- 39.Witteles RM, Kao D, Christopherson D, Matsuda K, Vagelos RH, Schreiber D, Fowler MB. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2007;50:1835–1840. doi: 10.1016/j.jacc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 40.Owan TE, Chen HH, Frantz RP, Karon BL, Miller WL, Rodeheffer RJ, Hodge DO, Burnett JC, Jr, Redfield MM. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail. 2008;14:267–275. doi: 10.1016/j.cardfail.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb SS, Stebbins A, Voors AA, Hasselblad V, Ezekowitz JA, Califf RM, O’Connor CM, Starling RC, Hernandez AF. Effects of nesiritide and predictors of urine output in acute decompensated heart failure: Results from ascend-hf. J Am Coll Cardiol. 2013 Jun 6; doi: 10.1016/j.jacc.2013.04.073. pii: S0735–1097(13)02175-X Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Redfield MM, Edwards BS, Heublein DM, Burnett JC., Jr Restoration of renal response to atrial natriuretic factor in experimental low-output heart failure. Am J Physiol. 1989;257:R917–923. doi: 10.1152/ajpregu.1989.257.4.R917. [DOI] [PubMed] [Google Scholar]
- 44.Chen HH, Cataliotti A, Schirger JA, Martin FL, Harstad LK, Burnett JC., Jr Local renal delivery of a natriuretic peptide a renal-enhancing strategy for b-type natriuretic peptide in overt experimental heart failure. J Am Coll Cardiol. 2009;53:1302–1308. doi: 10.1016/j.jacc.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riter HG, Redfield MM, Burnett JC, Chen HH. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47:2334–2335. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Mentzer RM, Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr, Luber JM, Jr, Smedira NG. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:The napa trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 47.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: A double-blind placebo-controlled pilot study. Circulation. 2007;116:I134–138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 48.Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, Braunwald E, O’Connor CM, Felker GM. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin c is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 50.Campbell CY, Clarke W, Park H, Haq N, Barone BB, Brotman DJ. Usefulness of cystatin c and prognosis following admission for acute heart failure. Am J Cardiol. 2009;104:389–392. doi: 10.1016/j.amjcard.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 51.Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, Gronda EG, Colombo P, Felker GM, Cas LD, Kremastinos DT, O’Connor CM, Cotter G, Davison BA, Dittrich HC, Velazquez EJ. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: An international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (measure-ahf) J Card Fail. 2008;14:777–784. doi: 10.1016/j.cardfail.2008.07.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, Stewart G, Nohria A, Rogers JG, Heywood JT, Gheorghiade M, Lewis EF, Mi X, Setoguchi S. The worst symptom as defined by patients during heart failure hospitalization: Implications for response to therapy. J Card Fail. 2012;18:524–533. doi: 10.1016/j.cardfail.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang PS, Cleland JG, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, Peacock WF, Fonarow GC, Aldeen AZ, Kirk JD, Storrow AB, Tavares M, Mebazaa A, Roland E, Massie BM, Maisel AS, Komajda M, Filippatos G, Gheorghiade M. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: The need for a uniform approach. Euro Heart J. 2008;29:816–824. doi: 10.1093/eurheartj/ehn048. [DOI] [PubMed] [Google Scholar]


