Abstract
Alignment of 36 MinC sequences revealed four completely conserved C-terminal glycines. As MinC inhibits cytokinesis in Neisseria gonorrhoeae and Escherichia coli, the functional importance of these glycines in N. gonorrhoeae MinC (MinCNg) and E. coli MinC (MinCEc) was investigated through amino acid substitution by using site-directed mutagenesis. Each mutant was evaluated for its ability to arrest cell division and to interact with itself and MinD. In contrast to overexpression of wild-type MinC, overexpression of mutant proteins in E. coli did not induce filamentation, indicating that they lost functionality. Yeast two-hybrid studies showed that MinCEc interacts with itself and MinDEc; however, no interactions involving MinCNg were detected. Therefore, a recombinant MinC protein, with the N terminus of MinCEc and the C terminus of MinCNg, was designed to test for a MinCNg-MinDNg interaction. Each MinC mutant interacted with either MinC or MinD but not both, indicating the specificity of glycine residues for particular protein-protein interactions. Each glycine was mapped on the C-terminal surfaces (A, B, and C) of the solved Thermotoga maritima MinC structure. We found that MinCEc G161, residing in close proximity to the A surface, is involved in homodimerization, which is essential for MinC function. Glycines corresponding to MinCEc G135, G154, and G171, located within or adjacent to the B-C surface junction, are critical for MinC-MinD interactions. Circular dichroism revealed no gross structural perturbations of the mutant proteins, although the contribution of glycines to protein flexibility and stability cannot be discounted. Using molecular modeling, we propose that exposed conserved MinC glycines interact with exposed residues of the α-7 helix of MinD.
The MinC protein plays a pivotal role in determining where cell division occurs in several bacterial species. In gram-negative bacteria, such as Neisseria gonorrhoeae and Escherichia coli, this protein acts as a cell division inhibitor in concert with MinD and MinE (5, 23). In E. coli, MinC has been shown to block cell division by destabilizing FtsZ rings (10), a protein involved in septal ring formation (20). Overexpression of MinC in E. coli produces a filamentous phenotype indicative of cell division inhibition (5, 23). In contrast, inactivation of this protein results in minicell morphology, which is mediated by irregular cell division at the cell poles (5). In the diplococcus N. gonorrhoeae, which, unlike E. coli, divides in alternating perpendicular planes (32), inactivation of minC results in enlarged cells with disrupted placement of the septum (23). Interestingly, gonococcal MinC complements an E. coli minC mutant, and its overexpression causes E. coli cells to filament, indicating the common functionality of MinC in the genera Neisseria and Escherichia (23).
Size exclusion chromatography, as well as limited proteolytic analyses, indicated that E. coli MinC (MinCEc) exists as a dimer (30). The MinCEc monomer consists of two separate domains joined by a flexible linker; the N-terminal domain comprises amino acid residues 1 to 99, and the C-terminal domain includes residues 125 to 231 (11). The N terminus of MinCEc interacts with FtsZEc (10), while the C terminus has been implicated in self-interaction and interaction with MinDEc (11). It has been proposed that the interaction of MinCEc with MinDEc brings the N terminus of MinCEc close to its target, FtsZEc (15).
The crystal structure of Thermotoga maritima MinC (MinCTm) indicated that it comprises four molecules arranged as two dimers (4). The N- and C-terminal domains of MinCTm are bound by a flexible linker, which is hypervariable between species (4). The N-terminal domain of each monomer contains two α-helices and five β-strands, and the C-terminal domain consists of a right-handed β-helix, which resembles a triangular barrel with three surfaces, A, B, and, C (4). The dimerization interface for MinCTm was found to be located on the hydrophobic A surface, although no specific amino acid residues were implicated in this function (4). The MinC residues implicated in the MinC-MinD interaction have not been identified.
In a current E. coli model explaining how the Min proteins act to determine the septation site, it is proposed that MinD dimerizes in the cytoplasm in the presence of ATP and then binds to a MinC dimer (12, 13). This MinCDEc complex then interacts with the cell membrane (12, 21, 31). The subsequent binding of MinEEc to the MinCDEc complex causes the displacement of MinCEc and stimulates the ATPase activity of MinDEc (13, 19, 21). As a result of ATP hydrolysis, MinDEc and MinEEc are released from the membrane. Through membrane-associated coiled structures, MinDEc oscillates from one end of the cell to the other and at the same time recruits MinCEc away from the cell center, allowing accumulation of FtsZ in this region (19, 21, 28). Gonococcal Min proteins also exhibit dynamic behavior in both rod-shaped and round E. coli cells, which divide in alternating perpendicular planes as N. gonorrhoeae does (24). Since the MinCEc and N. gonorrhoeae MinC (MinCNg) proteins play a critical role in the functioning of the min system in their hosts and since the C-terminal domains of these proteins are highly conserved, we were interested in establishing what role could be attributed to specific completely conserved residues in the function of MinCNg or MinCEc.
Alignment of MinC sequences from 36 different bacterial species indicated that while the MinC C terminus is highly conserved, only five residues (four glycines and one arginine) are identical. Glycine is unique among the amino acids due to its small size, the enhanced backbone flexibility that it confers (1), and its involvement in protein stability (16) or in protein-protein interactions (2). For these reasons, we investigated the functional roles of the conserved glycines in the C terminus of MinCNg and MinCEc by amino acid substitution using site-directed mutagenesis.
In this study, we found that the completely conserved C-terminal glycines are essential for maintaining MinC functionality as a cell division inhibitor and for the interaction of MinC with other Min proteins. On the basis of existing crystal structures of the MinC and MinD proteins and taking into account which MinC glycine residues should be surface exposed, we propose a specific interaction between residues on the α-7 helix of MinD and the B-C surface junction of MinC.
MATERIALS AND METHODS
Strains and growth conditions.
Strains and plasmids used in this study are shown in Table 1. E. coli DH5α was used for cloning purposes. E. coli PB103 (wild type) and E. coli DR105 (minC) were used for expression and complementation studies, respectively. E. coli C41(DE3) was used as an expression strain for purification of six-His-tagged MinCNg. E. coli cells were grown at 37°C for 6 to 8 h in Luria-Bertani broth (Difco) supplemented with 100 μg of ampicillin per ml and 40 μM isopropyl-β-d-thiogalactopyranoside (IPTG), when required. Saccharomyces cerevisiae strain SFY526 (Clontech) was used in yeast two-hybrid assays to examine protein-protein interactions. Yeast was grown at 30°C on yeast extract-peptone-adenine-dextrose medium or on the appropriate synthetic dropout media to select for transformants, as described in the Clontech yeast two-hybrid manual.
TABLE 1.
Strains and plasmids used in this study
| Strains or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | supE44ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco-BRL |
| E. coli PB103 | dadR1 trpE61 trpA62 tna-5 purB+ | 23a |
| E. coli DR105 | zcf-117::Tn10/dadR1 trpE61 trpA62 tna-5 minC3 Tetr | 23a |
| E. coli C41 (DE3) | F−ompT hsdSB(rB− mB−) gal dcm Δ(srl-recA)306::Tn10 (Tetr) (DE3) | 23b |
| S. cerevisiae SFY526 | MATa ura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3 112 Canrgal4-542 gal80-538 URA3::GAL1UAS− GAL1TATA-lacZ | Clontech |
| Plasmids | ||
| pUC18 | Ampr Plac | Amersham Pharmacia |
| pEGFP | Ampr Plac::gfp | Clontech |
| pSR2 | Ampr Plac::minCNg | 23 |
| pSR23 | Ampr Plac::minC(MinCEc1-99) | This study |
| pSR24 | Ampr Plac::minC(MinCCh:MinCEc1-99-MinCNg103-237) | This study |
| pSR32 | Ampr Plac::minCEc | This study |
| pSR32G135 | Ampr Plac::minCEc(G135D) | This study |
| pSR32G154 | Ampr Plac::minCEc(G154D) | This study |
| pSR32G161 | Ampr Plac::minCEc(G161S) | This study |
| pSR32G171 | Ampr Plac::minCEc(G171E) | This study |
| pSR32P141 | Ampr Plac::minCEc(P141A) | This study |
| pVG4 | Ampr Plac::minCNg(G138D) | This study |
| pVG6 | Ampr Plac::minCNg(G157D) | This study |
| pVG8 | Ampr Plac::minCNg(G164S) | This study |
| pVG10 | Ampr Plac::minCNg(G174E) | This study |
| pPF1 | Ampr Plac::minCNg(E144I) | This study |
| pGAD424 | Ampr PADH1::gal4 (AD)c,d | Clontech |
| pSRAD-C | Ampr PADH1::gal4 (AD)-minCEc | This study |
| pSRAD-D | Ampr PADH1::gal4 (AD)-minDEc | 29 |
| pGADminC | Ampr PADH1::gal4 (AD)-minCNg | This study |
| pGADminD | Ampr PADH1::gal4 (AD)-minDNg | 29 |
| pSRAD-G135 | Ampr PADH1::gal4 (AD)-minCEc (G135D) | This study |
| pSRAD-G154 | Ampr PADH1::gal4 (AD)-minCEc (G154D) | This study |
| pSRAD-G161 | Ampr PADH1::gal4 (AD)-minCEc (G161S) | This study |
| pHDAD-G171 | Ampr PADH1::gal4 (AD)-minCEc (G171E) | This study |
| pSRAD-P141 | Ampr PADH1::gal4 (AD)-minCEc (P141A) | This study |
| pSRAD-Ch | Ampr PADH1::gal4 (AD)-minC(MinCEc1-99-MinCNg103-237) | This study |
| pGBT9 | Ampr PADH1::gal4 (BD)e | Clontech |
| pSRBD-C | Ampr PADH1::gal4 (BD)-minCEc | This study |
| pSRBD-D | Ampr PADH1::gal4 (BD)-minDEc | 29 |
| pGBT9minC | Ampr PADH1::gal4 (BD)-minCNg | This study |
| pGBT9minD | Ampr PADH1::gal4 (BD)-minDNg | 29 |
| pSRBD-G135 | Ampr PADH1::gal4 (BD)-minCEc (G135D) | This study |
| pSRBD-G154 | Ampr PADH1::gal4 (BD)-minCEc (G154D) | This study |
| pSRBD-G161 | Ampr PADH1::gal4 (BD)-minCEc (G161S) | This study |
| pHDBD-G171 | Ampr PADH1::gal4 (BD)-minCEc (G171E) | This study |
| pSRBD-P141 | Ampr PADH1::gal4 (BD)-minCEc (P141A) | This study |
| pSRBD-Ch | Ampr PADH1::gal4 (BD)-minC(MinCEc1-99-MinCNg103-237) | This study |
| pSRBD-ChG135 | Ampr PADH1::gal4 (BD)-minCCh(G135D) | This study |
| pSRBD-ChG154 | Ampr PADH1::gal4 (BD)-minCCh(G54D) | This study |
| pSRBD-ChG161 | Ampr PADH1::gal4 (BD)-minCCh(G161S) | This study |
| pSRBD-ChG171 | Ampr PADH1::gal4 (BD)-minCCh(G171E) | This study |
| pPF2 | Ampr PADH1::gal4 (BD)-minCCh(E141I) | This study |
| pET30a | Kanr PT7::6-His | Novagen |
| pHLCC | Kanr PT7:: minCNg-6-His | This study |
| pETminCG138 | Kanr PT7:: minCNg(G138D)-6-His | This study |
| pETminCG157 | Kanr PT7:: minCNg(G157D)-6-His | This study |
| pETminCG164 | Kanr PT7:: minCNg(G164S)-6-His | This study |
| pETminCG174 | Kanr PT7:: minCNg(G174E)-6-His | This study |
Gift from P. de Boer (Case Western Reserve University, Cleveland, Ohio).
Gift from J. E. Walker (Medical Research Council, Cambridge, England).
PADH1 is yeast promoter which regulates expression of gal4.
Encodes the AD of GAL4.
Encodes the DNA BD of GAL4.
PCR and IPCR.
Oligonucleotide primers used for PCR and inverse PCR (IPCR) in this study are listed in Table 2. Primers were designed by using Primer Designer (Scientific and Education Software) and were synthesized by the University of Ottawa Biotechnology Research Institute. All PCRs and IPCRs were carried out with a Perkin-Elmer GenAmp PCR System 9600 thermocycler (Perkin-Elmer Corp.) as follows: 3 min at 94°C; 30 cycles of denaturation for 15 s at 94°C, annealing for 15 s at temperatures varying from 48 to 51°C (depending on the primer pair used), and extension for 1 to 7 min (depending on the expected product size) at 72°C; 5 min at 72°C; and hold at 4°C. The PCRs were carried out in 100-μl (final volume) mixtures containing 75.5 μl of double-distilled H2O (ddH2O), 10 μl of 10× PCR buffer containing 1.5 mM MgCl2 (Roche Diagnostics Corp.), 2 μl of deoxynucleoside triphosphates (Boehringer Mannheim Corp.), 1 μl of each primer (0.2 μg/ml), 0.5 μl of Taq DNA polymerase for PCR amplification (Roche) or 0.1 μl of Vent DNA polymerase (New England Biolabs) for IPCR amplification, and 10 μl of template DNA. N. gonorrhoeae CH811 and E. coli PB103 cell suspensions were prepared by diluting cells from overnight cultures in ddH2O. Cell concentrations were adjusted by using McFarland equivalence turbidity standard 0.5 (Remel) to provide chromosomal DNA templates for PCRs. The concentrations of plasmid DNA templates were adjusted to 0.01 μg/ml with ddH2O for IPCRs.
TABLE 2.
Oligonucleotide primers used for PCRs in this study
| Primer | Sequence | Product |
|---|---|---|
| min10 | 5′ GCG CCT GCA GAT GAT GGT TTA TAT AAT 3′ | minCNg |
| min29 | 5′ GCG CGG ATC CCA AAC AAT TAC TCT GAG CC 3′c | |
| NEc-up | 5′ GCG CCT GCA GAT GTC AAA CAC GCC AAT C 3′ | N terminus of minCEc |
| NEc-down | 5′ GCG CAC TAG TCA GGA TAG GCA GCC C 3′a,c | |
| CNg-up | 5′ GCG CAC TAG TCA TTC GGA AAA TGT TAA AG 3′a | C terminus of minCNg |
| CNg-down | 5′ GCG CGG GCC CTT ACT CTG AGC CAA TTG C 3′c | |
| EcminC-up | 5′ GCG CGA ATT CAT GTC AAA CAC G 3′a | minCEc |
| EcminC-down | 5′ GCG CGG ATC CTC AAT TTA ACG GTT GAA CGG 3′a,c | |
| EC G135-1 | 5′ TCC GAT CAG CGT ATT TAT GCT 3′b | minCEc G135D |
| EC G135-2 | 5′ ACG CAC CGG GGT ATC TAT TAA 3′c | |
| EC G154-1 | 5′ GCT GAC GCC GAA TTG ATT GC 3′b | minCEc G154D |
| EC G154-2 | 5′ GCT AAC GTG GCT TGT AAC AA 3′c | |
| EC G161-1 | 5′ GAT TCG AAC ATT CAT GTC TAT GG 3′b | minCEc G161S |
| EC G161-2 | 5′ GGC AAT CAA TTC GGC CCC AGC GC 3′c | |
| EC G171-1 | 5′ GCG GAA CGT GCG CGT GCA GGG G 3′b | minCEc G171E |
| EC G171-2 | 5′ CAT CAT GCC ATA GAC ATG AAT G 3′c | |
| EC P141-1 | 5′ GCT GCT CAA TGT GAT CTG ATT GTT AC 3′b | minCEc P141A |
| EC P141-2 | 5′ ATA AAT ACG CTG ACC GGA ACG CAG CG 3′c | |
| minCG138-1 | 5′ ACC GAT CAG CAG GTT TAT GCC 3′b | minCNg G138D |
| minCG138-2 | 5′ ACG GAC AGG GGA TGT AAT CAA 3′c | |
| minCG157-1 | 5′ CAG GAC GCG GAA TTG ATT GCA 3′b | minCNg G157D |
| minCG157-2 | 5′ GCT GAC CGC CCC CGT AAC AAT 3′c | |
| minCG164-1 | 5′ GAT TCG AAT ATG CAT ATT TAT 3′b | minCNg G164S |
| minCG164-2 | 5′ TGC AAT CAA TTC CGC CCC CTG 3′c | |
| minCG174-1 | 5′ AGA GAT CGT GCT TTG GCC GGC 3′b | minCNg G174E |
| minCG174-2 | 5′ CAT CGG CGC ATA AAT ATG CAT 3′c | |
| minCE144-1 | 5′ GCC ATC GAT GGC GAT TTG ATT GTT ACG 3′b | minCNg E144I |
| minCE144-2 | 5′ ATA AAC CTG ACC GGT ACG GAC AGG 3′c | |
| HLC2 | 5′ GCG CCA TAT GAT GGT TTA TAT AAT TGA ATG 3′a | minCNg |
| HLC3 | 5′ GCG CAA GCT TCT CTG AGC CAA TTG CAC TG 3′a,c |
Restriction sites for the following enzymes are underlined: SpeI (CNg-up, NEc-down), EcoRI (EcminC-up), BamHI (EcminC-down, min29), PstI (min10, NEc-up), ApaI (CNg-down), ClaI (minCE144-1), TseI (EC P141-1), NcoI (HLC2), and HindIII (HLC3).
Novel restriction sites introduced for screening PCR products are underlined. Recognition sites for the following enzymes were used: Sau3AI (EC G135-1); BsaHI (EC G154-1), HinfI (EC G161-1), loss of AciI (EC G171-1), Sau3AI (minCG138-1), HgaI (minCG157-1), TaqI (minCG164-1), and Sau3AI (minCG174-1).
Anneals to complementary strand.
Site-directed mutagenesis of minCEc and minCNg.
Wild-type minCEc was PCR amplified by using primers EcminC-up and EcminC-down containing EcoRI and BamHI restriction sites, respectively (Table 2). This gene was ligated with pUC18, which had been previously digested with the same restriction enzymes, to generate pSR32 (Table 1). Wild-type minC in pSR32 was mutagenized by IPCR by using primers containing point mutations that extended in opposite directions around pSR32, as described by Sambrook and Russell (25). Five mutations in minCEc (G135D, G154D, G161S, G171E, and P141A) were introduced into pSR32 by using primer pairs EC G135-1 plus EC G135-2, EC G154-1 plus EC G154-2, EC G161-1 plus EC G161-2, EC G171-1 plus EC G171-2, and EC P141-1 plus EC P141-2 (Table 2), generating plasmids pSR32G135, pSR32G154, pSR32G161, pSR32G171, and pSR32P141, respectively (Table 1).
Similarly, wild-type minCNg was mutagenized in pSR2 (Table 1) by IPCR. Mutated minCNg genes with G138D, G157D, G164S, G174E, and E144I substitutions were created by using primer pairs minCG138-1 plus minCG138-2, minCG157-1 plus minCG157-2, minCG164-1 plus minCG164-2, minCG174-1 plus minCG174-2, and minCE144-1 plus minCE144-2 (Table 2), generating plasmids pVG4, pVG6, pVG8, pVG10, and pPF1 (Table 1), respectively. Plasmids containing mutant minC genes were screened for mutations by PCR amplification of minCNg with primers min10 and min29 (Table 2) and by PCR amplification of minCEc with primers EcminC-up and EcminC-down (Table 2). Purified amplicons were screened for mutations by digestion with appropriate enzymes since each site-directed mutation introduced a novel restriction site in the mutated gene that was not present in wild-type minCEc or minCNg (Table 2). It was thus possible to screen for minC mutants by examining the restriction endonuclease digestion patterns of plasmid-encoded minC genes from transformants and comparing them with the pattern of the wild-type minC gene. All mutant minC genes were sequenced to confirm point mutations, frame conservation, and gene integrity at the Biotechnology Research Institute, University of Ottawa.
Construction of recombinant MinCCh containing the N terminus of MinCEc and the C terminus of MinCNg.
Since no interactions of gonococcal MinC with itself or with MinDNg could be detected by using yeast two-hybrid methods, a chimeric MinC protein (MinCCh) was constructed to determine whether combined N- and C-terminal domains from MinCEc and MinCNg could restore these interactions. Plasmid pEGFP (Clontech) was used to make these constructs since it had suitable restriction sites needed for sequential cloning; however, the gfp gene was deleted, leaving only the chimeric minC gene (minCCh) cloned in the vector. The chimeric protein, MinCCh, comprised the N-terminal domain of MinCEc and the flexible linker and C-terminal domain of MinCNg. A 314-bp fragment which encoded amino acids 1 to 99 of MinCEc was PCR amplified with primers NEc-up and NEc-down, which contained PstI and SpeI restriction sites at their 5′ and 3′ ends, respectively (Table 2). This fragment replaced a PstI-SpeI fragment in pEGFP, deleting gfp and generating pSR23 (Table 1). A 425-bp fragment encoding amino acids 103 to 237 of MinCNg was PCR amplified with primers CNg-up and CNg-down containing SpeI and ApaI restriction sites at their 5′ and 3′ ends, respectively (Table 2). This partial minCNg fragment was cloned into pSR23 that had previously been digested with SpeI and ApaI, and pSR24 was generated with a chimeric minC gene encoding MinCCh (Table 1). minCCh was confirmed to be in frame by DNA sequencing at the Biotechnology Research Institute, University of Ottawa.
Morphological studies of E. coli transformants to determine MinC functionality.
To determine whether mutations in conserved glycine residues of MinC allowed the protein to retain its functionality, E. coli PB103 was transformed with plasmids containing each point mutation in minCEc (pSR32G135, pSR32G154, pSR32G161, pSR32G171, and pSR32P141), as well as with plasmids containing point mutations in gonococcal minC (pVG4, pVG6, pVG8, pVG10, and pPF1). Plasmids pSR2 (wild-type minCNg) and pSR32 (wild-type minCEc) were used as positive controls, and pUC18 was used as a negative control. Induction of filamentation due to the inability to form proper septa at the middle of the cell in E. coli has been used previously as an indicator of MinC functionality (5, 8, 12, 23). Similarly, to determine whether MinCCh retains functionality as a cell division inhibitor, E. coli PB103 was transformed with plasmid pSR24 encoding MinCCh. As no significant morphological differences were observed in cells with and without IPTG induction, all expression assays were conducted without induction and were repeated at least three times.
E. coli filamentation, defined as production of cells that were at least fourfold longer than wild-type rods (5), was assessed by both phase-contrast microscopy and flow cytometry. For phase-contrast microscopy, samples from each transformant were fixed to 0.1% polylysine-coated coverslips by using the protocol of Ramirez-Arcos et al. (23). Cells were visualized at a magnification of ×100 with oil immersion by phase-contrast microscopy by using a Zeiss Axioskop microscope. At least 10 microscope fields were examined for each transformant, and each field contained a minimum of 100 cells, which were analyzed to determine the percentage of filaments or normal-size short rods. Photographs were obtained with a Sony Power HAD 3CCD color video camera and Northern Eclipse software, version 6.0. Flow cytometry was used to determine the percentages of filamentous cells (length, >10 μm) and wild-type rods (length, 2 to 5 μm) in the whole population of E. coli cells. Cells were suspended in 1 ml of filter-sterilized phosphate-buffered saline to reduce the presence of background particles. Transformants were analyzed with a Beckman Coulter Epics XL-MCL flow cytometer at a voltage and gain of 50 V and 1.0, respectively, for side scatter and at a voltage and gain of 127 or 459 V and 1.0, respectively, for forward scatter. The event retrieval time was set to 300 s, and a maximum of 100,000 events were examined. Data retrieval and analysis were performed by using EXPO32 ADC XL 4 Color software and EXPO32 v.1.2 software, respectively. Gate A was created around wild-type rods by using PB103 transformed with pUC18 as a negative control. Gate B was created around filamentous cells by using PB103 transformed with pSR2 and pSR32 expressing MinCNg and MinCEc, respectively, as positive controls. The percentage of cells within each gate was calculated by determining the percentage of total gated cells within each gate and excluding background noise and particulate matter. A Student’s t test was used to determine significant differences in percentages of filamentation between wild-type and mutant proteins.
E. coli DR105 (minC) was used for complementation studies with gonococcal MinC having mutations at the conserved glycines, using previously described protocols (23).
Yeast two-hybrid assays.
Wild-type minCNg and minCEc, as well as minCCh, were PCR amplified individually by using primers incorporating EcoRI and BamHI restriction sites at the 5′ and 3′ ends, respectively (Table 2). Each gene was ligated in frame with the GAL4 DNA binding domain (BD) and the GAL4 activation domain (AD) of pGBT9 and pGAD424 (Clontech), respectively. The plasmids containing wild-type minCNg and minCEc were pGADminC (AD-MinCNg), pGBT9minC (BD-MinCNg), pSRAD-C (AD-MinCEc), and pSRBD-C (BD-MinCEc) (Table 1). The plasmids containing mutated minCEc were pSRAD-G135 (AD-MinCEc G135D), pSRBD-G135 (BD-MinCEc G135D), pSRAD-G154 (AD-MinCEc G154D), pSRBD-G154 (BD-MinCEc G154D), pSRAD-G161 (AD-MinCEc G161S), pSRBD-G161 (BD-MinCEc G161S), pHDAD-G171 (AD-MinCEc G171E), pHDBD-G171 (BD-MinCEc G171E), pSRAD-P141 (AD-MinCEc P141A), and pSRBD-P141 (AD-MinCEc P141A) (Table 1). Wild-type MinCEc interacted with MinDEc whether it was fused to the GAL4 DNA AD or the GAL4 DNA BD; however, the interaction was much stronger when MinCEc was fused to the GAL4 DNA BD. Hence, wild-type and mutant MinCEc fusions to this domain were used to examine their interactions with MinDEc, which was fused to the GAL4 DNA AD. The plasmids containing minCCh were pSRAD-Ch (AD-MinCCh) and pSRBD-Ch (BD-MinCCh) (Table 1). MinCCh interacted with MinDNg only when it was fused to the DNA BD of GAL4. Therefore, we constructed plasmids encoding mutant MinCCh proteins that were fused only to this domain. The gonococcal minC fragment encoding the C terminus of MinCCh in pSRBD-Ch (BD-MinCCh) was replaced with PCR fragments encoding the MinCNg G138D, G157D, G164S, G174E, and E144I mutants. These fragments were amplified from plasmids pVG4, pVG6, pVG8, pVG10, and pPF1 by using primers CNg-up and min29, containing SpeI and BamHI restriction sites at their 5′ and 3′ ends, respectively (Table 2). After SpeI and BamHI digestion of these fragments, they were ligated to a DNA fragment corresponding to pSRBD-Ch that had previously been digested with the same enzymes that excluded the region encoding the C terminus of wild-type MinCNg in this plasmid, and plasmids pSRBD-ChG135, pSRBD-ChG154, pSRBD-ChG161, pSRBD-ChG171, and pPF2 were generated (Table 1). These plasmids were transformed into E. coli PB103 for overexpression studies of the MinCCh mutant proteins. Filamentation was evaluated by phase-contrast microscopy. Plasmids pGADminD (AD-MinDNg), pGBT9minD (BD-MinDNg), pSRAD-D (AD-MinDEc), and pSRBD-D (BD-MinDEc) had been constructed previously in our laboratory (29) (Table 1).
All plasmids were transformed into S. cerevisiae SFY526 singly as negative controls or in pairs to test for protein-protein interactions by using the lithium acetate method (Clontech yeast two-hybrid manual). Yeast transformants were assayed for β-galactosidase activity by using the colony lift method with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) as a substrate and by liquid assays with o-nitrophenyl-d-galactopyranoside as a substrate as described in the Clontech yeast two-hybrid manual. Both colony lift and liquid assays were performed in triplicate at least twice for each interaction. Standard deviations were determined for the averages of the β-galactosidase activities. A Student’s t test was used to determine significant differences between β-galactosidase activities representing the strength of protein-protein interactions.
Protein analysis and Western blotting of bacterial and yeast extracts.
Protein extracts from E. coli PB103 expressing MinCNg, MinCEc, and MinCCh proteins with point mutations were prepared as described previously (23). Protein extracts from S. cerevisiae expressing mutant MinCEc and MinCCh fused to the GAL4 AD and the GAL4 BD were prepared by using the method described by Horváth and Riezman (9). Cells were grown to optical density at 600 nm of 1.5, harvested, washed in sterile ddH2O, and resuspended in 100 μl of sample buffer (0.06 M Tris-HCl [pH 6.8], 10% glycerol, 2% sodium dodecyl sulfate, 0.0025% bromophenol blue). Samples were boiled for 5 min and then centrifuged at 14,000 rpm for 5 min (Sorvall MC 12V centrifuge) and the supernatants were designated the total-protein fractions. Bacterial and yeast protein fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and protein concentrations were standardized prior to membrane transfer by densitometry as described previously (23). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis-resolved proteins were transferred to Immobilon-P membranes (Millipore Corporation), and Western blotting was performed by using methods described previously (23). The membranes were incubated with 1:100 anti-MinCNg antiserum overnight at 4°C. Differences in protein expression levels were calculated by densitometry.
Purification of His6-MinCNg and circular dichroism (CD) analysis.
To purify wild-type and mutant MinCNg, the coding region of minCNg was PCR amplified with primers HLC2 and HLC3, which incorporated NdeI and HindIII restriction sites at the 5′ and 3′ ends, respectively (Table 2), by using pSR2 (wild-type MinCNg), pVG4 (MinCNg G138D), pVG6 (MinCNg G157D), pVG8 (MinCNg G164S), and pVG10 (MinCNg G174E) as templates. The genes were fused in frame to the C-terminal His6 tag of pET30a (Novagen), generating pHLCC, pETminCG138, pETminCG157, pETminCG164, and pETminCG174, respectively (Table 1). The fusions were confirmed to be in frame by DNA sequencing. Log-phase cultures (500 ml) of E. coli C41(DE3) carrying these plasmids were induced with 0.4 mM IPTG for 0.5 h at 37°C with shaking at 250 rpm. Protein purification was performed as previously described (29), with a few modifications: proteins were washed with a buffer containing 30 mM imidazole, 0.5 M NaCl, and 20 mM Tris-HCl (pH 7.9) and were eluted with the same buffer containing 250 mM imidazole, as described by Novagen. The protein concentration was determined by the Bradford method by using the Bio-Rad protein assay dye reagent.
CD spectroscopy was performed with a model 62DS CD spectrometer (AVIV Instruments, Inc., Lakewood, N.J.) with 1-mm-path-length quartz cell. The sample temperature was maintained at 20 ± 0.2°C. Far-UV CD spectra were recorded for both the wild-type protein and the mutants at protein concentrations between 0.1 and 0.2 mg/ml in 10 mM ammonium bicarbonate buffer (pH 8.0). All CD experiments were repeated three times to ensure spectral reproducibility. Following baseline subtraction, the CD spectra were normalized based on protein concentrations and were expressed in molar ellipticity per mean residue.
Protein alignment and modeling.
MinC sequences from 36 different prokaryotic microorganisms were obtained from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). These sequences were aligned by using Clustal W software (http://searchlauncher.bcm.tmc.edu/multi-align/multi-align.html).
Molecular modeling was performed by using SWISS-MODEL (http://www.expasy.org/swissmod/SWISS-MODEL.html). Briefly, we used MinCTm (PDB 1HF2) (4) and Pyrococcus furiosus MinD (MinDPf) (PDB 1G3R) (8) solved structures as templates for molecular modeling of a MinCTm-MinDPf complex. MinDPf has been previously modeled as a dimer by using the dimeric structure of the nitrogenase iron protein NifH (PDB 1N2C) (12, 21). Using this method, we superimposed MinDPf onto NifH to obtain a MinDPf dimer. We positioned this MinDPf dimer with the C terminus of a MinCTm monomer in a situation where a MinCTm-MinDPf interaction could occur between the MinDPf α-7 helix and the B-C surface junction of MinCTm. We calculated the overall exposure (fraction of solvent-accessible surface area) of the conserved glycine residues (G111, G129, G136, and G146) of MinCTm using the computer program VADAR (33). We also analyzed the structural flexibility that the conserved glycines conferred to MinCTm by measuring their specific temperature factors or B values (B factors) using Swiss-PDB (http://au.expasy.org/spdbv/). The B factors are linearly related to the mean square displacement of an atom and give an indication of atomic flexibility in the crystalline state of a protein.
RESULTS
Conserved C-terminal glycine residues in the MinCNg and MinCEc proteins are implicated in the function of MinC as a cell division inhibitor.
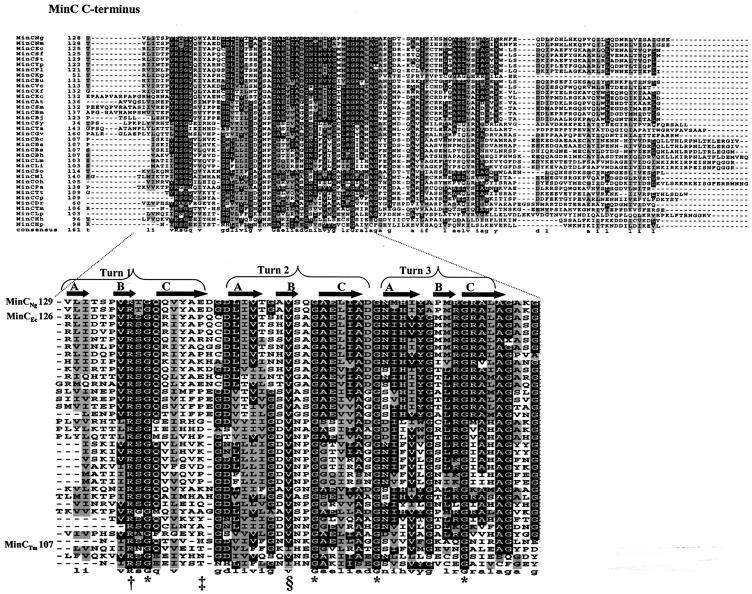
As of October 2003, 36 MinC sequences were available from the National Center for Biotechnology Information for comparison by protein alignment. Our analysis revealed that the C-terminal domain of MinC contains five completely conserved residues (one arginine and four glycines) (Fig. 1). The alignment also showed that the C terminus of MinC is more conserved than the N terminus and the flexible linker region of the protein (data not shown). A valine residue (corresponding to V154 of MinCNg) is conserved in all but 2 of the 36 species analyzed (Fig. 1). Due to the unique role that glycines may have in protein flexibility, protein stability, or protein-protein interactions, we investigated the functional effects of the conserved glycines in the C terminus of both MinCNg and MinCEc.
FIG. 1.
Multiple alignment of the conserved C terminus of prokaryotic MinC. Ng, Neisseria gonorrhoeae; Nm, Neisseria meningitidis; Ec, Escherichia coli; Sf, Shigella flexneri; St, Salmonella enterica serovar Typhi; Yp, Yersinia pestis; Pl, Photorabdus luminescens; Kp, Klebsiella pneumoniae; Bu, Buchnera sp.; Vc, Vibrio cholerae; So, Shewanella oneidensis; Xf, Xylella fastidiosa; Xc, Xanthomonas campestris; Pa, Pseudomonas aeruginosa; At, Agrobacterium tumefaciens; Sm, Sinorhizobium sp.; Bm, Brucella melitensis; Bj, Bradyrhizobium japonicum; Ml, Mesorhizobium sp.; Sy, Synechocystis sp.; Ta, Thermosynechococcus elongatus; Gv, Gleobacter violaceus; Bc, Bacillus cereus; Ba, Bacillus anthracis; Bs, Bacillus subtilis; Bh, Bacillus halodurans; Lm, Listeria monocytogenes; Li, Listeria innocua; Oh, Oceanobacillus iheyensis; Tt, Thermoanaerobacter tengcongensis; Cp, Clostridium perfringens; Dr, Deinococcus radiodurans; Tm, Thermotoga maritima; Lp, Lactobacillus plantarum; Hh, Helicobacter hepaticus; Hp, Helicobacter pylori. White type on a black background indicates residues that are conserved, while black type on a gray background indicates related amino acids. Asterisks indicate the four completely conserved glycine residues in the C terminus of MinC. The dagger, double dagger, and section sign indicate a completely conserved arginine, the position of the nonconserved residues selected for site-directed mutagenesis as controls, and a highly conserved valine, respectively. Predicted surfaces A, B, and C, as well as turns of the β-helix of the MinC C terminus, are labeled.
A number of single-point mutants were generated in these MinC proteins by site-directed mutagenesis, including G138D, G157D, G164S, and G174E MinCNg mutants and corresponding G135D, G154D, G161S, and G171E MinCEcmutants. The specific glycine substitutions selected in the present study were based on previous reports in which minCEc mutants were characterized after mutagenesis with nitrosoguanidine (12), ethyl methanesulfonate (18), or hydroxylamine (22). While many induced MinCEc mutants had nonsense and missense mutations, several mutations involved changes from glycine (G) to charged amino acids, such aspartic acid (G10D and G171D), glutamic acid (G161E), lysine (G161K), and arginine (G176R) (18, 22). In the present study, mutations at nonconserved (i.e., neutral) C-terminal residues (E144I mutation in MinCNg and P141A mutation in MinCEc) were also created to validate the functional significance of mutations in conserved glycine residues of MinC.
The functionalities of the different glycine mutants were determined by their abilities to inhibit cell division, as observed by filamentation, when they were overexpressed in wild-type E. coli PB103 (Table 1). Induction of filamentation in E. coli upon overexpression of MinC has often been used as an indicator of MinC functionality (5, 8, 12, 23). After transformation of E. coli PB103 with plasmids encoding the MinCNg or MinCEc mutants, phase-contrast microscopy and flow cytometry were used to assess the presence and extent of filamentation compared to the filamentation of wild-type rod-shaped cells.
In the analysis of MinC overexpression in E. coli by phase-contrast microscopy, at least 1,000 cells from random microscopic fields were counted. Approximately 90% of the E. coli cells overexpressing wild-type MinCNg from pSR2 (which encodes MinCNg cloned into pUC18) were filamentous, as were cells overexpressing the neutral MinCNg mutant E144I (Table 3). Most (∼99%) of the E. coli cells carrying pUC18, which served as a negative control, had a typical wild-type, short-rod morphology, indicating that normal cell division occurred. Mutant MinCNg proteins with mutations at each of the individual conserved glycines did not result in significant filamentation upon overexpression in E. coli, indicating that each mutant was not able to function as a cell division inhibitor (Table 3). The percentage of filamentation for the MinCNg glycine mutants varied from 0.07 ± 0.03 to 1.28 ± 0.38% (Table 3). Overexpression of the four glycine mutants of MinCEc also failed to produce filamentation in E. coli PB103, indicating that the mutations produced nonfunctional MinCEc proteins. The percentage of filamentation for these mutants varied between 0.75 ± 0.08 and 3.12 ± 0.39%, (Table 3). By contrast, overexpression of the MinCEc P141A mutant protein resulted in a filamentous phenotype similar to that of wild-type MinCEc, providing further evidence that the P141A mutation was neutral in affecting functionality (Table 3). Thus, the four conserved glycine residues in MinCEc or MinCNg are implicated in the function of MinC as a cell division inhibitor.
TABLE 3.
Summary of functionality of MinCNg, MinCEc, and MinCh mutants
| Protein | Mutation | % Filamentation upon overexpression in E. coli PB103a | Protein interactions determined by yeast two-hybrid assays
|
|
|---|---|---|---|---|
| Self | MinD | |||
| MinCEc | Wild type | 76.5 ± 0.71 | + | + |
| G135D | 3.12 ± 0.39 | + | − | |
| G154D | 0.75 ± 0.08 | + | − | |
| G161S | 1.86 ± 0.15 | − | + | |
| G171E | 1.12 ± 0.49 | Weak | − | |
| P141A | 75.2 ± 0.08 | + | + | |
| MinCNg | Wild type | 87.82 ± 13.61 | −b | −b |
| G138D | 1.28 ± 0.38 | NTc | NT | |
| G157D | 0.14 ± 0.04 | NT | NT | |
| G164S | 0.09 ± 0.04 | NT | NT | |
| G174E | 0.07 ± 0.03 | NT | NT | |
| E144I | 86.25 ± 2.52 | NT | NT | |
| MinCCh | Wild type | 75.5 ± 3.9 | −d | + |
| G135D | 1.23 ± 0.09 | NT | − | |
| G154D | 4.1 ± 1.56 | NT | − | |
| G161S | 0.09 ± 0.03 | NT | + | |
| G171E | 0.07 ± 0.01 | NT | − | |
| E141I | 77.3 ± 5.46 | NT | + | |
E. coli cells overexpressing wild-type and mutant MinC proteins were analyzed for the presence of filamentation by phase-contrast microscopy. At least 1,000 cells were counted for each transformant.
MinCNg did not interact with itself or with MinDNg. Therefore, MinCNg mutants were not tested for these interactions.
NT, not tested.
Since self-interaction of wild-type MinCCh was not detected, we did not test self-interactions of the MinCCh mutants.
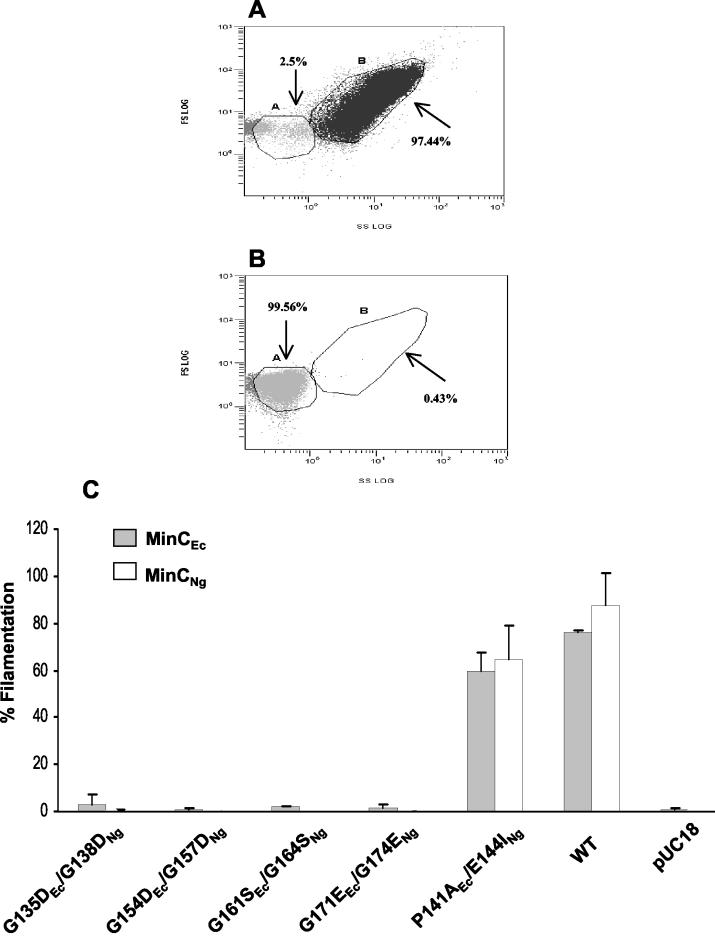
Flow cytometry, an alternative to microscopic cell counting, permits analysis of larger numbers of bacterial cells (100,000 cells) in a short period of time (3). The phenotypic changes due to MinC overexpression as determined by flow cytometry were compared to the results obtained with phase-contrast microscopy in three independent assays. Positive and negative controls are shown in Fig. 2A and B, respectively. Flow cytometry dot plots showed that 97.44% of the E. coli cells transformed with pSR2 (wild-type MinCNg) were filamentous (Fig. 2A) and that a negligible percentage (0.43%) of cells carrying pUC18 were filamentous (Fig. 2B). As observed by phase-contrast microscopy, flow cytometry analysis demonstrated that overexpression of each of the MinCNg and MinCEc proteins bearing substitutions at conserved C-terminal glycines did not induce filamentation, in contrast to wild-type proteins (Fig. 2C). Mutations at nonconserved residues (MinCNg E144I and MinCEc P141A) did not significantly affect the abilities of the proteins to induce filamentation in E. coli (Fig. 2C), further supporting the premise that such mutations are functionally neutral.
FIG. 2.
Flow cytometry analysis of E. coli PB103 cells overexpressing MinCEc and MinCNg mutant proteins. (A and B) Flow cytometry dot plots of E. coli populations transformed with pSR2 (wild-type MinCNg) as a positive control (A) and with pUC18 as a negative control (B). The plots of side scatter (SS) versus forward scatter (FS) show two population sizes, the wild-type rods within gate A and long filaments within gate B. The percentages of cells are expressed as percentages of the gated events. (C) Graph showing the percentages of filamentation in a population of 100,000 cells expressing wild-type (WT) MinCEc and MinCNg proteins, as well as MinCEc mutants G135D, G154D, G161S, G171E, and P141A and MinCNg mutants G138D, G157D, G164S, G174E, and E144I. An unpaired t test analysis showed that the differences in the percentage of filamentation between wild-type MinCNg and MinCNg E144I and between wild-type MinCEc and MinCEc P141A were not significant (P = 0.063 and P = 0.097, respectively). The error bars indicate standard errors.
We previously demonstrated that wild-type MinCNg restored wild-type morphology to E. coli DR105 (minC) (23). None of the gonococcal MinC glycine mutants was able to complement E. coli DR105, and these cells retained the characteristic minicell phenotype (data not shown).
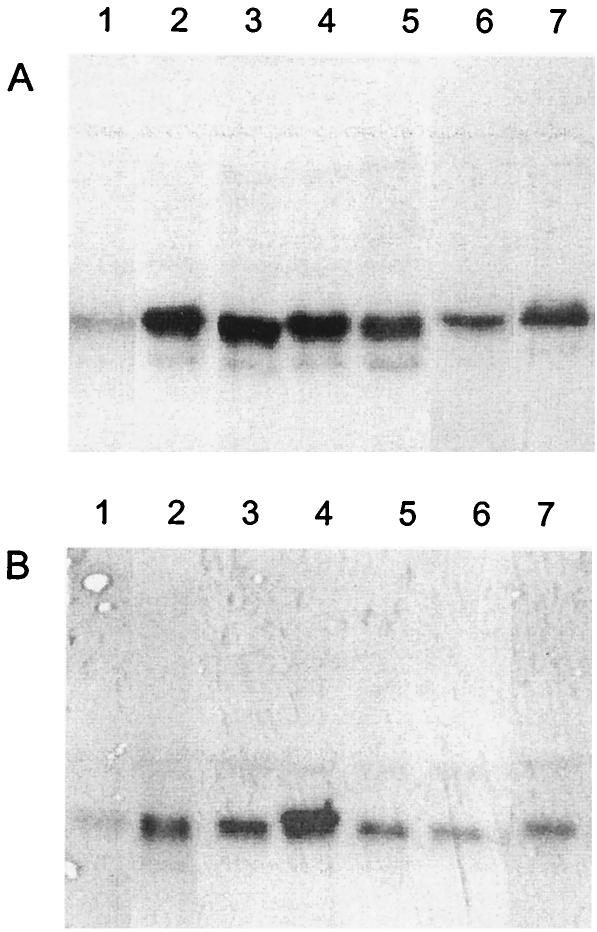
Although the abrogated function of MinC glycine mutants is consistent with a critical role for the glycine residues, it was possible that the mutants exhibited altered functions due to low levels of MinC expression. We determined the levels of expression of the wild-type and mutant MinCEc and MinCNg proteins in E. coli PB103 by Western blotting using antiserum to MinCNg, which cross-reacted with MinCEc (23). Densitometric analysis of MinC bands showed that wild-type MinCEc and MinCEc mutants G135D, G154D, and G161S had similar expression levels, which overall were ∼3.5-fold greater (Fig. 3A, lanes 2 to 5) than the expression levels of native MinCEc (Fig. 3A, lane 1). MinCEc mutants G171E and P141A had 2.5- and 3.0-fold-greater protein levels, respectively (Fig. 3A, lanes 6 and 7) than cells with native MinCEc (Fig. 3A, lane 1). Our results demonstrate that between 3.0-fold (MinCEc P141A) and 3.5-fold (wild-type MinCEc) overexpression of MinCEc is enough to cause filamentation in wild-type E. coli PB103 (Table 3). Since the levels of overexpression in MinCEc mutants G135D (Fig. 3A, lane 2), G154D (Fig. 3A, lane 3), and G161S (Fig. 3A, lane 4) were comparable to levels observed when the wild-type protein was overexpressed, the lost functionality was not due to altered levels of protein expression. It is still possible that the decreased expression of the MinCEc G171E mutant relative to the level of expression of wild-type MinCEc resulted in its inability to induce filamentation in E. coli PB103.
FIG. 3.
Expression of MinC mutant proteins in E. coli PB103: Western blots obtained with anti-MinCNg. (A) E. coli PB103 was transformed with pUC18 (control) (lane 1), pSR32 (wild-type minCEc control) (lane 2), pSR32G135 (minCEc encoding the G135D mutation) (lane 3), pSR32G154 (minCEc encoding the G154D mutation) (lane 4), pSR32G161 (minCEc encoding the G161S mutation) (lane 5), pSR32G171 (minCEc encoding the G171E mutation) (lane 6), and pSR32P141 (minCEc encoding the P141A mutation) (lane 7). (B) E. coli PB103 was transformed with pUC18 (control) (lane 1), pSR2 (wild-type minCNg control) (lane 2), pVG4 (minCNg encoding the G138D mutation) (lane 3), pVG6 (minCNg encoding the G157D mutation) (lane 4), pVG8 (minCNg encoding the G164S mutation) (lane 5), pVG10 (minCNg encoding the G174E mutation) (lane 6), and pPF1 (minCNg encoding the E144I mutation) (lane 7).
In the case of gonococcal MinC overexpression in E. coli PB103, wild-type and mutant G138D MinCNg proteins had similar overexpression levels (Fig. 3B, lanes 2 and 3). The levels of the MinCNg mutant G164S, G174E, and E144I proteins were 15, 33, and 14% less, respectively (Fig. 3B, lanes 5 to 7), than the level of overexpressed wild-type MinCNg (Fig. 3B, lane 2). Interestingly, the level of the gonococcal G157D MinC mutant protein (Fig. 3A, lane 4) was 30% higher than the level of the wild-type MinCNg (Fig. 3A, lane 2), and this mutant exhibited slightly retarded mobility in protein gels. It has been shown previously that overexpression of wild-type MinCNg from pSR2 induces filamentation in E. coli PB103 (23). Hence, it was expected that the MinCNg G138D mutant, which exhibited overexpression levels similar to those of the wild-type MinCNg, would not have altered functionality. It should be noted that the neutral mutant E144I could still cause cell division arrest despite 14% less expression than wild-type MinCNg. Thus, since the G164S mutant had a similar expression level, its nonfunctionality cannot be attributed to its expression level. However, it is possible that the MinCNg G174E mutant is nonfunctional because it does not reach a critical concentration required to arrest cell division in E. coli PB103. Therefore, with the exception of the MinCEc G171E and MinCNg G174E mutants, we are certain that the failure to cause filamentation of the MinC glycine mutants was not due to a lack of overexpression at adequate levels.
Specific MinCEc glycine mutants have altered interactions with themselves or MinDEc.
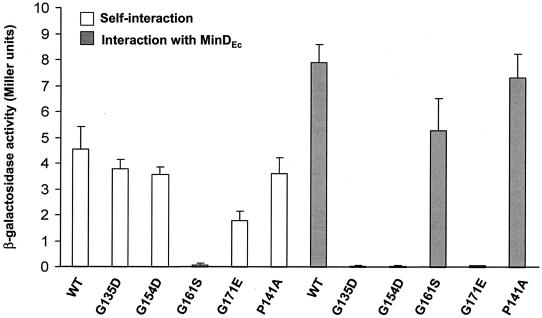
Interactions of MinCEc with itself and with MinDEc have been determined previously by using yeast two-hybrid methods (11). This method was therefore used to analyze the self-interactions of the four MinCEc glycine mutants, as well as their interactions with MinDEc (Table 3). MinCEc mutant G161S did not have the ability to self-interact (Fig. 4), in contrast to MinCEc mutants G135D, G154D, G171E, and P141A, which were capable of homodimer formation (although the self-interaction of the G171E mutant was significantly decreased compared to that of wild-type MinCEc[Fig. 4]). However, unlike MinCEc G161S, the MinCEc G135D, G154D, and G171E mutants were not able to interact with MinDEc, indicating that MinC homodimerization can be preserved while MinC-MinD interactions are selectively disrupted. It should be noted that the strength of the interaction of MinCEc G161S with MinDEc was significantly less than the strength of the wild-type interaction (Fig. 4). The neutral P141A mutation did not significantly alter the MinCEc-MinDEc interaction (Fig. 4).
FIG. 4.
Protein-protein interactions of MinCEc mutant proteins. The graph shows the interactions of wild-type (WT) and mutant E. coli MinC proteins (G135D, G154D, G161S, G171E, and P141A) with themselves (open bars) and with MinDEc (grey bars). An unpaired t test analysis showed that the differences in the strengths of self-interaction between wild-type MinCEc and the G135D, G154D, and P141A mutants were not significant (P = 0.128, P = 0.07, and P = 0.153, respectively). However, the difference in the strength of the self-interaction between wild-type MinCEc and the G171E mutant was significant (P = 0.005). The difference in the strength of the interaction between wild-type MinCEc or MinCEc G161S and MinDEc was significant (P = 0.005), whereas the interaction between the MinCEc P141A mutant and MinDEc was not significantly different (P = 0.091). The error bars indicate standard errors.
Creation of a recombinant MinC containing the N terminus of MinCEc and the C terminus of MinCNg (MinCCh) permitted detection of interactions between MinCNg and MinDNg.
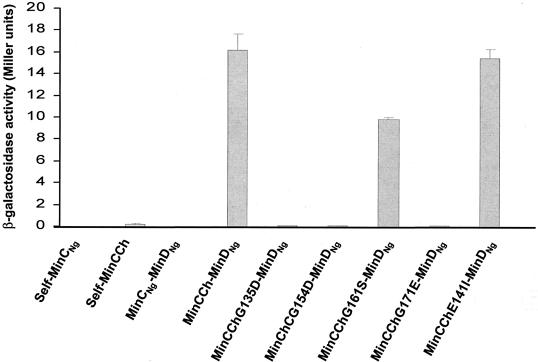
Since yeast two-hybrid studies of MinC glycine mutants of E. coli distinguished specific glycine residues involved in homodimer formation or interactions with MinD, we proposed that MinC mutants with mutations in conserved glycines from N. gonorrhoeae should have identical interactions. However, interactions of MinCNg with itself or with MinDNg were not detected by yeast two-hybrid methods, in contrast to MinCEc interactions (Fig. 5). The failure to detect protein-protein interactions involving MinCNg could have been due either to low levels of expression or to the instability of the proteins in the yeast reporter strain. Western blot analysis of yeast extracts expressing MinCNg or MinDNg failed to detect these proteins, despite the fact that MinDNg showed positive self-association and interaction with MinENg in the yeast two-hybrid system (29). This suggests that the expression levels of Min proteins in yeast extracts may not be high enough to be detected by polyclonal antisera to either MinCNg or MinDNg. In addition, the manufacturer of the system (Clontech) recognizes that the level of expression from the yeast two-hybrid vectors is inherently low in this particular yeast two-hybrid system.
FIG. 5.
Protein-protein interactions of MinCNg mutants. The graph shows the interactions of wild-type MinCNg and MinCCh with themselves and with MinDNg and the interactions of mutant MinCCh proteins with MinDNg. An unpaired t test analysis showed that the differences in the strengths of the interactions of wild-type MinCCh and mutant MinCCh G161S with MinDNg were significant (P = 0.000), while the interaction of the MinCCh E141I mutant with MinDNg was not significant (P = 0.155). The error bars indicate standard errors.
Since interactions involving MinCEc were detectable in this system, we hypothesized that this protein was more stably expressed than MinCNg in yeast cells and, furthermore, that the stability might be maintained by constructing a chimeric MinC protein. We therefore created a recombinant MinC protein (MinCCh) comprising the N terminus of MinCEc (for stability) and the C terminus of MinCNg (to examine the role of conserved glycines in the gonococcal protein). A similar approach was taken in studies with the β-galactosidase of Cellvibrio gilvus, which became stable once its C terminus was fused to the N terminus of the β-galactosidase from T. maritima (7).
It has been demonstrated previously that the N-terminal domain of MinCEc by itself does not interact with either MinCEc or MinDEc (11). Therefore, the MinCCh protein should impart MinCNg functionality since the interactions with itself and with MinD should be mediated by the C terminus. Plasmid pSR24, which encodes MinCCh, was constructed to contain the first 99 N-terminal residues of MinCEc and the flexible linker and C terminus of MinCNg (residues 103 to 237). Chimeric MinC mutants containing substitutions at the conserved glycines (G135, G154, G161, and G171) and at the nonconserved E141 residue were constructed. The residues of the C terminus of MinCCh were renumbered to correspond to the MinCEc numbering. As expected, overexpression of the wild-type MinCCh protein and overexpression of the neutral E141I mutant protein were able to induce filamentation in E. coli PB103 (Table 3). By contrast, overexpression of the G135D, G154D, G161S, and G171E chimeric mutants indicated that they were nonfunctional since they could not induce filamentation (Table 3). Western blotting revealed that the levels of expression of chimeric proteins in E. coli were similar to the level of expression of the wild-type MinCNg (data not shown).
Despite the inability of wild-type MinCCh to self-interact in yeast two-hybrid assays, this protein interacted with MinDNg (Fig. 5 and Table 3), and therefore interactions between MinCCh mutants and MinDNg were tested. As observed for the analogous MinCEc mutants, MinCCh G135D, G154D, and G171E mutants were not able to interact with MinDNg, in contrast to the MinCCh G161S mutant, which was able to interact with MinDNg (Fig. 5 and Table 3). As expected, the neutral E141I mutation did not significantly affect the interaction of MinCCh with MinDNg (Fig. 5 and Table 3). Western blotting was again ineffective in detecting the MinCCh mutants from yeast extracts with anti-MinCNg antiserum. These results show that the same three glycine residues that are important for an E. coli MinC-MinD interaction are implicated in N. gonorrhoeae MinC-MinD interactions as well.
MinC glycine residues residing in the B-C surface junction of the MinC C terminus are involved in the MinC-MinD interaction.
We positioned the conserved glycines in the C terminus of MinCTm (G111, G129, G136, and G146) using the crystal structure of this protein (4). Due to the high level of conservation of MinC C-terminal residues, it is likely that the homologous glycines of MinCEc (G135, G154, G161, and G171) and MinCNg (G138, G157, G164, and G174) are located at similar positions. The G111 and G129 glycines of MinCTm are in the first and second turns, respectively, of the β-helix, in the junction between the B and C surfaces (4) (Fig. 1). Using the software program VADAR (33), we found that the overall levels of exposure (fractions of the solvent-accessible surface area) for these two residues are 35 and 44%, respectively. G136 of MinCTm is probably not exposed since its overall level of exposure is only 2%. This residue is located in a turn in close proximity to the A surface, which was predicted to be the region of MinC dimerization (4) (Fig. 1). Residue G146 of MinCTm is located in the C surface in close proximity to the B-C surface junction, in the third turn of the β-helix (4) (Fig. 1), and it is not surface exposed. In MinCTm, the homologue of the nonconserved residues P141 of MinCEc and E144 of MinCNg is the H116 residue, which is located within the C surface, in close proximity to the A-C surface junction (4) (Fig. 1). Our results show that MinCEc G161, which is located in proximity to the A surface, is required for proper protein self-interaction. In addition, analysis of the B factor for this glycine revealed that it is not flexible and is completely buried in the dimer interface. However, the other three conserved glycine residues, which are located within or near the B-C surface junction in the MinC C terminus and which have B factors indicating that they are relatively flexible, are not involved in MinC homodimerization but are essential for the MinC-MinD interaction.
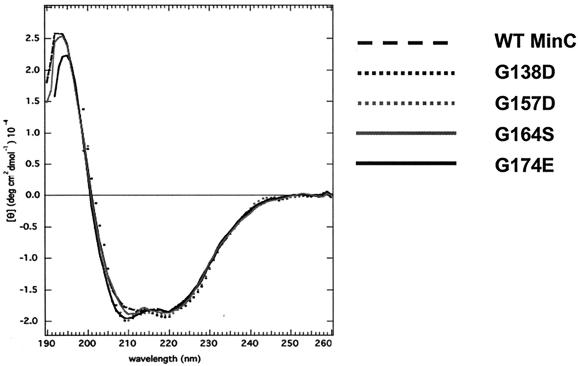
CD reveals that MinCNg glycine mutants have an unchanged secondary structure.
Glycine is a small residue that can adopt a wide range of backbone torsion angles and is often found in β-turns (1). Since the completely conserved residues G111, G129, G136, and G146 of MinCTm are found within or in close proximity to β-turns (4), the structural properties of these residues may be important for the overall MinC structure. Therefore, the folded state of the MinCNg glycine mutants was evaluated by far-UV CD. Analysis of these proteins indicated that the secondary structure of the mutant proteins was essentially identical to that of the wild-type protein (Fig. 6). Although this assay did not demonstrate that the tertiary structure of the proteins is unchanged, significant structural perturbations are unlikely since each glycine substitution in MinC selectively affects its interaction with either MinC or MinD but not its interaction with both proteins.
FIG. 6.
Far-UV CD spectra of wild-type MinCNg and its single-glycine mutants. All samples were dissolved in 10 mM ammonium bicarbonate buffer (pH 8.0), and the measurements were obtained at 20°C. [θ], molar ellipticity per mean residue; WT, wild type.
DISCUSSION
We have shown that four completely conserved glycine residues in the C-terminal domain of MinC are important and that they have a key role in the functioning of MinC as a cell division inhibitor. This is true irrespective of the bacterial origin of MinC since both MinCEc and MinCNg glycine mutants (MinCEc G135D, G154D, G161S, and G171E and the corresponding MinCNg G138D, G157D, G164S, and G174E proteins) were unable to induce filamentation upon overexpression in E. coli. By contrast, mutations at nonconserved amino acids (MinCNg E144I or MinCEc P141A) preserved the ability of the protein to inhibit septation. The lack of functionality of the mutant proteins, with the exception of MinCEc G171E and MinCNg G174E, could not be attributed to a diminution of the protein levels.
To investigate whether the conserved glycine residues of MinC are essential for protein function due to their critical structural locations, each glycine was mapped on the C-terminal surfaces (A, B, and C) of the crystal structure of MinCTm. Our analysis revealed that MinCEc G135 and G154 and the corresponding residues MinCNg G138 and G157 are in the first and second turns, respectively, of the β-helix, in the junction between the B and C surfaces. MinCEc G171 and the corresponding residue MinCNg G174 are located within the C surface near its junction with the B surface (4) (Fig. 1). Interestingly, our protein-protein interaction assays demonstrated that these glycine residues are involved in the interaction between MinC and MinD but not in MinC homodimerization. Therefore, the B-C surface junction of MinC is probably the region responsible for the interaction of MinC with MinD. By contrast, MinCEc G161 and the corresponding residue MinCNg G164 are located in a turn in close proximity to the A surface, the region of MinC dimerization (4) (Fig. 1). Yeast two-hybrid assays provided direct experimental evidence for the involvement of the A surface in MinC dimerization since the MinCEc G161S mutant was not able to self-interact. In addition, our results demonstrate that MinC dimerization is required for the protein function as a cell division inhibitor because the MinCEc G161 or MinCNg G164 mutant was also unable to inhibit cell division.
As determined by CD analyses, the mutated MinC proteins did not have gross structural changes in their secondary structures. In addition, the alteration of protein-protein interactions involving MinC was specific to certain glycine residues (i.e., interactions were lost with either MinC or MinD but not with both proteins simultaneously). Therefore, significant structural perturbations of the mutant proteins cannot explain their loss of function as cell division inhibitors. Another study, involving a mutation in rat insulin-like growth factor-binding protein 5, has also shown that replacement of a critical glycine with glutamine causes the protein to lose activity, although the mutation did not result in gross conformational changes in the protein (27).
It is still possible that local structural perturbations that are transparent to CD analysis but nevertheless affect protein-protein interactions are present in the MinC glycine mutants. Such structural changes include altered protein flexibility and/or stability or changes in the electrostatic nature of the mutant MinC proteins. Glycine, which is often present in β-turns, may confer flexibility to a protein backbone due to its single hydrogen atom side chain (1). Although the four conserved glycines of MinC are located within or in close proximity to the C-terminal β-helix turns, the B factors for the solved structure of MinCTm indicate that only G111, G129, and G146 (G138, G157, and G174 in MinCNg) are relatively flexible. Therefore, mutation of these residues may have resulted in reduced protein flexibility, thereby affecting the movement of the MinC C-terminal domain necessary for mediating interactions with MinD. By contrast, MinCTm G136 (G164 in MinCNg) is not flexible and remains buried in the homodimer interface. Alternatively, replacement of glycine with either aspartic acid (MinCEc G135D and G154D and MinCNg G138D and G157D) or glutamic acid (MinCEc G171E and MinCNg G174E) may have introduced electrostatic repulsion at the protein-protein interfaces.
Two mutant proteins, MinCEc G171E and MinCNg G174E, had decreased levels of expression compared to the levels of expression of wild-type proteins, suggesting that these proteins had decreased stability. Sen and Rothfield (26) have also shown that a G171S mutation of MinCEc led to protein instability. Therefore, low MinCEc G171E protein levels could explain why this protein exhibited decreased self-interaction compared to that of wild-type MinCEc and did not interact with MinDEc. Kong et al. (17) showed that a G145A substitution in the human glutathione transferase P1-1 resulted in loss of enzyme activity due to altered stability of the protein, despite crystallographic analyses which revealed that the structure of the mutant protein was identical to the structure of the wild-type protein.
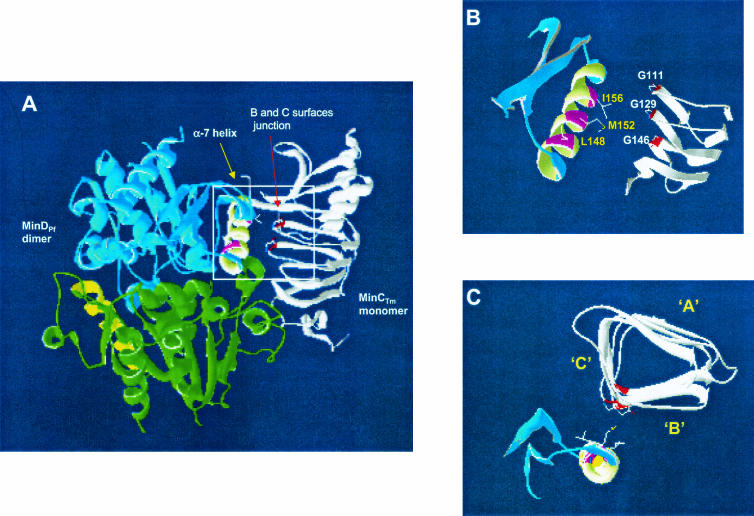
In order to identify regions of MinD involved in its interaction with the C terminus of MinC, a MinC-MinD association was modeled by using the available structures of MinCTm (4) and MinDPf (8) (Fig. 7). The α-7 helix of MinD has been implicated previously in the interaction of this protein with MinC (8). We modeled MinDPf as a dimer and observed that part of the α-7 helix of each MinDPf monomer is exposed, suggesting that this region may be available for interaction with MinC. Our structural analysis showed that three hydrophobic amino acids of the α-7 helix of MinDPf (L148, M152, and I156) may remain surface exposed after formation of a MinDPf dimer. However, sequence alignments of MinD (8, 29) revealed that only L148 and I156 of MinDPf have homologous aliphatic amino acids at the same positions in other species. Most of the amino acids that align with L148 are valines (e.g., in the MinDNg and MinDEc sequences) or isoleucines. Similarly, the majority of MinD sequences, including the MinDNg and MinDEc sequences, have a glycine at the position that aligns with I156. We propose that the glycine residues corresponding to MinCTm G111 and G129, which are surface exposed and are located in the B-C surface junction of the C terminus, may participate in hydrophobic associations with the surface-exposed residues of the α-7 helix of MinD corresponding to MinDPf L148 and I156 (Fig. 7B and C). It has been reported previously that glycine residues can participate directly in protein-protein interactions (2, 6). Lutkenhaus and Sundaramoorthy (21) recently proposed that the interaction between MinC and MinD probably occurs in the cytoplasm and that the MinD-ATP-MinC complex persists because MinC does not stimulate the ATPase activity of MinD.
FIG. 7.
Molecular model of MinC-MinD interaction obtained by using the solved structures of MinCTm and MinDPf. (A) The MinD monomers in the MinDPf dimer are blue and green, while MinCTm is white. Yellow indicates α-7 helix residues of MinDPf. Residues present at the B-C surface junction of monomeric MinCTm are red. (B) Magnification of the putative region of interaction between the exposed glycines (G111 and G129) of MinCTm and the exposed residues (L148 and I156) of the α-helix of MinDPf. (C) 90° view of panel B, with a barrel showing the A, B, and C surfaces of the β-helix of the C terminus of MinCTm.
Several spontaneous MinCEc C-terminal mutants, including MinCEc G161E, G171D, and G171S, have been isolated and analyzed to determine their morphological effects when they are expressed in E. coli (14, 18, 22, 26). However, these chromosomal mutants were not tested for functionality by overexpression in E. coli or by yeast two-hybrid analyses. The present study is the first systematic investigation of the roles of completely conserved glycine residues in MinC function. We propose that the conserved glycines located within or near the B-C surface junction of the MinC C terminus are implicated in its interaction with MinD. We also provide experimental evidence that a conserved glycine, which is near the MinC C-terminal A surface, is essential for MinC self-interaction. The conserved MinC glycines are probably involved in protein flexibility, stability, and steric hindrance. Ultimately, these residues are responsible for specific topological arrangements of the secondary structure of MinC that are necessary for MinC dimerization and MinC-MinD interactions to occur.
Acknowledgments
This project was funded by grant 200209MOP-106682-MI-CECA-22130 from the Canadian Institutes of Health Research to J. R. Dillon. V. Greco was supported by a Natural Sciences and Engineering Research Council, Canada, postgraduate scholarship.
We thank P. Fabre and H. Li for assisting with plasmid construction, as well N. Goto and N. Eng for their helpful suggestions regarding the manuscript.
REFERENCES
- 1.Alix, A. J. 2001. A turning point in the knowledge of the structure-function-activity relations of elastin. J. Soc. Biol. 195:181-193. [PubMed] [Google Scholar]
- 2.Bogan, A. A., and K. S. Thorn. 1998. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier, T., M. Troussellier, A. Anzil, C. Courties, and P. Servais. 2001. Using light scatter signal to estimate bacterial biovolume by flow cytometry. Cytometry 44:188-194. [DOI] [PubMed] [Google Scholar]
- 4.Cordell, S. C., R. E. Anderson, and J. Lowe. 2001. Crystal structure of the bacterial cell division inhibitor MinC. EMBO J. 20:2454-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer, P., R. Crossley, and L. Rothfield. 1989. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in Escherichia coli. Cell 56:641-649. [DOI] [PubMed] [Google Scholar]
- 6.Dogovski, C., J. Pi., and J. Pittard. 2003. Putative interhelical interactions within the PheP protein revealed by second-site suppressor analysis. J. Bacteriol. 185:6225-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal, K., B. Jo Kim, J. D. Kim, Y. K. Kim, M. Kitaoka, and K. Hayashi. 2002. Enhancement of transglycosylation activity by construction of chimeras between mesophilic and thermophilic beta-glucosidase. Arch. Biochem. Biophys. 407:125-134. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, I., T. Oyama, and K. Morikawa. 2001. Structural and functional studies of MinD ATPase: implications for the molecular recognition of the bacterial cell division apparatus. EMBO J. 20:1819-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horváth, A., and H. Riezman. 1994. Rapid protein extraction from Saccharomyces cerevisiae. Yeast 10:1305-1310. [DOI] [PubMed] [Google Scholar]
- 10.Hu, Z., A. Mukherjee, S., Pichoff, and J. Lutkenhaus. 1999. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc. Natl. Acad. Sci. 96:14819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, Z., and J. Lutkenhaus. 2000. Analysis of MinC reveals two independent domains involved in interaction with MinD and FtsZ. J. Bacteriol. 182:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, Z., and J. Lutkenhaus. 2003. A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol. Microbiol. 47:345-355. [DOI] [PubMed] [Google Scholar]
- 13.Hu, Z., C., Saez, and J. Lutkenhaus. 2003. Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J. Bacteriol. 185:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe, A., R. D'Ari, and S. Hiraga. 1988. Minicell-forming mutants of Escherichia coli: production of minicells and anucleate rods. J. Bacteriol. 170:3094-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, J. E., L. L. Lackner, and P. A. de Boer. 2002. Targeting of DMinC/MinD and DMinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings. J. Bacteriol. 184:2951-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiger, G., and D. Eisenberg. 2002. GXXXG and GXXXA motifs stabilize FAD and NAD(P)-binding Rossmann folds through C(alpha)-H…O hydrogen bonds and van der Waals interactions. J. Mol. Biol. 323:69-76. [DOI] [PubMed] [Google Scholar]
- 17.Kong, G. K., G. Polekhina, W. J. McKinstry, M. W. Parker, B. Dragani, A. Aceto, D. Paludi, D. R. Principe, B. Mannervik, and G. Stenberg. 2003. Contribution of glycine 146 to a conserved folding module affecting stability and refolding of human glutathione transferase p1-1. J. Biol. Chem. 278:1291-1302. [DOI] [PubMed] [Google Scholar]
- 18.Labie, C., F. Bouché, and J. P. Bouché. 1990. Minicell-forming mutants of Escherichia coli: suppression of both DicB- and MinD-dependent division inhibition by inactivation of the minC gene product. J. Bacteriol. 172:5852-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lackner, L. L., D. M. Raskin, and P. A. de Boer. 2003. ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J. Bacteriol. 185:735-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutkenhaus, J., and S. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 21.Lutkenhaus, J., and M. Sundaramoorthy. 2003. MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation. Mol. Microbiol. 48:295-303. [DOI] [PubMed] [Google Scholar]
- 22.Mulder, E., C. Woldringh, F. Tetart, and J. Bouché. 1992. New minC mutations suggest different interactions of the same region of division inhibitor MinC with proteins specific for minD and dicB coinhibition pathways. J. Bacteriol. 174:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez-Arcos, S., J. Szeto, T. J. Beveridge, C. Victor, F. Francis, and J. R. Dillon. 2001. Deletion of the cell division inhibitor MinC results in lysis of Neisseria gonorrhoeae. Microbiology 147:225-237. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez-Arcos, S., J. Szeto, J-A, Dillon, and W. Margolin. 2002. Conservation of dynamic localization among MinD and MinE orthologues: oscillation of Neisseria gonorrhoeae proteins in Escherichia coli. Mol. Microbiol. 46:493-504. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sen, M., and L. Rothfield. 1998. Stability of the Escherichia coli division inhibitor protein MinC requires determinants in the carboxy-terminal region of the protein. J. Bacteriol. 180:175-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shand, J. H., J. Beattie, H. Song, K. Phillips, S. M. Kelly, D. J. Flint, and G. J. Allan. 2003. Specific amino acid substitutions determine the differential contribution of the N- and C-terminal domains of insulin-like growth factor (IGF)-binding protein-5 in binding IGF-I. J. Biol. Chem. 278:17859-17866. [DOI] [PubMed] [Google Scholar]
- 28.Shih, Y. L., T. Le, and L. Rothfield. 2003. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc. Natl. Acad. Sci. 100:7865-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeto, J., S. Ramirez-Arcos, C. Raymond, L. D. Hicks, C. M. Kay, and J.-A Dillon. 2001. Gonococcal MinD affects cell division in Neisseria gonorrhoeae and Escherichia coli and exhibits a novel self-interaction. J. Bacteriol. 183:6253-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szeto, T. H., S. L. Rowland, and G. F. King. 2001. The dimerization function of MinC resides in a structurally autonomous C-terminal domain. J. Bacteriol. 183:6684-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szeto, T. H., S. L. Rowland, L. I. Rothfield, and G. F. King. 2002. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc. Natl. Acad. Sci. 99:15693-15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westling-Häggström, B., T. Elmros, S. Normark, and B. Winblad. 1977. Growth pattern and cell division in Neisseria gonorrhoeae. J. Bacteriol. 129:333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wishart, D. S., L. Willard, F. M. Richards, and B. D. Sykes. 1994. VADAR, version 1.2. Edmonton, Alberta, Canada. [DOI] [PubMed]