Abstract
Functionalized nano-graphene– and graphene-based nanocomposites have gained tremendous attention in the area of biomedicine in recent years owing to their biocompatibility, the ease with which they can be functionalized and their properties such as thermal and electrical conductivity. potential applications for functionalized nanoparticles range from drug delivery and multimodal imaging to exploitation of the electrical properties of graphene toward the preparation of biosensing devices. this protocol covers the preparation, functionalization and bioconjugation of various graphene derivatives and nanocomposites. starting from graphite, the preparations of graphene oxide (GO), reduced GO (RGO) and magnetic GO–based nanocomposite, as well as how to functionalize them with biocompatible polymers such as polyethylene glycol (PEG), are described in detail. We also provide procedures for 125I radiolabeling of PEGylated GO and the preparation of GO-based gene carriers; other bioconjugation approaches including drug loading, antibody conjugation and fluorescent labeling are similar to those described previously and used for bioconjugation of PEGylated carbon nanotubes. We hope this article will help researchers in this field to fabricate graphene-based bioconjugates with high reproducibility for various applications in biomedicine. the sample preparation procedures take various times ranging from 1 to 2 d.
INTRODUCTION
In recent years, functionalized nano-graphene– and graphene-based nanocomposites have attracted attention in biomedical applications because of their unique and highly enriched physical and chemical properties1–7. GO and RGO can be functionalized by biocompatible polymers such as PEG, by either covalent conjugation or noncovalent surface coating, to acquire improved stability in physiological environments8,9. Numerous graphene-based biosensing platforms have been reported in the past few years to detect different biological molecules on the basis of various mechanisms10–12. Graphene-coated substrate or graphene-containing composite materials have also shown promise in a number of tissue engineering applications, such as enhancing the growth and differentiation of human mesenchymal stem cells13,14 and promoting neuronal differentiation of human neural stem cells15. Graphene-based devices can also be used as a sensitive platform for interfacing with biological cells to detect the change in the electrical potential of cell membrane16,17. Owing to their ultra-high surface area (single sheets of carbon atoms), functionalized nanoscale GO or RGO bioconjugates have been widely explored as drug and gene carriers18–23. Because of their high near-IR (NIR) absorbance, functionalized graphene derivatives can be used as photothermal agents for cancer photothermal therapy, and they have shown excellent tumor ablation therapeutic effects in a number of animal studies8,24,25. Targeting ligands such as an antibody or peptide can be conjugated to functionalized nano-graphene for targeted drug delivery23,26,27, specific cell labeling,19 and in vivo tumor positron emission tomography (PET) imaging28–30. In addition, inorganic nanoparticles, such as magnetic iron oxide nanoparticles (IONPs), can be grown on the surface of GO, obtaining functional graphene-based nanocomposites (GO-IONPs) with interesting optical and magnetic properties that can be useful for multimodal imaging and imaging-guided cancer therapy24,31. Moreover, we and others have shown that well-functionalized graphene derivatives are nontoxic in vitro to cells13–15,32 and in vivo to mice via i.v. injection, oral feeding and i.p. injection at the tested doses2,33–35 (Supplementary Table 1).
Despite the explosion of interest in the development of graphene-based materials for a wide range of biomedical applications, the descriptions of methods to prepare those materials vary substantially from article to article, resulting in substantial confusion for newcomers. For example, the detailed procedures used to synthesize GO can be inconsistent in the literature36,37. There are also a number of different methods for preparing RGO by reducing GO with various chemical reagents (e.g., hydroxylamine hydrochloride, formaldehyde, vitamin C, sodium borohydrate, sodium hydrosulfite and hydrazine hydrate)25,38–42. Although those samples have the same names (GO or RGO), their sizes and properties may have large variations. A detailed protocol to describe how precisely those interesting graphene-based bioconjugates are made is therefore highly desired.
In the past few years, our group has made major efforts to explore the biomedical applications of graphene, particularly as a potential cancer theranostic agent. In this article, we will systematically summarize the procedures used in our laboratory to make functionalized nano-graphene bioconjugates for various biomedical applications1,8,24,28.
The preparation of various GO derivatives and their surface functionalization, particularly PEG coating, is the main focus of this protocol. This protocol could be particularly helpful to researchers working or interested in the field of graphene bio-research or nanomedicine in general. The major goal of this article is to provide standard and reliable protocols to guide researchers in this field toward fabricating graphene-based bioconjugates with high reproducibility, which is often lacking in nano-biotechnology.
Step 1 covers the preparation of GO and its derivatives, and their quality control is described in Step 2. Step 3 covers the preparation and use of two example GO-based bioconjugates. Considering the similarities in bioconjugation methods between PEGylated GO derivatives and PEGylated single-walled carbon nanotubes (SWNTs), as well as the fact that protocols for the latter have been carefully described in an earlier Nature Protocols article43, we will only introduce the 125I labeling of PEGylated GO derivatives and the preparation of GO-based gene carriers in this article, as these two parts are not covered in the previous paper.
Step 1: preparation of GO and its derivatives
Preparation of GO
GO is probably the most commonly used graphene material for biomedical applications, as it can be synthesized in large scales using relatively easy and inexpensive methods, and it contains many functional groups on the surface that are available for further conjugation of polymer or biomolecules. The synthesis of GO starting from graphite is typically based, with slight modifications, on a method (called Hammer’s method) developed in 1958 (ref. 44; this synthesis is described in Step 1A).
Covalent PEGylation of GO
Covalently conjugating GO with carboxyl groups to amine-terminated branched PEG (e.g., six-armed-PEG-NH2), with the help of N-(3-dimethylaminopropyl-N′-ethylcarbodiimide) hydrochloride (EDC) as the catalyst, is the most commonly used approach in our group to prepare PEGylated nano-GO (nGO-PEG) with ultra-small sizes (10–50 nm)8,9,18,22. Compared with as-made GO, nGO-PEG shows much smaller sizes owing to the sonication involved during the PEG conjugation step that greatly breaks down the nanosheets. We describe this in Step 1B. Note that RGO with the majority of carboxyl groups removed cannot be effectively conjugated with amine-terminated PEG by such a covalent chemistry.
Preparation of RGO and nRGO with different sizes
GO with abundant oxygen-containing groups on its surface can be reduced by various reducing chemicals to obtain RGO. Compared with GO, RGO shows a much lower oxygen content with the majority of oxygen-containing groups such as carboxyl, hydroxyl and ketone moieties removed during the reduction process and thus usually has a poor water solubility. However, RGO with largely restored aromatic structure shows markedly enhanced absorbance in the NIR region and is a powerful agent in photothermal therapy of cancer25,45. Refluxing GO in hydrazine hydrate is one of the most commonly used approaches to prepare RGO. This is described in Step 1C. In order to obtain nanoscale RGO, or nRGO, with ultra-small sheet sizes (<50 nm), nGO-PEG can be used to replace GO as the starting material25,45. In this process, the coating PEG polymer would be removed and the resulting nRGO sample would become water insoluble. This process is also described in Step 1C.
Preparation of magnetic GO nanocomposites
Magnetic GO-based nanocomposites can be prepared by a hydrothermal growth method starting from GO. In this process, with sodium acrylate and sodium acetate acting as the reducing agents, FeCl3 is hydrolyzed and reduced to form Fe3O4 IONPs attached on the surface of GO. Notably, GO can also be partially reduced during this reaction, yielding RGO-IONPs with strong superparamagnetism24,31. This procedure is described in Step 1E.
Noncovalent functionalization of RGO, nRGO and RGO-IONP
RGO, nRGO and RGO-IONP with limited numbers of available -COOH groups can be noncovalently functionalized by sonication in solutions of amphiphilic polymers such as our home-made C18PMH-PEG or commercially available phospholipid-PEG25,45. The hydrophobic chains of C18PMH-PEG are strongly anchored onto the RGO surface, whereas the hydrophilic PEG chains offer those materials great water solubility and excellent stability in various biological solutions. It is worth noting that RGO-PEG prepared by sonicating RGO in the C18PMH-PEG solution would show much larger sheet sizes (atomic force microscopy (AFM)-measured average diameter ~67 nm) compared with nGO-PEG with covalent PEG coating (AFM-measured average diameter ~23 nm). Therefore, to obtain ultra-small PEGylated RGO (nRGO-PEG), a reliable approach is to reduce nGO-PEG to make nRGO, which is then sonicated in C18PMH-PEG to yield nRGO-PEG with similarly ultra-small sizes (AFM-measured average diameter ~25 nm) as that of nGO-PEG25,45. The PEG coating density of RGO-PEG and nRGO-PEG, however, is thicker than that of nGO-PEG, as evidenced by the increased sheet thickness revealed by AFM, as well as the remarkably prolonged blood circulation half-life of the former after i.v. injection. The concentrations of RGO and nRGO are determined by their UV-visible absorption at 270 nm with a weight extinction coefficient of 70 mg ml−1 cm−1. This process is described in Steps 1D and 1F.
Compared with other reported methods for preparing functionalized GO derivatives, our approach based on PEGylation offers a number of different advantages8,25,33: (i) compared with GO without surface coating, the PEGylation derivatives have much lower lung uptake and show remarkably reduced toxicity in vitro and in vivo; (ii) the use of amine-terminated branched PEG to functionalize GO allows us to use the amino groups for further conjugation of antibody, peptide, fluorescence dye and radiolabeling linker; and (iii) compared with other polymers such as amine-modified dextran or chitosan9,46, PEG has a defined structure and molecular weight. However, successful PEGylation requires careful controlling of the preparation procedures (e.g., appropriate treating of GO with base, adding the right amount of catalyst during reaction and so on) and thus needs a detailed protocol as a guideline.
Step 2: quality control steps
It is important to check the quality of functionalized graphene derivatives on the basis of various characterization approaches, such as stability in saline solution (Step 2A), UV-visible-NIR spectra (Step 2B) and AFM images (Step 2C).
RGO and nRGO usually have limited water solubility. GO and RGO-IONP, although soluble in water, are not stable in the presence of salts. Therefore, the most straightforward method to determine whether PEGylation is successful or not is to test the stability of PEGylated samples in saline. Generally speaking, we usually add the PEGylated samples into solutions containing 9% (wt/vol) sodium chloride, which is 10× physiological saline, and then centrifuge the samples to check their saline stability. GO derivatives with acceptable PEG coating show few precipitates under such conditions.
UV-visible NIR spectra of various GO derivatives should also be recorded in the quality check step. The UV/NIR absorbance ratios of various samples calculated by the UV peak absorbance (~230 nm for GO and nGO-PEG, ~270 nm for RGO-PEG and nRGO-PEG) versus their NIR absorbance at 800 nm are important in order to determine whether the samples have been correctly prepared or not (e.g., with sufficient reduction for RGO samples). We also use the UV absorbance peaks of those samples to determine the concentrations.
Finally, AFM imaging of various GO derivatives can be carried out to determine the sizes and thicknesses of various samples. Compared with other nanoscale imaging techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM), AFM is probably the most convenient method to characterize the morphology of this type of 2D nanomaterial, as AFM shows a much higher resolution compared with SEM and offers better contrast compared with TEM.
Step 3: Preparation of GO-based bioconjugates
125I labeling of nGO-PEG, nRGO-PEG and RGO-PEG
125I labeling as a relatively accurate method to track the in vivo behaviors of GO and its derivatives has been intensively used in our group. The half-life of 125I is ~60 d, which is much longer than many other isotopes used for nanomaterial labeling, such as 64Cu (t1/2 = 12.7 h), thereby allowing us to track the in vivo behaviors of PEGylated GO over a much longer period of time. In contrast, 125I can be easily labeled to the defect sites of PEGylated GO without a need to use the functional groups on PEG. However, on the basis of our observation, although 125I labeling on PEGylated GO is quite stable, 125I labeled on uncoated GO could gradually detach from GO in physiological solutions, likely due to the instability of GO in the presence of salts and proteins. We compared the biodistribution of free 125I with that of 125I-labeled nGO-PEG in our previous study33 and found that free 125I was rapidly excreted within 6 h of i.v. injection (Supplementary Fig. 1).
Labeling of PEGylated GO with radioactive iodine happens on the defect sides of the GO sheets (e.g., on the edges or within the GO sheet with dangling bonds and broken six-member rings) by an electrophilic addition reaction, and thus it does not require the amino groups on the PEG coating. Different PEGylated GO derivatives including nGO-PEG, nRGO-PEG, RGO-PEG and even RGO-IONP-PEG can all be labeled by 125I using a standard chloramine-T oxidation method (Step 3A).
Use of nGO-PEG-PEI for transfection
In our and others’ previous work, polyethyleneimine (PEI)-functionalized GO (GO-PEI) complexes prepared via either the electrostatic interaction between GO sheets and PEI or the covalent conjugation between carboxyl groups of GO and amino groups of PEI have been found to be effective for in vitro plasmid DNA and siRNA delivery20,47. However, we discovered that GO-PEI would aggregate in serum-containing environment, resulting in markedly decreased transfection efficiency when cells were transfected in the presence of serum. Inspired by the well-known serum stealth effect of PEG, we fabricated a dualpolymer–coated gene delivery carrier, nGO-PEG-PEI. Compared with GO-PEI, nGO-PEG-PEI exhibited superior physiological stability, reduced cytotoxicity and remarkably improved gene transfection efficiency without serum interference (Step 3B)21.
MATERIALS
REAGENTS
Preparation of GO and its derivatives
Graphite powder (Huadong graphite factory, China)
Potassium permanganate (KMnO4; Sinopharm Chemcial Reagent, cat. no. 7722-64-7).
N-(3-dimethylaminopropyl-N′-ethylcarbodiimide) hydrochloride (EDC; Sigma-Aldrich, cat. no. 25952)
Ultrapure water (18.2 MΩ cm; e.g., Milli-Q)
Concentrated sulfuric acid (H2SO4; Sinopharm Chemical Reagent)
Hydrochloric acid (HCl; Sinopharm Chemical Reagent, cat. no. 7647-01-0)
Sodium hydroxide (NaOH; Sinopharm Chemical Reagent, cat. no. 1310-73-2)
Hydrogen peroxide (H2O2; Sinopharm Chemical Reagent, cat. no. 7722-84-1) ! CAUTION H2SO4, HCl, NaOH and H2O2 are capable of causing very severe burns, especially when they are at high concentrations. Wear goggles, a lab coat and a face mask during experiments.
Ethylene glycol (EG; Sinopharm Chemical Reagent, cat. no. 107-21-1)
Diethylene glycol (DEG; Sinopharm Chemical Reagent, cat. no. 111-46-6)
Dichloromethane (CH2Cl2; Sinopharm Chemical Reagent, cat. no. 750902)
Triethylamine (TEA; Sinopharm Chemical Reagent, cat. no. 121448)
Trifluoroacetic acid (TFA; Sinopharm Chemical Reagent, cat. no. 76051) ! CAUTION TFA is capable of causing very severe burns, especially when it is at a high concentration. Wear goggles, a lab coat and a face mask during experiments.
PEI branched, average MW ~25,000 Da by light scattering, number average molecular weight (MN) ~10,000 by gel permeation chromatography, ≤1% water (Sigma-Aldrich, cat. no. 408727) ! CAUTION PEI may cause eye, skin and respiratory irritation and is harmful if swallowed. Wear goggles, a lab coat and a face mask during experiments.
Six-armed PEG-NH2, 10 kDa (SunBio, cat. no. P6AM-10)
mPEG5000-NH2 (Biomatrik, cat. no. 5672)
Poly(maleic anhydride-alt-1-octadecene) (C18PMH; Sigma-Aldrich, cat. no. 419117)
MF-Millipore membrane filters (Millipore, cat. no. VCWP04700)
Sodium acetate
Quality checking
Silicon wafer, p-type, boron-doped, 8–13_Ω cm, 500-µm thickness, 100-mm diameter (LijingKeji China)
(3-Aminopropyl)triethoxysilane (APTES; Sigma-Aldrich, cat. no. A3648)
Preparation of GO-based gene carrier
Enhanced GFP plasmid (EGFP; Clontech, cat. no. 632470)
Tris-EDTA buffer (Sigma-Aldrich, cat. no. T9285-100 ml)
HeLa human cervical cancer cells (ATCC, cat. no. CCl-2; see Reagent Setup)
PBS, 1× (GIBCO, cat. no. 10010-056)
PBS, 10× (GIBCO, cat. no. 70011-044)
RPMI-1640 medium (Invitrogen, cat. no. 11875-093)
High-glucose DMEM medium (Invitrogen, cat. no. 21063-045)
Low-glucose DMEM medium (Invitrogen, cat. no. 11885-084)
FBS (Invitrogen, cat. no. 10437-028)
Penicillin-streptomycin, liquid, 10,000 U penicillin; 10,000 mg streptomycin (Invitrogen, cat. no. 15140-163)
Thiazolyl blue tetrazolium bromide (MTT; Sigma-Aldrich, cat. no. 298-93-1)
CellTiter 96 kit, for cell viability MTS assay (Promega, cat. no. G3580)
Radiolabeling with iodine-125
Na125I(Chengdu nuclear isotope, Qualcomm) ! CAUTION Undergo appropriate training for handling radioactive materials. Wear goggles, a lab coat, a mask, and a radiation dosimeter badge and rings during experiments. Handling of radioactive isotopes should only be performed in designated rooms for those experiments. Check for any possible radioactive contamination after the experiments.
Chloramine-T (Sigma-Aldrich, cat. no. 857319)
Mice (Nanjing Peng Sheng Biological Technology; see Reagent Setup for details)
EQUIPMENT
AFM instrument (Multi-Mode V; Veeco)
Standard silicon cantilevers (Olympus, cat. no. OMCL-AC160TS-R3)
Zetasizer nano ZS (Malvern)
Sorvall Legend Micro 21 microcentrifuge (Thermo, cat. no. 75002435)
Sorvall Primo R benchtop centrifuges (Thermo, cat. no. 75005453)
Heraeus Primo R centrifuge (Thermo, cat. no. 75005440)
Ultrapure water system (Thermo)
DHG heating and drying oven (Shanghai Jinghong Laboratory Instrument, 9076A)
UV765 absorbance spectrometer (Shanghai Precision and Scientific Instrument)
Gel imaging analysis system (Peiqing Science & Technology)
Confocal laser-scanning microscope (Leica, TCS SP5II)
Flow cytometer (FACScan; Becton Dickinson)
Amicon centrifugal filter device, 4 ml, 100,000-Da MWCO (Millipore, cat. no. UFC810024)
Amicon centrifugal filter device, 15 ml, 100,000-Da MWCO (Millipore, cat. no. UFC901008)
Vacuum filtration device (Beijing North Branch of Analytic Instrument Company HG08-VF-204A)
Filter membrane, pore size 100 nm (Millipore, cat. no. VCTP04700)
Thermomixer Comfort, 1.5 ml (Eppendorf, cat. no. 5355 000.011)
Instant thin-layer chromatography strips (ITLC; Biodex, cat. no. 150–771)
Cyclone storage phosphor screen system (PerkinElmer, C431200)
REAGENT SETUP
Ultrapure water
Prepare ultrapure water by purifying deionized (DI) water using the Easypure RoDi system (Thermo) according to the standard protocol. The ultrapure water can be stored in autoclaved clean glass vials at room temperature (25 °C) for 1 month if appropriately sealed.
C18PMH-PEG synthesis
Prepare C18PMH-PEG according to a previously reported protocol48. In brief, weigh 143 mg of mPEG-NH2 (5k) and 10 mg of poly(maleic anhydride-alt-1-octadecene) into a 25-ml glass vial, and add 2 ml of dichloromethane containing triethylamine (200 µl). Allow the reaction to proceed at room temperature under magnetic stirring with the glass vial tightly sealed. Afterward, dry the solvent under a nitrogen stream. Dissolve the yielded solid product in 5 ml of water. Dialyze the solution against water (1 liter) using a 14-kDa MWCO membrane for 1 d to remove the excess mPEG-NH2, and then lyophilize the solution. The solid powder is the product of C18PMH-PEG, which can be stored at −20 °C for 6 months.
Phosphate buffer, 1× PB, pH 7.5
Dissolve 6.04 g of Na2HPO4·12H2O and 1.56 g of NaH2PO4·12H2O in 100 ml of ultrapure water. It can be stored at room temperature for 1 month.
Phosphate buffer, 1× PB, pH 8.0
Dissolve 3.21 g of Na2HPO4·12H2O and 2.96 g of NaH2PO4·12H2O in 100 ml of ultrapure water. It can be stored at room temperature for 1 month.
Phosphate buffer, 10× PB, pH 7.5
Dissolve 60.4 g of Na2HPO4·12H2O and 15.6 g of NaH2PO4·12H2O in 100 ml of ultrapure water. It can be stored at room temperature for 1 month.
Phosphate buffer, 10× PB, pH 8.0
Dissolve 32.1 g of Na2HPO4·12H2O and 29.6 g of NaH2PO4·12H2O in 100 ml of ultrapure water. It can be stored at room temperature for 1 month.
EGFP plasmid
Dissolve 50 µg of EGFP plasmid in 100 µl of Tris-EDTA (10 mM, pH 8.0) buffer at a concentration of 500 ng µl−1 before use. The dissolved mixture can be stored at −20 °C for 6 months. Avoid repeated freeze-thaw cycles.
Cell culture
Culture HeLa cells in DMEM (high glucose) supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin-streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. For transfection experiments, seed 8 × 104 cells in 500 µl of DMEM medium into each well of the 24-well plate, and incubate the plate at 37 °C for 24 h to ensure each well has reached ~70% confluency before transfection.
Animals
Healthy female BALB/c mice ages between 6 and 8 weeks, which are one of the most commonly used inbred strains, are used in our experiments. ! CAUTION Make sure to undergo appropriate training regarding animal handling and have animal protocols in place before performing animal studies.
EQUIPMENT SETUP
Atomic force microscope
AFM characterization is performed using a Veeco NanoScope IV AFM instrument. Standard silicon cantilevers purchased from Olympus (cat. no. OMCL-AC160TS-R3) are used as the AFM tip during imaging.
Confocal laser-scanning microscope
The confocal imaging experiment is conducted using a Leica SP5 confocal laser-scanning microscopy (CLSM) instrument. EGFP-transfected HeLa cells cultured in 35-mm culture dishes are imaged by CLSM under the ×10 objective with an excitation of 488 nm and an emission band between 500 and 600 nm.
Flow cytometry (FACS) machine
A FACSCalibur (BD) is used in our experiments for the flow cytometry assay. Cells are stained using propidium iodide (PI) to exclude dead cells. EGFP-transfected HeLa cells are analyzed on the basis of the fluorescence signals recorded in the channel 1 (EGFP fluorescence) and channel 3 (PI fluorescence). A flow rate of about 500 cells per second is used in the measurement.
PROCEDURE
Preparation of GO and its derivatives
1| The first step is to prepare GO derivatives with different surface chemistry and sizes (Table 1) for varied aims of applications (Fig. 1). The options include preparation of GO (Step 1A), nGO-PEG (Step 1B), RGO or nRGO (Step 1C), RGO-PEG or nRGO-PEG (Step 1D), RGO-IONP (Step 1E) and RGO-IONP-PEG (Step 1F).
- Preparation of GO ● TIMING 2 d
-
Mix 1g of graphite powder with 60 g of NaCl in a glass beaker. Grind this mixture in a mortar until a gray powder is formed. NaCl is added to increase the efficiency during grinding.! CAUTION When you are grinding, wear a lab coat and a face mask to avoid inhalation of the powder.
-
Suspend the powder in 1 liter of water (the NaCl will dissolve), and remove the dissolved NaCl by filtration. Put the filter paper containing graphite powder into oven and heat at 90 °C overnight to remove the water.! CAUTION Please ensure that the temperature of the oven is accurately controlled. Approximately 20% of the graphite will be lost during this step owing to grinding and washing.
- Dissolve the remaining power in 98% (vol/vol) H2SO4 (23 ml) under magnetic stirring in a three-necked flask (250 ml) for 8 h. ! CAUTION Sulfuric acid is highly corrosive. Wear a lab coat, gloves and goggles during experiments.
-
Place the three-necked flask in an ice-water bath. Gradually add 3 g of KMnO4 to it.! CAUTION Keep at least one neck open, and keep the temperature at <20 °C to avoid an explosion.
-
Move the three-necked flask into an oil bath at 40 °C and stir for 30 min.! CAUTION Keep the temperature precisely at 40 ± 2 °C. If the reaction temperature is too high or too low, the quality of GO may be affected.
-
Heat the mixture to 70 °C for 45 min. After heating for 15 min, the color of the mixture should change to a dark brown slurry.? TROUBIES HOOTING
-
Add 3 ml of DI water into the three-necked flask. Heat the mixture to 105 °C for 5 min. Add another 3 ml of DI water into the mixture with the temperature kept at 105 °C for 5 min. Thereafter, add another 40 ml of DI water with the temperature maintained at 100 °C for 15 min.▲ CRITICAL STEP Water should be added slowly. Make sure that the temperature is kept below 108 °C. If water is added too fast, the temperature of the reaction may be quickly increased to over 110 °C.
-
Terminate the reaction by adding DI water (140 ml) and 30% (vol/vol) H2O2 solution (10 ml).! CAUTION H2O2 is a dangerous chemical. Wear a lab coat, gloves and goggles during experiments.
- Centrifuge the product at 9,000g for 5 min at room temperature. Discard the supernatant and wash the precipitated solid twice using 5% (vol/vol) HCl; next, wash three times with DI water.
-
Re-disperse and store the final GO product in DI water at room temperature. The concentration of GO solution can be determined by its UV-visible NIR absorbance spectrum with a weight extinction coefficient of 47.6 mg ml−1 cm−1 at 230 nm.? TROUBLESHOOTING■ PAUSE POINT The GO can be stored at room temperature for 6 months without obvious changes in properties.
-
- Preparation of NGO-PEG ● TIMING 1–2 d
-
Add 1.8 g of NaOH into 10 ml of GO solution at the concentration of 10 mg ml−1 (the final product of Step 1A). Heat the mixture at 55 °C for 4 h.! CAUTION NaOH is highly corrosive. Wear lab coat, gloves and goggles during experiments.
-
Add 37% (vol/vol) HCl solution (6–7 ml) into the solution to neutralize NaOH. Wash the product four times with DI water to remove the salt. Collect the product (base-treated GO) during washing by centrifugation at 9,000g for 5 min.! CAUTION HCl is highly corrosive. Wear lab coat, gloves and goggles during experiments.
-
Add 25 mg of six-armed PEG-NH2 into the base-treated GO solution (1 mg ml−1, 5 ml) in a 25-ml glass vial. Sonicate the vial vigorously in a bath sonicator at room temperature for 15 min, and then add 5 mg of EDC. Sonicate the solution for 30 min at room temperature, and then add another 5 mg of EDC. After further sonication for 20 min, stir the reaction solution overnight at room temperature.! CAUTION Keep the temperature in the water bath <35 °C.▲ CRITICAI STEP Make sure that the vial is at the best position in the bath sonicator to ensure the most efficient sonication.
- Centrifiuge the solution at 21,000g for 30 min to remove any aggregates. Collect the supernatant.
- Add the above supernatant solution into a 15-ml Amicon centrifugal filter device (MWCO = 100 kDa). Centrifuge the device at 2,200g for ~10 min. The leftover solution volume in the filter should be less than ~1 ml. Fill the filter device with water to 15 ml. Wash the solution in the device five or six times by repeating the centrifuging and water-adding steps, to completely remove excess PEG and EDC in the obtained NGO-PEG solution. After the final wash step, add water and collect the solution retained in the filter device.
-
Record the UV-visible NIR absorbance of obtained NGO-PEG solution to determine its concentration (47.6 mg ml−1 cm−1 at 230 nm). Store the nGO-PEG solution at 4 °C.? TROUBIESHOOTING■ PAUSE POINT The obtained nGO-PEG can be stored at 4 °C for ~2 months without obvious changes in stability and other properties.
-
- Preparation of RGO or nRGO ● TIMING 1–2 d
- Add 1 ml of hydrazine hydrate into 10 ml of GO solution at the concentration of 10 mg ml−1 (the final product of Step 1A), or 10 ml of nGO-PEG solution at the concentration of 2 mg ml−1 (the final product of Step 1B), in a 50-ml round-bottomed flask. Reflux the solution at 95 °C for 24 h under stirring.
-
After cooling down the above solution to room temperature, use a vacuum filtration device to remove hydrazine hydrate through a 100-nm filter membrane. Wash the solid retained in the filtration device four or five times using DI water, yielding RGO or nRGO product in black solid.■ PAUSE POINT The as-made RGO or nRGO can be stored at room temperature for 6 months without obvious changes in properties.
- Preparation of RGO-PEG or nRGO-PEG ● TIMING 1–2 d
-
Add 5 ml of C18PMH-PEG (2 mg ml−1) into 1 mg of RGO or nRGO in a 25-ml glass vial. Sonicate the vial for 90 min.! CAUTION Make sure that the vial is at the best position in the bath sonicator to ensure the most efficient sonication. Keep the temperature in the water bath <35 °C.
- Centrifuge the obtained solution at 21,000g for 3 h to remove any unstable aggregates.
- Collect the supernatant and filter it through a 100-nm filter membrane with a vacuum filtration device. Wash the product three times with DI water to remove excess C18PMH-PEG.
-
Re-disperse the obtained RGO-PEG or nRGO-PEG product in DI water. Determine the concentration of RGO-PEG or nRGO-PEG by UV-visible NIR absorbance spectrum with a weight extinction coefficient of 70 mg ml−1 cm−1 at 270 nm.■ PAUSE POINT The obtained RGO-PEG and nRGO-PEG can be stored at 4 °C for ~2 months without obvious changes in stability and other properties.
-
- Synthesis of RGO-IONP composite ● TIMING 2 d
-
Mix 20 mg of GO (the final product of Step 1A), 270 mg of FeCl3·6H2O, 750 mg of sodium acrylate (CH2=CHCOONa) and 750 mg of sodium acetate in a 25-ml glass vial. Add 0.5 ml of EG and 9.5 ml of DEG. Sonicate the vial in a bath sonicator for 180 min at room temperature until a homogeneous dispersion is formed.! CAUTION Make sure that the vial is at the best position in the bath sonicator to ensure the most efficient sonication. Keep the temperature in the water bath <35 °C.▲ CRITICAL STEP The GO sample used here should have a very high concentration (~10 mg ml−1) to minimize the amount of water introduced into the reaction system.
-
Transfer the mixture to a Teflon-lined stainless steel autoclave and heat it at 200 °C for 10 h in an oven.! CAUTION Make sure the autoclave is tightly sealed before the mixture is transferred into the oven. This experiment should be carried out in a separate room without other people working around.
-
Let the autoclave cool down to room temperature. Wash the product, RGO-IONP in solid, three times with ethanol and three times with DI water to remove any impurities.? TROUBIESHOOTING
-
Re-disperse the obtained RGO-IONP product in DI water. Determine the concentration of the RGO-IONP solution (in terms of RGO concentration) by its UV-visible NIR absorbance spectrum with a weight extinction coefficient of 21.2 mg ml−1 cm−1 at 900 nm.■ PAUSE POINT The as-made RGO-IONP can be stored at room temperature for 6 months without obvious changes in properties.
-
- Preparation of rGO-IONP-PEG ● TIMING 1 d
- Add 50 mg of C18PMH-PEG5000 into the solution of RGO-IONP (1 mg ml−1, 5 ml) in a 25-ml glass vial.
-
Sonicate the vial in a bath sonicator for 30 min.! CAUTION Make sure that the vial is at the best position in the bath sonicator to ensure the most efficient sonication. Keep the temperature in the water bath <35 °C.
-
Centrifuge the solution at 2,200g for 5 min to remove any unstable aggregates.! CAUTION Select a suitable centrifugal speed to remove the aggregates without losing much product. A higher centrifugation speed may lead to a much lower yield.
- Collect the supernatant, and filter it through a 100-nm filter membrane using a vacuum filtration device. Wash the product three times with DI water to remove excess C18PMH-PEG.
-
Re-disperse the product retained in the filtration device in DI water. Store the obtained RGO-IONP-PEG at 4 °C.■ PAUSE POINT The obtained RGO-IONP-PEG may be stored at 4 °C for ~2 months without obvious changes in stability and other properties.
Table 1.
Sizes of various GO derivatives determined by AFM.
| Diameter (nm)a mean ± s.d. |
Thickness (nm) mean ± s.d. |
|
|---|---|---|
| GO | 495 ± 119 | 0.94 ± 0.12 |
| nGO-PEG | 24 ± 4.1 | 1.2 ± 0.45 |
| RGO-PEG | 55 ± 17 | 4.4 ± 0.86 |
| NRGO-PEG | 26 ± 6.1 | 5.7 ± 1.1 |
| RGO-IONP | 202 ± 87 | 11.9 ± 3.6 |
| RGO-IONP-PEG | 67 ± 24 | 13.5 ± 5.4 |
The values are presented as means ± s.d.
The diameter of a nano-sheet is calculated by (width × length) ×1/2.
Figure 1.
A schematic of Step 1 that includes preparation and surface functionalization of GO derivatives including GO, RGO, nRGO and RGO-IONP, as well as nGO-PEG, RGO-PEG, nRGO-PEG and RGO-IONP-PEG.
Quality control steps ● TIMING 1 d
2| Before using various PEGylated GO derivatives for further bioconjugation, it is important to ensure that they are of sufficient quality. Possible quality control tests include the saline stability test (option A), analysis by UV-visible-NIR (option B) and AFM (option C). Because the GO and RGO-IONP, as well as other samples with failed surface functionalization, would rapidly aggregate in the presence of salts owing to the electron-screening effect, we usually use the saline stability test as a convenient quality check approach to determine whether the PEG functionalization is successful or not.
- saline stability test ● TIMING 1 h
- Mix 0.5 ml each of nGO-PEG, RGO-PEG, nRGO-PEG and RGO-IONP-PEG with 0.5 ml of high-concentration saline (9% (wt/vol) NaCl).
- Centrifuge the nGO-PEG, RGO-PEG and nRGO-PEG samples at 21,000g for 5 min at room temperature, or centrifuge the RGO-IONP-PEG sample at 2,200g for 5 min at room temperature. PEGylated GO derivatives must be highly stable without substantial precipitation (<10%) under such harsh conditions.
- UV-visible NIR absorbance spectra ● TIMING 1 h
- Record UV-visible NIR absorbance spectra for the obtained GO, nGO-PEG, RGO-PEG and nRGO-PEG solutions. Make sure that the characteristic peaks of GO and nGO-PEG are at ~230 nm; their 230-nm/800-nm absorbance ratio should be ~7–8. Make sure that the characteristic peaks of RGO-PEG and nRGO-PEG are at ~270 nm; their 270-nm/800-nm absorbance ratios should be ~3–3.5.
- AFM characterization ● TIMING 2 h
- Prepare a silicon substrate by cutting a small piece (~5 × 5 mm) of silicon wafer.
- Treat the silicon substrate with APTES (12 µl in 10 ml of water) for 15 min, and wash it with acetone, methanol and isopropanol.
- Drop the solution of GO, nGO-PEG, RGO-PEG, nRGO-PEG or RGO-IONP-PEG with a concentration of ~0.05 mg ml−1 onto the surface of silicon substrate. After 10 min, wash the substrate with DI water and dry it by blowing nitrogen.
- Use AFM to image the various GO derivatives deposited on the silicon substrate.
Preparation of GO-based bioconjugates
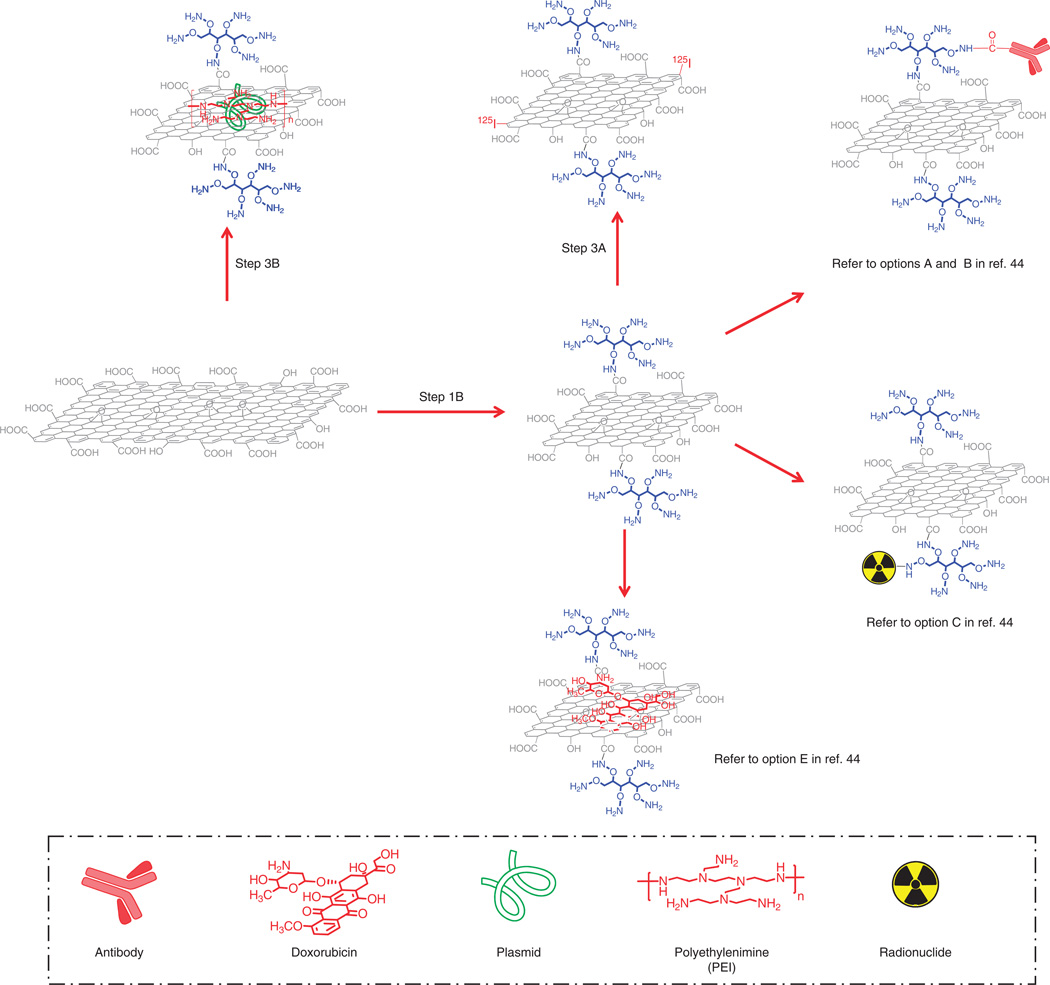
3| To realize specific biomedical applications, functionalized GO derivatives prepared in Step 1 can be further modified with various different approaches, as illustrated in Figure 2. However, as many of those procedures are quite similar to the bioconjugation of PEGylated SWNTs as described in detail in our previously published protocol, we will only focus on option 3A (125I labeling) and option 3B (preparation of GO-based gene carriers), which were not included in the earlier paper43. Although option 3A is generally applicable to all PEGylated GO derivatives including nGO-PEG, GO-IONP-PEG, nRGO-PEG, RGO-PEG and RGO-IONP-PEG, option 3B, which includes the preparation of the gene delivery system, uses GO as the starting material.
- 125I labeling ● TIMING 1 d
-
Mix 120 µl of nGO-PEG at 2 mg ml−1 (the final product of Step 1B) with Na125I (120 µCi), 100 µl of chloramine-T (4 mg ml−1) and 30 µl of 10× PB solution (pH 7.5). Incubate the mixture solution at room temperature for 30 min.! CAUTION Undergo appropriate training for handling radioactive materials. Wear goggles, a lab coat, a mask, and a radiation dosimeter badge and rings during these experiments. Handling of radioactive isotopes should only be performed in designated rooms for those experiments. Check for any possible radioactive contamination after the experiments.
-
Purify the solution through an Amicon centrifugal filter device (MWCO = 100 kDa), and wash it four or five times with DI water to remove excess 125I. A radiolabeling yield of 50% is usually achieved.! CAUTION Radioisotope waste, including contaminated devices such as filters, should be collected and disposed of in designated containers shielded with lead.? TROUBIESHOOTING■ PAUSE POINT 125I has a half-life of ~60 d. Store 125I-nGO-PEG at 4 °C until it is used. The 125I-nGO-PEG can be stored for 3–5 d without obvious loss or detachment of radioactivity.
- Inject 100 µl of 0.8 mg ml−1 125I-nGO-PEG into each of BALB/c mice through the tail vein.
-
For biodistribution studies, kill mice by cervical dislocation at 1 h, 6 h, 1 d, 3 d, 7 d, 15 d, 30 d and 60 d after injection. Collect and weigh the major organs and tissues. Use a gamma counter to measure the radioactivity levels in different tissue samples. Measure the radioactivities of diluted injection solutions (100 µl for each) with a series of dilutions (20–200 times) to get the calibration curve and determine the injection dose.! CAUTION The animal bodies should be collected in a designated freezer for radioactive-contaminated biohazardous waste. Label all animal cages clearly with radioactive marks. Leave the contaminated cages in the radioactive work-designated animal room for a week until the complete decay of 125I (half-life 60 d) before cleaning.
- Check for any possible radioactive contamination after the experiments (v) Calculate the %ID per g (injection dose per gram of tissue):
-
- Preparation of GO-based gene carriers (nGO-peG-peI) ● TIMING 1–2 d
-
Dilute base-treated GO (the product of Step 1B(ii)) to a concentration of 0.5 mg ml−1 with DI water. Mix 10 ml of GO (0.5 mg ml−1) with 5 mg of six-armed PEG-NH2 pre-dissolved in 0.5 ml. After sonication for ~5 min, add ~5 mg of EDC in 0.2 ml of water into the mixture, and sonicate it for another 5 min. Stir the mixture at room temperature for 10 min.▲ CRITICAL STEP Base-treated GO (product of Step 1B(ii)) instead of untreated GO (final product of Step 1A) should be used here. Make sure that you add EDC solution into the mixture immediately after it is dissolved in water to avoid hydrolysis of EDC.
- Add 0.5 ml of 50 mg ml−1 PEI stock solution into the mixture. After sonication for 5 min, add ~10 mg of EDC in 0.2 ml of water into the mixture. Sonicate the solution for another 5 min, and then let it react at room temperature for ~6 h under stirring.
-
Remove excess polymers by filtration using a vacuum filtration device through 100-nm filter membrane. Wash the product three to five times with 4 ml of DI water each time. Re-disperse the obtained nGO-PEG-PEI in 1 ml of DI water.? TROUBIESHOOTING■ PAUSE POINT nGO-PEG-PEI is stable at 4 °C for 2 months. It is recommended to use it for the gene transfection experiment within 1–2 weeks.
- Dilute 1 µg of EGFP plasmid in 100 µl of the medium without FBS, mix gently and incubate it at room temperature for 5 min.
- Add the designated amount of nGO-PEG-PEI (2.1, 4.3, 8.5 and 17.0 µg) to the diluted plasmid DNA mixture to make four samples, respectively. Mix gently and incubate them at room temperature for 20 min.
- Add the prepared mixtures into each well containing HeLa cells at ~70% confluency and 900 µl of FBS-free or FBS-containing medium. Mix gently by rocking the plate back and forth.
- Incubate the cells with nGO-PEG-PEI/plasmid DNA complexes at 37 °C for 4 h, and then wash the cells twice with PBS and re-incubate them in 500 µl of fresh complete cell medium at 37 °C for another 44 h.
- To detect EGFP fluorescence, image the cells by CLSM with excitation at 488 nm and at an emission wavelength between 500 nm and 600 nm.
- To analyze the transfection efficiency of cells with the flow cytometer, collect the cells after imaging and stain them with PI at 1 µg ml−1 for 15 min to exclude dead cells. Record EGFP and PI fluorescence signals by channel 1 (green) and 3 (red), respectively. Exclude PI-positive cells (dead cells) in the data analysis. Determine the EGFP transfection efficiency at each nitrogen/phosphorous (N/P) ratio on HeLa cells by calculating EGFP-positive cells in each cell sample.
-
Figure 2.
A schematic to show further bioconjugation of PEGylated nano-GO (Step 3). Step 3A is 125I labeling of nGO-PEG, and Step 3B is the preparation of nGO-PEG-PEI and the subsequent complexing with pDNA for gene transfection. The procedures for antibody conjugation, 64Cu labeling and drug loading to PEGylated nano-GO can be found in the preparation of corresponding bioconjugates based on PEGylated SWNTs, as described in detail in ref. 44.
? TROUBIESHOOTING
Troubleshooting advice can found in Table 2
Table 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A(vi) | The color of the reacted solution is black | Reaction temperature may have exceeded 70 °C | Keep the reaction temperature at 70 °C |
| 1A(x) | The GO yield is very low | Grinding in Step 1A(i) is not efficient | Increase grinding force and time |
| 1B(vi) | GO aggregates during reaction | The amount of added EDC in a single portion into the solution is excessive | Add EDC in smaller portions |
| 1E(ii) | The RGO-IONP product has low stability in the aqueous suspension | The temperature of the autoclave is <200 °C There is too much water in the added as-made GO sample | Make sure the reaction temperature is ~200 °C Use a GO solution with a higher concentration to minimize its water content |
| 3A(ii) | The radiolabeling yield is very low | Na125I and/or chloramine-T used in the experiment might have been stored too long | Use fresh 125I and chloramine-T in the experiment |
| 3B(iii) | nGO-PEG-PEI is very toxic to cells | The excess PEI was not completely removed | Use 100-nm filter membranes, not Amicon centrifugal filter devices (MWCO = 100 kDa) to purify the final product. Wash the sample extensively with DI water |
● TIMING
Step 1A, preparation of GO: 2 d
Step 1B, preparation of nGO-PEG: 1–2 d
Step 1C, preparation of RGO and nRGO: 1–2 d
Step 1D, preparation of RGO-PEG and nRGO-PEG: 1–2 d
Step 1E, synthesis of RGO-IONP composite: 2 d
Step 1F, preparation of RGO-IONP-PEG: 1 d
Step 2A, saline stability test: 1 h
Step 2B, UV-visible NIR absorbance spectra: 1 h
Step 2C, AFM characterization: 2 h
Step 3A, 125I labeling of nGO-PEG, nRGO-PEG and RGO-PEG: 1 d
Step 3B, preparation of GO-based gene carriers (nGO-PEG-PEI): 1–2 d
ANTICIPATED RESULTS
Functionalization of GO and its derivatives
All GO derivatives prepared in Step 1 are water soluble, except RGO and nRGO before PEGylation, which have limited water solubility. GO and RGO-IONPs are water soluble but would precipitate in the presence of salts.
In contrast, PEGylated GO derivatives including nGO-PEG, RGO-PEG, nRGO-PEG and RGO-IONP-PEG are all highly stable in various biological solutions including saline and serum (Fig. 3a). Although GO and nGO-PEG show the UV-visible absorbance peak at ~230 nm, the absorbance peaks of RGO-PEG and nRGO-PEG are located at ~270 nm (Fig. 3b).
Figure 3.
Characterization of various functionalized GO derivatives. (a) The photos show various functionalized GO derivative in different solutions including DI water, saline and serum. (b) UV-visible NIR spectra of GO, nGO-PEG, RGO-PEG and NRGO-PEG. (c) UV-visible spectra of GO and RGO-IONP-PEG. RGO-PEG, nRGO-PEG and RGO-IONP-PEG exhibit much higher NIR absorbance compared with GO. (d) AFM images of various functionalized GO derivatives. This figure is adapted from refs. 24,25 and reproduced with permission from Elsevier.
Compared with GO and nGO-PEG, RGO-PEG, nRGO-PEG and RGO-IONP-PEG exhibit greatly enhanced NIR absorbance (Fig. 3b,c).
AFM is used to determine the sizes of GO derivatives (Fig. 3d). The size of GO before PEGylation has a wide distribution around 100–500 nm, whereas nGO-PEG sheets after PEGylation are much smaller with an average size of ~23 nm. The average sizes of RGO-PEG and nRGO-PEG are ~65 and ~27 nm, respectively. With covalent functionalization, the sheet thickness of nGO-PEG is 1–2 nm; in contrast, the sheet thickness of RGO-PEG and nRGO-PEG with noncovalent functionalization is increased to ~4–6 nm due to polymer (C18PMH-PEG) coating on RGO or nRGO surface23. Similarly to GO and nGO-PEG, the average size of PEGylated RGO-IONP became much smaller (~67 nm) than RGO-IONP (~202 nm)22 (Fig. 3d).
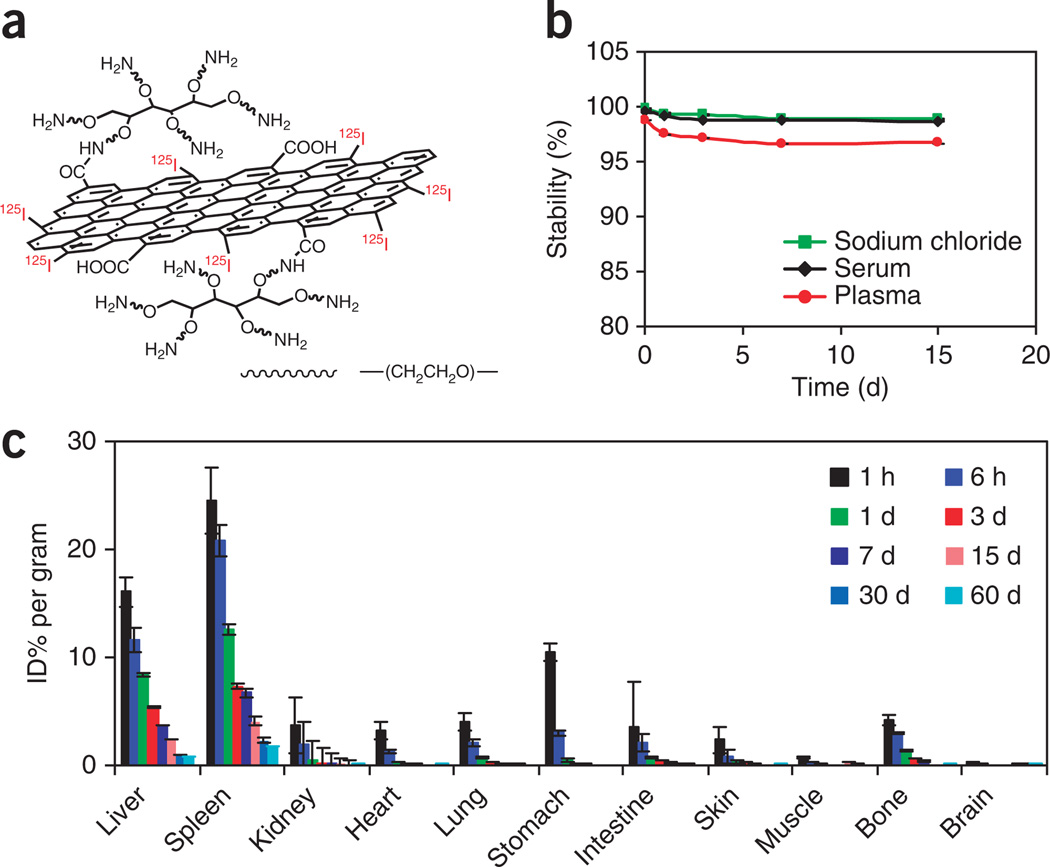
125I-labeled nGO-PEG for long-term biodistribution studies
In this labeling method, 125I atoms are anchored on the defect sites or edges of the GO sheet (Fig. 4a). The obtained 125I-nGO-PEG would show great radiolabeling stability without marked detachment of labeled 125I in saline, serum and plasma under 37 °C within 2 weeks (Fig. 4b). BALB/c mice i.v. injected with 125I-nGO-PEG are killed at different time points after being injected. Biodistribution data based on radioactivity measurement reveal that nGO-PEG would mainly accumulate in the reticuloendothelial system organs including liver and spleen after i.v. administration (consistent with PET imaging data based on 64Cu-labeled nGO-PEG)29 and can be gradually flushed out from the mouse body over time31 (Fig. 4c).
Figure 4.
125I-labeled nGO-PEG for the in vivo biodistribution study. (a) A schematic of 125I-nGO-PEG. (b) The radiolabeling stability of 125I-nGO-PEG in saline, serum and mouse plasma at 37 °C. (c) Biodistribution of 125I-nGO-PEG in mice measured at different time points after i.v. injection. Error bars show s.d., n = 4 or 5 mice per group. This figure is adapted from ref. 33 and reproduced with permission from ACS Publications.
GO-based gene transfection
Prepared nGO-PEG-PEI with an ultra-small size (Fig. 5a) shows excellent stability in physiological solutions (Fig. 5b), as well as lower cytotoxicity, when compared with bare PEI polymer (Fig. 5c). nGO-PEG-PEI shows high EGFP pDNA transfection efficiency, which is not markedly affected by adding FBS during transfection. In contrast, although satisfied transfection efficiencies of bare PEI polymer and GO-PEI can be achieved in FBS-free medium, the existence of serum would lead to substantially decreased transfection efficiencies when these two are used as the pDNA transfection agents19 (Fig. 5d,e).
Figure 5.
Graphene-based gene transfection. (a) An AFM image of nGO-PEG-PEI (Step 3B). (b) Photos of nGO-PEG-PEI in saline and serum-containing cell medium. (c) Relative viabilities of cells treated with nGO-PEG-PEI, GO-PEI and free PEI. Error bars show s.d. on the basis of triplicate samples. (d) Confocal images of EGFP-transfected HeLa cells using nGO-PEP-PEI, GO-PEI or free PEI as the transfection agent in the absence and presence of 10% FBS. (e) Relative EGFP transfection efficiencies of nGO-PEP-PEI, GO-PEI and free PEI in the presence of different concentrations of FBS. Error bars show s.d. of at least four parallel measurements. This figure is adapted from our previously published work21 and reproduced with permission from Wiley-VCH.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China (973 Program, 2012CB932600 and 2011CB911002), the National Science Foundation of China (NSFC, 51002100, 51222203, 51132006) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the University of Wisconsin at Madison, the US National Institute of Biomedical Imaging and Bioengineering/National Cancer Institute (1R01CA169365), and the US Department of Defense (W81XWH-11-1-0644).
Footnotes
Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS Z.L. and W.C. designed the experiments and wrote the manuscript; K.Y., L.F. and H.H. performed the experiments, analyzed the results and wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2013;42:530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 2.Yang K, Li Y, Tan X, Peng R, Liu Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small. 2013;9:1492–1503. doi: 10.1002/smll.201201417. [DOI] [PubMed] [Google Scholar]
- 3.Feng LZ, Liu ZA. Graphene in biomedicine: opportunities and challenges. Nanomedicine. 2011;6:317–324. doi: 10.2217/nnm.10.158. [DOI] [PubMed] [Google Scholar]
- 4.Bitounis D, Ali-Boucetta H, Hong BH, Min D-H, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013;25:2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 5.Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics. 2012;2:283–294. doi: 10.7150/thno.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Nayak TR, Hong H, Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4:3833–3842. doi: 10.1039/c2nr31040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng L, Wu L, Qu X. New horizons for diagnosis and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013:168–186. doi: 10.1002/adma.201203229. [DOI] [PubMed] [Google Scholar]
- 8.Yang K, et al. Graphene in mice: ultra-high in vivo tumor uptake and photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SA, Yang K, Feng LZ, Liu Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon. 2011;49:4040–4049. [Google Scholar]
- 10.Lu C-H, Yang H-H, Zhu C-L, Chen X, Chen G-N. A graphene platform for sensing biomolecules. Angew. Chem. Int. Ed. 2009;48:4785–4787. doi: 10.1002/anie.200901479. [DOI] [PubMed] [Google Scholar]
- 11.He S, et al. A Graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv. Funct. Mater. 2010;20:453–459. [Google Scholar]
- 12.Tang LAL, Wang J, Loh KP. Graphene-based SELDI probe with ultrahigh extraction and sensitivity for DNA oligomer. J. Am. Chem. Soc. 2010;132:10976–10977. doi: 10.1021/ja104017y. [DOI] [PubMed] [Google Scholar]
- 13.Lee WC, et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5:7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 14.Nayak TR, et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5:4670–4678. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, et al. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011;23:H263–H267. doi: 10.1002/adma.201101503. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Karni T, Qing Q, Li Q, Fang Y, Lieber CM. Graphene and nanowire transistors for cellular interfaces and electrical recording. Nano Lett. 2010;10:1098–1102. doi: 10.1021/nl1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen P, Berry V. Graphene interfaced with biological cells: opportunities and challenges. J. Phys. Chem. Lett. 2012;3:1024–1029. doi: 10.1021/jz300033g. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Robinson JT, Sun XM, Dai HJ. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3:1252–1257. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, et al. Polyethylene glycol and polyethylenimine dualfunctionalized nano-graphene oxide for photothermally enhanced gene delivery. Small. 2013;9:1989–1997. doi: 10.1002/smll.201202538. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 24.Yang K, et al. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012;24:1868–1872. doi: 10.1002/adma.201104964. [DOI] [PubMed] [Google Scholar]
- 25.Yang K, et al. The influence of surface chemistry and particle size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials. 2012;33:2206–2214. doi: 10.1016/j.biomaterials.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 26.Huang P, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–250. doi: 10.7150/thno/v01p0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, et al. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013;135:4799–4804. doi: 10.1021/ja312221g. [DOI] [PubMed] [Google Scholar]
- 28.Shi S, et al. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials. 2013:3002–3009. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong H, et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong H, et al. In vivo targeting and positron emission tomography imaging of tumor vasculature with Ga-66-labeled nano-graphene. Biomaterials. 2012;33:4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, et al. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012;5:199–212. [Google Scholar]
- 32.Li Y, et al. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33:402–411. doi: 10.1016/j.biomaterials.2011.09.091. [DOI] [PubMed] [Google Scholar]
- 33.Yang K, et al. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5:516–522. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 34.Duch MC, et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011;11:5201–5207. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang K, et al. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials. 2013;34:2787–2795. doi: 10.1016/j.biomaterials.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Yan L, Bangal PR. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon. 2010;48:1146–1152. [Google Scholar]
- 37.Hernandez Y, et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotech. 2008;3:563–568. doi: 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- 38.Mao S, et al. A new reducing agent to prepare single-layer, high-quality reduced graphene oxide for device applications. Nanoscale. 2011;3:2849–2853. doi: 10.1039/c1nr10270b. [DOI] [PubMed] [Google Scholar]
- 39.Tang X-Z, Cao Z, Zhang H-B, Liu J, Yu Z-Z. Growth of silver nanocrystals on graphene by simultaneous reduction of graphene oxide and silver ions with a rapid and efficient one-step approach. Chem. Commun. 2011;47:3084–3086. doi: 10.1039/c0cc05613h. [DOI] [PubMed] [Google Scholar]
- 40.Ding YH, et al. A green approach to the synthesis of reduced graphene oxide nanosheets under UV irradiation. Nanotechnology. 2011;22:215601. doi: 10.1088/0957-4484/22/21/215601. [DOI] [PubMed] [Google Scholar]
- 41.Gurunathan S, Han JW, Eppakayala V, Jeyaraj M, Kim J-H. An environmentally friendly approach to the reduction of graphene oxide by Escherichia fergusoni . J. Nanosci. Nanotechnol. 2013;13:2091–2098. doi: 10.1166/jnn.2013.6738. [DOI] [PubMed] [Google Scholar]
- 42.Zhou T, et al. A simple and efficient method to prepare graphene by reduction of graphite oxide with sodium hydrosulfte. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/4/045704. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009;4:1372–1382. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hummers WS, Offeman RE. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80:1339–1339. [Google Scholar]
- 45.Robinson JT, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133:6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 46.Bao HQ, et al. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small. 2011;7:1569–1578. doi: 10.1002/smll.201100191. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, et al. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small. 2011;7:460–464. doi: 10.1002/smll.201001522. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, et al. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials. 2011;32:144–151. doi: 10.1016/j.biomaterials.2010.08.096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.