Abstract
σB, the stress-activated σ factor of Bacillus subtilis, requires the RsbT protein as an essential positive regulator of its physical stress pathway. Stress triggers RsbT to both inactivate the principal negative regulator of the physical stress pathway (RsbS) by phosphorylation and activate a phosphatase (RsbU) required for σB induction. Neither the regions of RsbT that are involved in responding to stress signaling nor those required for downstream events have been established. We used alanine scanning mutagenesis to examine the contributions of RsbT's charged amino acids to the protein's stability and activities. Eleven of eighteen rsbT mutations blocked σB induction by stress. The carboxy terminus of RsbT proved to be particularly important for accumulation in Bacillus subtilis. Four of the five most carboxy-terminal mutations yielded rsbT alleles whose products were undetectable in B. subtilis extracts. Charged amino acids in the central region of RsbT were less critical, with four of the five substitutions in this region having no measurable effect on RsbT accumulation or activity. Only when the substitutions extended into a region of kinase homology was σB induction affected. Six other RsbT variants, although present at levels adequate for activity, failed to activate σB and displayed significant changes in their ability to interact with RsbT's normal binding partners in a yeast dihybrid assay. These changes either dramatically altered the proteins' tertiary structure without affecting their stability or defined regions of RsbT that are involved in multiple interactions.
σB is a Bacillus subtilis transcription factor that controls the bacterium's general stress regulon. This is a collection of more than 200 genes whose transcription is elevated after exposure to physical (e.g., heat shock, ethanol, or osmotic shock) or nutritional (e.g., glucose limitation, phosphate limitation, or azide treatment) stress (7, 18, 24, 25, 31). Induction of the general stress regulon is triggered by the activation of σB (4-6, 8, 9). σB is encoded by the seventh gene of an eight-gene operon, with the remaining genes specifying regulators of σB activity (20, 36). All eight genes are constitutively expressed from a promoter (PA) that is likely recognized by the cell's principal σ factor (σA). An internal σB-dependent promoter (PB) elevates the expression of the sigB operon's downstream four genes during periods of σB activity (i.e., PA ΔrsbR rsbS rsbT rsbU PB ΔrsbV rsbW sigB rsbX) (4, 8, 9, 20).
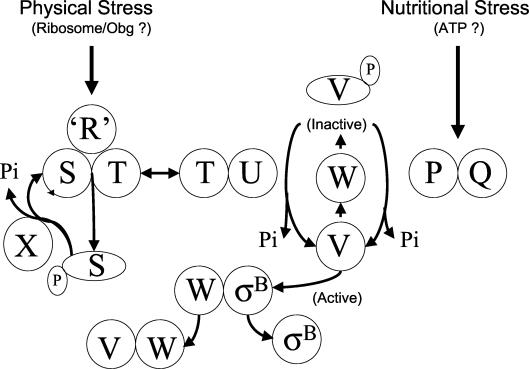
As illustrated in Fig. 1, the primary regulators of σB are RsbV and RsbW. RsbW is an anti-σB protein that binds σB and makes it unavailable to RNA polymerase (6, 14). RsbV is the σB release factor (6, 8). RsbW forms mutually exclusive complexes with either the RsbV protein or σB (13, 14). RsbV availability determines the amount of σB that is released from RsbW, with the phosphorylation state of RsbV controlling its activity (14). RsbW is both an RsbV/σB binding protein and an RsbV-specific kinase. In unstressed B. subtilis, an RsbW-dependent phosphorylation of RsbV blocks its ability to extract σB from RsbW (3, 14). RsbV-P is reactivated by either of two stress-specific phosphatases: one in a pathway that responds to nutritional stress and the other responsive to physical stress (21, 30, 32, 33, 37). Either of these enzymes can dephosphorylate RsbV-P and allow it to again displace σB from the RsbW-σB complex.
FIG. 1.
Model of σB activation. σB is held inactive in unstressed B. subtilis as a complex with an anti-σB protein, RsbW (W). σB is freed from RsbW when a release factor, RsbV (V), binds to RsbW. In unstressed B. subtilis, RsbV is inactive due to an RsbW-catalyzed phosphorylation (V-P). Physical stress activates an RsbV-P phosphatase, RsbU (U), which reactivates RsbV. RsbT (T) is the RsbU activator. RsbT is normally bound to a negative regulator RsbS (S), which inhibits its activity. RsbR and a family of related proteins (“R”) also bind to RsbS and RsbT and are believed to facilitate their interactions. Upon exposure to stress, RsbT phosphorylates and inactivates RsbS then activates the RsbU phosphatase. A ribosome-mediated process and Obg, a ribosome-associated GTPase that can bind to RsbT, play essential, but unknown roles in the activation of RsbT. RsbS-P is dephosphorylated and reactivated by a phosphatase RsbX (X) that is encoded by one of the genes downstream of the sigB operon's σB-dependent promoter. RsbX levels become elevated when σB is active, which may facilitate a return of RsbT to an inactive complex with RsbS. Energy depletion activates a separate pathway in which a novel RsbV-P phosphatase (P) and an associated protein (Q) are triggered, by unknown means, to reactivate RsbV. The model is based on references (1, 4, 6, 8, 14, 20, 27, 35-37, 40).
The nutritional stress phosphatase (RsbP) is cotranscribed with a predicted hydrolase or acryltransferase (RsbQ) that is needed for RsbP activity (10, 30). The metabolic inducers of the RsbP/Q phosphatase are unknown, but the conditions that trigger this pathway are associated with a decrease in the cell's ATP levels, suggesting that change in nucleotide pools may be involved in triggering the activation (33, 39).
The phosphatase that responds to physical stress (RsbU) requires an additional factor, the RsbT protein, for activity (37). In unstressed B. subtilis, RsbT is held inactive in a complex with its negative regulator, RsbS. Exposure to stress empowers RsbT to phosphorylate and inactivate RsbS and then activate RsbU (1, 12, 35, 37). Interactions between RsbS and RsbT are believed to be modulated by RsbR and a family of related proteins (1, 2, 16). Recent experiments (12) have demonstrated that RsbR, and presumably its homologs, can self-associate into large-molecular-mass complexes (∼106 Da) that can incorporate RsbS and RsbT. These complexes may represent the normal state of RsbR, RsbS, and RsbT proteins in unstressed B. subtilis. In vitro, the phosphorylation state of RsbR, which, like RsbS can be phosphorylated by RsbT, determines the effectiveness with which RsbT can phosphorylate and escape from RsbS (12, 16). σB activity returns to its prestress levels through the activity of RsbX, a RsbS-P-specific phosphatase that reactivates RsbS and allows it to again sequester RsbT into an inactivating complex (32, 37). The mechanisms by which the presence of physical stress is conveyed to RsbR, RsbS, and RsbT are unknown. There is evidence, however, that a ribosome-associated event may contribute to the process. σB activation by physical stress fails to occur in B. subtilis strains that are either missing ribosome protein L11 or are deficient in a ribosome-associated GTP-binding protein (Obg) (27, 28, 40).
Although RsbT is a critical positive regulator of the physical stress pathway, neither the regions that are involved in responding to stress nor those which catalyze downstream events have been established. In the present study we use alanine scanning mutagenesis to examine the contributions of RsbT's charged amino acids to its stability and activities.
MATERIALS AND METHODS
Bacterial strains, plasmids and cultivation.
The B. subtilis strains and the plasmids used for the present study are listed in Table 1. All BSA and BSW strains are derivatives of PY22. Bacteria were routinely grown in LB (26) at 37°C. Physical stress induction of σB was triggered by exposure of B. subtilis to ethanol during exponential growth at a final concentration of 4% (35). PSPAC promoter fusions were induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to 0.1 mM.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype | Construction or source |
|---|---|---|
| Bacillus strains | ||
| BSA46 | trpC2 SPβ ctc::lacZ | 4 |
| PY22 | trpC2 | 29 |
| XS352 | trpC2 aph3′ 5′′/sigB ΔrsbST SPβ ctc::lacZ | 29 |
| BSZ11 | trpC2 sigB ΔrsbST rsbX::pWH25 SPβ ctc::lacZ | pWH25 → XS352 → BSA46 |
| BSA661-T0 | trpC2 aph3′5′′/sigB rsbT SPβ ctc::lacZ | pDRNT → XS352 |
| BSA661-T1 | trpC2 aph3′5′′/sigB rsbT1 SPβ ctc::lacZ | pT1 → XS352 |
| BSA661-T2 | trpC2 aph3′5′′/sigB rsbT2 SPβ ctc::lacZ | pT2 → XS352 |
| BSA661-T3 | trpC2 aph3′5′′/sigB rsbT3 SPβ ctc::lacZ | pT3 → XS352 |
| BSA661-T4 | trpC2 aph3′5′′/sigB rsbT4 SPβ ctc::lacZ | pT4 → XS352 |
| BSA661-T5 | trpC2 aph3′5′′/sigB rsbT5 SPβ ctc::lacZ | pT5 → XS352 |
| BSA661-T6 | trpC2 aph3′5′′/sigB rsbT6 SPβ ctc::lacZ | pT6 → XS352 |
| BSA661-T7 | trpC2 aph3′5′′/sigB rsbT7 SPβ ctc::lacZ | pT7 → XS352 |
| BSA661-T8 | trpC2 aph3′5′′/sigB rsbT8 SPβ ctc::lacZ | pT8 → XS352 |
| BSA661-T9 | trpC2 aph3′5′′/sigB rsbT9 SPβ ctc::lacZ | pT9 → XS352 |
| BSA661-T10 | trpC2 aph3′5′′/sigB rsbT10 SPβ ctc::lacZ | pT10 → XS352 |
| BSA661-T11 | trpC2 aph3′5′′/sigB rsbT11 SPβ ctc::lacZ | pT11 → XS352 |
| BSA661-T12 | trpC2 aph3′5′′/sigB rsbT12 SPβ ctc::lacZ | pT12 → XS352 |
| BSA661-T13 | trpC2 aph3′5′′/sigB rsbT13 SPβ ctc::lacZ | pT13 → XS352 |
| BSA661-T14 | trpC2 aph3′5′′/sigB rsbT14 SPβ ctc::lacZ | pT14 → XS352 |
| BSA661-T15 | trpC2 aph3′5′′/sigB rsbT15 SPβ ctc::lacZ | pT15 → XS352 |
| BSA661-T16 | trpC2 aph3′5′′/sigB rsbT16 SPβ ctc::lacZ | pT16 → XS352 |
| BSA661-T17 | trpC2 aph3′5′′/sigB rsbT17 SPβ ctc::lacZ | pT17 → XS352 |
| BSA661-T18 | trpC2 aph3′5′′/sigB rsbT18 SPβ ctc::lacZ | pT18 → XS352 |
| BSW13B | trpC2 PSPACrsbT SPβ ctc::lacZ | pRW13 → BSA46 |
| BSW14 | trpC2 PSPACrsbT3 SPβ ctc::lacZ | pRW10 → BSA46 |
| BSW15 | trpC2 PSPACrsbT4 SPβ ctc::lacZ | pRW11 → BSA46 |
| BSW16 | trpC2 PSPACrsbT5 SPβ ctc::lacZ | pRW12 → BSA46 |
| BSW17 | trpC2 PSPACrsbT7 SPβ ctc::lacZ | pRW14 → BSA46 |
| BSW18 | trpC2 PSPACrsbT13 SPβ ctc::lacZ | pRW15 → BSA46 |
| BSW19 | trpC2 PSPACrsbT16 SPβ ctc::lacZ | pRW16 → BSA46 |
| BSW44 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT SPβ ctc::lacZ | pRW22 → XS352 |
| BSW45 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT5 SPβ ctc::lacZ | pRW23 → XS352 |
| BSW46 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT7 SPβ ctc::lacZ | pRW24 → XS352 |
| BSW47 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT16 SPβ ctc::lacZ | pRW25 → XS352 |
| BSW48 | trpC2 ΔrsbST PSPACrsbT3 SPβ ctc::lacZ | pRW10 → BSZ11 |
| BSW49 | trpC2 ΔrsbST PSPACrsbT4 SPβ ctc::lacZ | pRW11 → BSZ11 |
| BSW50 | trpC2 ΔrsbST PSPACrsbT5 SPβ ctc::lacZ | pRW12 → BSZ11 |
| BSW13A | trpC2 ΔrsbST PSPACrsbT SPβ ctc::lacZ | pRW13 → BSZ11 |
| BSW51 | trpC2 ΔrsbST PSPACrsbT7 SPβ ctc::lacZ | pRW14 → BSZ11 |
| BSW52 | trpC2 ΔrsbST PSPACrsbT13 SPβ ctc::lacZ | pRW15 → BSZ11 |
| BSW53 | trpC2 ΔrsbST PSPACrsbT16 SPβ ctc::lacZ | pRW16 → BSZ11 |
| BSW69 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT3 SPβ ctc::lacZ | pRW36 → XS352 |
| BSW70 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT4 SPβ ctc::lacZ | pRW37 → XS352 |
| BSW71 | trpC2 aph3′5′′/sigB PArsbRΔrsbS rsbT13 SPβ ctc::lacZ | pRW38 → XS352 |
| Plasmids | ||
| pACT-2 | Apr vector with AD of GAL4 | Clontech |
| pAS2-1 | Apr vector with BD of GAL4 | Clontech |
| pUV70 | Apr protein fusion of RsbS with AD of GAL4 | 34 |
| pUV134 | Apr protein fusion of RsbR with AD of GAL4 | 34 |
| pUV166 | Apr protein fusion of RsbT with AD of GAL4 | 34 |
| pUV187 | Apr protein fusion of RsbU with AD of GAL4 | 34 |
| pJMObg | Apr protein fusion of Obg with AD of GAL4 | 27 |
| pWH25 | Apr SpecrrsbX | 29 |
| pUK19 | Apr Kanr | 19 |
| pUK19-1.1 | Apr KanrspoIIB | This study |
| pDG148 | Apr KanrlacI PSPAC | P. Stagier |
| pRW10 | Apr KanrlacI PSPACrsbT3 | This study |
| pRW11 | Apr KanrlacI PSPACrsbT4 | This study |
| pRW12 | Apr KanrlacI PSPACrsbT5 | This study |
| pRW13 | Apr KanrlacI PSPACrsbT | This study |
| pRW14 | Apr KanrlacI PSPACrsbT7 | This study |
| pRW15 | Apr KanrlacI PSPACrsbT13 | This study |
| pRW16 | Apr KanrlacI PSPACrsbT16 | This study |
| pRW22 | Apr SpecrPArsbRΔrsbSrsbT | This study |
| pRW23 | Apr SpecrPArsbRΔrsbSrsbT5 | This study |
| pRW24 | Apr SpecrPArsbRΔrsbSrsbT7 | This study |
| pRW25 | Apr SpecrPArsbRΔrsbSrsbT16 | This study |
| pRW26 | Apr protein fusion of rsbT3 with BD of GAL4 | This study |
| pRW27 | Apr protein fusion of rsbT4 with BD of GAL4 | This study |
| pRW28 | Apr protein fusion of rsbT5 with BD of GAL4 | This study |
| pRW29 | Apr protein fusion of rsbT7 with BD of GAL4 | This study |
| pRW30 | Apr protein fusion of rsbT8 with BD of GAL4 | This study |
| pRW31 | Apr protein fusion of rsbT13 with BD of GAL4 | This study |
| pRW32 | Apr protein fusion of rsbT15 with BD of GAL4 | This study |
| pRW33 | Apr protein fusion of rsbT16 with BD of GAL4 | This study |
| pRW34 | Apr protein fusion of rsbT18 with BD of GAL4 | This study |
| pRW36 | Apr SpecrPArsbRΔrsbSrsbT3 | This study |
| pRW37 | Apr SpecrPArsbRΔrsbSrsbT4 | This study |
| pRW38 | Apr SpecrPArsbRΔrsbSrsbT13 | This study |
| pUS19 | Apr Specr | 5 |
| pUSD19-1 | Apr Specr NdeI removed | This study |
| pDRNT | Apr SpecrPArsbRST | This study |
| pT1 | Apr SpecrPArsbRST1 | This study |
| pT2 | Apr SpecrPArsbRST2 | This study |
| pT3 | Apr SpecrPArsbRST3 | This study |
| pT4 | Apr SpecrPArsbRST4 | This study |
| pT5 | Apr SpecrPArsbRST5 | This study |
| pT6 | Apr SpecrPArsbRST6 | This study |
| pT7 | Apr SpecrPArsbRST7 | This study |
| pT8 | Apr SpecrPArsbRST8 | This study |
| pT9 | Apr SpecrPArsbRST9 | This study |
| pT10 | Apr SpecrPArsbRST10 | This study |
| pT11 | Apr SpecrPArsbRST11 | This study |
| pT12 | Apr SpecrPArsbRST12 | This study |
| pT13 | Apr SpecrPArsbRST13 | This study |
| pT14 | Apr SpecrPArsbRST14 | This study |
| pT15 | Apr SpecrPArsbRST15 | This study |
| pT16 | Apr SpecrPArsbRST16 | This study |
| pT17 | Apr SpecrPArsbRST17 | This study |
| pT18 | Apr SpecrPArsbRST18 | This study |
PCR mutagenesis of rsbT.
Site-directed PCR mutagenesis was performed on rsbT by using high-fidelity Taq polymerase (Life Technologies, Rockville, Md.) to replace charged amino acids along the length of the protein with alanine. To prepare a substrate for rsbT mutagenesis and subsequent expression, the PArsbR-S and rsbT regions of the sigB operon were amplified in sequential steps from PY22 chromosomal DNA. Two sets of primers were used. The most upstream primer, which hybridizes 5′ to the sigB operon's promoter (PA) and incorporates a BamHI site, was paired for PCR with a primer containing the restriction site NdeI and designed to hybridize to the 3′ end of rsbS. A second pair of primers was designed to amplify rsbT, with the upstream primer containing the restriction site NdeI and the downstream primer, designed to hybridize to the 3′ end of rsbT, incorporating the restriction site SphI. Two separate PCR fragments were produced: PArsbR-S (1,359 bp) and rsbT (449 bp). The two fragments were mixed and used as templates for an additional amplification with the two most outboard primers from the original separate amplifications, i.e., the oligonucleotide that hybridized immediately upstream of PA and downstream of rsbT. This “long PCR” product contained PArsbR-S and rsbT as a single fragment, with a unique NdeI site at the 5′ end and SphI at the 3′ end of rsbT. The NdeI/SphI sites were to be used to introduce the mutant rsbT alleles into plasmid vectors for transfer to B. subtilis.
The PCR product containing PArsbR-S and rsbT was cloned into a variant of pUS19 from which the NdeI site, normally present in the vector, had been deleted. As a consequence, the only NdeI site remaining is the one engineered upstream of rsbT. By using this plasmid (pDRNT) as a template, unique primers were designed to introduce the alanine substitutions throughout the length of the protein.
Three individual PCR amplifications were used to create each rsbT mutant allele. First, a primer upstream of rsbT was paired with a mutagenic oligonucleotide designed to hybridize at the region within rsbT at which the mutation would be made. The mutagenic primer contained the sequence specifying the bases for the desired alanine substitutions. A separate PCR was set up by using a mutagenic oligonucleotide that was complementary to that used in the first amplification paired with a primer downstream of rsbT. These two fragments were then mixed together as a template for a third reaction in which the two outboard primers—one upstream and the other downstream of rsbT—were used to create a “long” PCR product encoding upstream DNA, as well as all of rsbT with the specific alanine substitution(s) at the intended locations. After sequencing of the cloned PCR products to verify the correct rsbT sequence, the rsbT alleles were cloned as NdeI and SphI fragments into a variant of the plasmid pDRNT, in which the wild-type rsbT had been removed by using these same restriction endonucleases. This created a collection of plasmids capable of expressing rsbR, rsbS, and one of the rsbT variants from the sigB operon's PA promoter in either Escherichia coli or B. subtilis.
Analysis of rsbT variants in E. coli and B. subtilis.
pDRNT and its related plasmids (pT1 to pT18, Table 1) carry the sigB operon promoter (PA) and the rsbR, rsbS and rsbT genes. The PA promoter is recognized by E. coli RNA polymerase. Hence, E. coli strains carrying the pDNRT family of plasmids express the B. subtilis rsbR, rsbS and rsbT genes. Although the pDNRT plasmids cannot replicate in B. subtilis, their transformation into B. subtilis, followed by selection for a plasmid-encoded antibiotic (spectinomycin) resistance, results in the isolation of clones in which the plasmid integrates into the chromosome by Campbell-like homologous recombination at sigB. If the recipient strain lacks a source of RsbS and RsbT (i.e., XS352 [ΔrsbS-T]), the plasmid-encoded alleles become the sole source of RsbS and RsbT in the transformants. To assess the ability of each variant RsbT to activate σB, strains were grown in Luria broth and exposed to 4% ethanol during exponential growth (optical density at 540 nm [OD540] = 0.2). The σB activities in these strains were monitored by measuring the accumulation of β-galactosidase from an E. coli lacZ gene fused to a σB-dependent promoter (ctc::lacZ).
To create B. subtilis strains that would express the rsbT variants but not RsbS, plasmids containing PArsbRST, with various rsbT alleles, were digested with HincII and NruI. HincII uniquely cleaves the plasmid at nucleotide 716 of the 821-nucleotide rsbR gene, whereas NruI cuts at nucleotide 186 of the 360-nucleotide rsbS gene. The resulting plasmid molecules, missing the 3′ end of rsbR and the 5′ region of rsbS, were religated and transformed into E. coli. Plasmids bearing the rsbR::rsbS deletion with either wild-type or variant rsbT alleles were transformed into a ΔrsbS-T B. subtilis strain (XS352). Integration of the plasmids into the sigB operon yields RsbR+ RsbS− strains expressing the rsbT allele of the incoming plasmid DNA.
RsbT induction studies.
rsbT expressed in excess to rsbS can activate σB in the absence of physical stress (21, 27). To test the ability of the variant rsbTs to activate in such a system, mutant and wild-type rsbT alleles were excised from their original plasmids with the restriction enzymes NruI and SphI and then cloned downstream of an IPTG-inducible promoter (PSPAC) in a plasmid capable of autonomous replication in both E. coli and B. subtilis (pDG148). These plasmids were transformed into B. subtilis strains either lacking rsbS and rsbT (BSZ11) or containing an intact sigB operon (BSA46). Cultures of B. subtilis carrying the plasmids were grown to an early exponential phase of growth (OD540 = 0.2) and exposed to 0.1 mM IPTG to induce the SPAC promoter and initiate RsbT synthesis. Samples were collected after 1 h and then analyzed for σB-dependent reporter gene (ctc::lacZ) expression. To evaluate possible further induction of σB by ethanol stress in these strains, reporter gene (ctc::lacZ) expression was also analyzed in cultures grown in the presence or absence of IPTG and exposed to 4% ethanol during growth (OD540 = 0.2).
Analysis of RsbT interactions in the yeast dihybrid system.
rsbT alleles were subcloned from the pDRNT/pT plasmids (Table 1) into the yeast “matchmaker” plasmid system in a two-step process. First, the pDRNT/pT plasmids were digested with SphI and the overhanging ends made blunt by using Klenow enzyme. The plasmids were then digested with NdeI to produce a rsbT DNA fragment suitable for cloning into the NdeI and SmaI sites of pAS2-1 (Clontech Laboratories, Inc., Palo Alto, Calif.). This created plasmids encoding translational fusions between the various rsbT alleles and the binding domain (BD) of the yeast GAL4 activator protein. These plasmids were transformed, according to established protocols (Clontech) into yeast strain Y190 harboring a resident plasmid that encoded the GAL4 activation domain fused to either RsbU, RsbR, RsbS, RsbT, or Obg (Table 1). Yeast strains carrying both plasmids were selected on the basis of plasmid-encoded prototrophic markers and screened for GAL4-dependent histidine prototrophy and β-galactosidase activity (34).
General methods.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and β-galactosidase activity assays were performed as previously described (14, 23). DNA manipulations followed standard protocols (26). Transformation of naturally competent B. subtilis cells was carried out as described by Yasbin et al. (38). Yeast β-galactosidase assays were performed as done previously (34). Sequencing of rsbT mutant alleles and plasmids was performed by the Center for Advanced DNA Technologies at the University of Texas Health Science Center. Quantitation of protein bands was performed on digital images by using an Alpha Imager 2000 (Alpha Innotech Corp., San Leandro, Calif.) and its associated software.
RESULTS AND DISCUSSION
Mutations in rsbT.
RsbT, an essential component of the σB physical stress pathway, displays multiple interactions and activities. In the yeast dihybrid assay, RsbT binds to itself, RsbR, RsbS, and RsbU, as well as to the GTP-binding protein Obg, with sufficient affinity to activate Gal4-dependent transcription (27, 34). In vitro, RsbT can form a high-molecular-weight complex with RsbR and RsbS, phosphorylate either of these proteins with ATP as a P04 donor and activate the RsbU-dependent dephosphorylation of RsbV-P (12, 16, 37).
Previously, three spontaneous rsbT mutations had been isolated as rsbT alleles with reduced activity (29). These included an isoluecine-to serine change at position 15, a nonsense mutation at position 63, and a valine-to-glycine substitution at position 107. An additional rsbT mutation was created at a residue critical for RsbT's kinase activity (Asp78) (22). This change, aspartate to asparagine, produced a mutant RsbT that was still able to activate RsbU but unable to inactivate RsbS by phosphorylation (22). The amino acid sequence of RsbT, including these four mutations, is illustrated in Fig. 2.
FIG. 2.
RsbT mutations. The amino acid sequence of RsbT is illustrated (36). Selected amino acids (underlined) were replaced with alanine as described in Materials and Methods. Mutant designations are indicated below the unlined amino acids that were changed (i.e., 1 = rsbT1, etc.). Mutations 1 to 17 are alanine substitutions, and mutation 18 introduced a termination codon at the underlined amino acid. Four previously described mutations in rsbT are indicated by the arrows at the sites affected. rsbT15IS, rsbT63Qterm, and, rsbT107VG have reduced RsbT activity and were isolated as suppressors of the heightened σB activity in RsbX−. B. subtilis (29). rsbT78DN was a specific alteration created to disrupt RsbT's kinase activity (22). Boldface sequences delineate RsbT regions that are conserved among protein kinases (21).
The small size of RsbT (133 amino acids) makes it suitable for a genetic analysis by alanine scanning mutagenesis. Relatively few directed changes could be used to span the entire length of the protein. In the present study, we replaced clusters of charged amino acids at sites throughout the length of the protein with alanine. Charged amino acids were chosen because they are likely residues to participate in protein-protein interactions, ligand binding, and/or catalysis. In addition, such amino acids have a high probability of being surfaced exposed and, as such, are less likely to be critical to the protein's general stability. Alanine was the chosen substitute because it is uncharged and compatible with most secondary structures.
A total of 17 dispersed positions (Fig. 2) where one or more charged amino acids are found along the length of RsbT were chosen as sites for alanine substitutions. The charged amino acids at each of these 17 sites were changed in unison to alanine by using standard PCR-based mutagenesis techniques (see Materials and Methods for details). In addition to the alanine substitutions, a termination codon was created at position 18 to remove the distal 14 amino acids and allow an assessment of the possible role of a putative carboxy-terminal alpha-helix on RsbT's activity. Substitutions 7, 8, and 13 fall within regions of the protein that are conserved among protein kinases (Fig. 2, boldface) and presumably could affect RsbT's ability to phosphorylate RsbR and RsbS.
Expression of the variant rsbTs in E. coli and B. subtilis.
The E. coli plasmids that were created to transfer the mutant rsbT alleles to B. subtilis (see Materials and Methods) encode rsbR and rsbS, as well as rsbT downstream of the sigB operon's PA promoter (i.e., PArsbR-S-T). PA is recognized by both B. subtilis and E. coli RNA polymerase. This circumstance allows us to express the rsbT variants in E. coli and test their ability to accumulate in this bacterium. The resulting RsbT levels may serve as an indication of the inherent stability of the mutant proteins, separate from any potential regulation of RsbT stability in B. subtilis.
E. coli transformed with the rsbT plasmids were inoculated into Luria broth and grown overnight. Whole-cell lysates of each culture were analyzed by Western blotting with antibodies reactive to RsbT and RsbR proteins. The relative abundance of the RsbR protein serving as a standard for normalizing expression in the different isolates. Variations in RsbT levels, relative to that of RsbR, can then be taken as a measure of the changes in RsbT stability caused by the particular mutation that it carries.
The results of the Western blot analyses of the expression of wild-type RsbT and the 18 RsbT variants in E. coli are summarized in Table 2. RsbT abundance was reduced in strain T3 and not detectable in strains T14 and T17. The remaining RsbT variants accumulated at levels that were similar to that observed for the wild-type RsbT protein.
TABLE 2.
Accumulation and activity of RsbT variantsa
| rsbT allele | Accumulation and/or activity (%)
|
||
|---|---|---|---|
| E. coli | B. subtilis | Inducibility | |
| Wild type | + | + | + |
| T1 | + | + | + |
| T2 | + | ± (38%) | + |
| T3 | + | ± (51%) | − |
| T4 | + | + | − |
| T5 | + | + | − |
| T6 | + | + | + |
| T7 | + | + | − |
| T8 | + | ± (20%) | − |
| T9 | + | + | + |
| T10 | + | ± (24%) | + |
| T11 | + | ± (42%) | + |
| T12 | + | + | + |
| T13 | + | ± (34%) | − |
| T14 | − | − | − |
| T15 | + | − | − |
| T16 | + | + | − |
| T17 | − | − | − |
| T18 | + | − | − |
The columns represent detection by Western blotting of the various rsbT products in E. coli and B. subtilis and their abilities to activate σB after ethanol treatment. For E. coli, “+” represents a detectable product regardless of level, and “−” represents undetectable RsbT. For B. subtilis, quantitation of the Western blots was performed with an Alpha Imager 2000 and its associated software. RsbT variants that accumulated at levels approximating that of wild-type RsbT are designated “+”; those displaying background signal levels are designated “−” and those with intermediate accumulation of RsbT are depicted as “±,” along with their relative abundances as a percentage of the wild-type RsbT levels. A “+” in the inducibility column reflects the ability of B. subtilis carrying the particular rsbT allele to display σB induction (5- to 10-fold increase in σB-dependent reporter gene activity) by 15 min after ethanol addition. The “−” strains displayed no difference in the activities of treated and untreated cultures.
XS352 is a strain of B. subtilis with an rsbS-T deletion that removes the last 96 codons of the 121 codon rsbS gene and the first 16 codons of the 133 codon rsbT gene (data not shown). Neither RsbS nor RsbT is detectable in XS352 by Western blotting. pDRNT-based plasmids, encoding either the wild-type or altered rsbT alleles, were transformed into XS352 and plated under conditions for the selection of transformants in which the plasmids had integrated into the chromosome by single-site homologous recombination at sigB. Although the relative lengths of homologous DNA on the plasmids (ca. 1 kbp upstream and 350 bp downstream of the ΔrsbS-T deletion) should favor plasmid integration upstream of the deletion, reformation of a wild-type rsbT allele is possible if the recombination event occurs within the residual rsbT sequences that were both downstream of the rsbS-T deletion in the chromosome and the upstream of the rsbT mutations on the plasmids. To preclude the inclusion of such a transformant in our analyses, the sigB regions of the transformants were examined by using a series of selective PCR analyses to verify that the plasmids had integrated upstream of the ΔrsbS-T deletion. All of the transformants that we analyzed contained the integrations at the upstream site (data not shown).
After transfer of the rsbT alleles to B. subtilis, Western blot analyses were undertaken on cultures of each transformant to determine whether the variant RsbTs would accumulate at levels similar to wild-type RsbT in B. subtilis. As was done with the E. coli extracts, the analyses included anti-RsbR antibody to normalize the Western blots of extracts with potentially different RsbT levels.
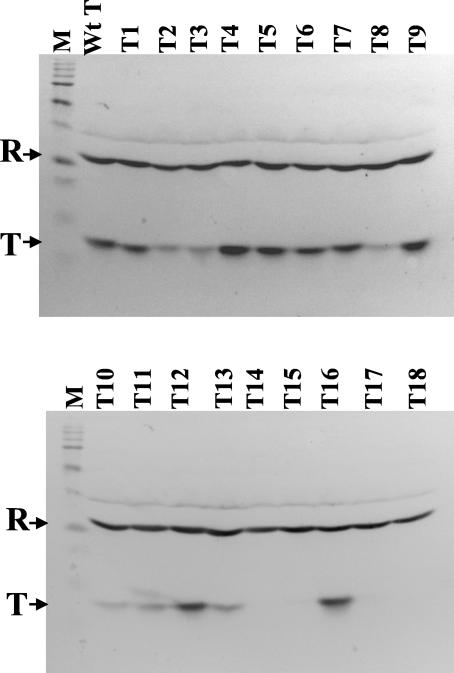
The results of an analysis on all 18 rsbT mutant strains and a wild-type rsbT control are shown in Fig. 3 and summarized in Table 2. RsbT is present at lower levels in T2, T3, T8, T10, T11, and T13 and not detectable in mutant strains T14, T15, T17, and T18. The remaining eight alleles accumulated RsbT at approximately wild-type levels. The most notable finding from this analysis is the apparent importance of RsbT's carboxy terminus to its stability. The deletion of the terminal 14 amino acids (T18) or substitutions in four of the five charged amino acid clusters that preceded the site of the deletion yielded RsbT variants that either failed to be detected (T14, T15, and T17) or were present at a reduced level (T13). The instability of RsbT 14 and 17 was common to both E. coli and B. subtilis; however, the reduced levels of the other RsbTs appeared to be Bacillus specific.
FIG. 3.
Western blot analysis of RsbT variants in B. subtilis. Stationary-phase cultures of B. subtilis (BSA661 T0 to T18), expressing rsbR-S and either wild-type rsbT (wtT) or one of the rsbT variants (i.e., T1, T2,…, etc.) from a pDRNT plasmid integrated at sigB were analyzed by Western blotting as described in Materials and Methods with anti-RsbR and anti-RsbT monoclonal antibodies as probes. The positions of the RsbR (R) and RsbT (T) proteins are indicated. The band that migrated above RsbR is a cross-reactive B. subtilis protein (ca. 40 kDa) present in stationary-phase extracts (15). Lane M contains “broad-range” prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis molecular mass standards (Bio-Rad, Hercules, Calif.) that were electrophoresed and transferred with the crude extracts.
Specific rsbT alleles fail to respond to stress.
Having determined the degree to which the products of the various rsbT alleles accumulate in B. subtilis, we examined their ability to activate σB after exposure to stress. B. subtilis strains expressing either a wild-type or mutant rsbT allele and a σB-dependent reporter system (ctc::lacZ) were grown in Luria broth and then exposed to ethanol at concentrations (4%) that normally activate σB. β-Galactosidase assay results for strains expressing each of the 18 rsbT mutants or a wild-type rsbT allele are summarized in Table 2. Strains containing 11 of the mutant alleles—T3, T4, T5, T7, T8, T13, T14, T15, T16, T17, and T18—failed to induce reporter gene activity after ethanol stress. Three of the six strains that accumulated less RsbT (T3, T8, and T13) and the four strains with undetectable RsbT (T14, T15, T17, and T18) in the Western blot analyses were included in this group. Mutants T3, T4, and T5 lay within a region at the amino-terminal portion of the protein that, based on a GOR4 analysis of its primary structure (http://abs.cit.nih.gov/gov/), is likely to form an alpha-helix. Mutants T7, T8, and T13 fall within regions of RsbT with high homology to protein kinases. Mutant T16 lies within a predicted alpha-helical domain near the carboxy-terminal portion of the protein, near where an RsbT-inactivating mutation (rsbT107VT) was found in an earlier study (29).
Activation of σB by induced RsbT synthesis.
Four of the rsbT mutants (T4, T5, T7, and T16) that made normal levels of RsbT in B. subtilis but did not respond to ethanol stress, as well as two additional nonresponsive mutants (T3 and T13) whose RsbT levels were greater than that of a stress-activatable rsbT variant (T10) with a reduced level of RsbT, were selected for further study. Elevated expression of rsbT can activate σB in the absence of stress (21, 27). The reason for this phenomenon is not certain, but presumably it represents a failure of RsbR/S to effectively inhibit the higher levels of RsbT. Plasmids, capable of replication in B. subtilis and carrying one of the rsbT alleles under the control of an IPTG-inducible promoter were constructed. Wild-type B. subtilis (BSA46), transformed with these plasmids and growing in Luria broth, was exposed to IPTG to induce the expression of rsbT. Cells were harvested after 1 h and then examined for the effects of rsbT expression on σB-dependent reporter gene activity (ctc::lacZ). B. subtilis strains carrying three (T5, T7, and T16) of the six rsbT variants activated σB when their expression was induced, although the degree of activation was still lower than that seen when the wild-type rsbT allele was expressed in this system (Table 3).
TABLE 3.
σB activation after induced expression of rsbTa
| Genotype | Mean β-galactosidase activity (Miller units) ± SD
|
|
|---|---|---|
| Uninduced | Induced | |
| rsbT/PSPACrsbT | 7 ± 2 | 25 ± 2 |
| rsbT/PSPACrsbT3 | 5 ± 2 | 6 ± 2 |
| rsbT/PSPACrsbT4 | 3 ± 2 | 4 ± 3 |
| rsbT/PSPACrsbT5 | 5 ± 3 | 19 ± 2 |
| rsbT/PSPACrsbT7 | 4 ± 2 | 14 ± 2 |
| rsbT/PSPACrsbT13 | 6 ± 2 | 5 ± 2 |
| rsbT/PSPACrsbT16 | 7 ± 2 | 12 ± 2 |
| ΔrsbS-T/PSPACrsbT | 2 ± 2 | 19 ± 4 |
| ΔrsbS-T/PSPACrsbT3 | 3 ± 3 | 3 ± 2 |
| ΔrsbS-T/PSPACrsbT4 | 2 ± 2 | 2 ± 2 |
| ΔrsbS-T/PSPACrsbT5 | 1 ± 1 | 1 ± 1 |
| ΔrsbS-T/PSPACrsbT7 | 1 ± 1 | 1 ± 1 |
| ΔrsbS-T/PSPACrsbT13 | 1 ± 1 | 2 ± 2 |
| ΔrsbS-T/PSPACrsbT16 | 1 ± 1 | 1 ± 1 |
B. subtilis strains with a wild-type rsbT allele at the sigB operon (rsbT) or a rsbS-T deletion at that locus (ΔrsbS-T) and one of the indicated rsbT alleles under the control of an IPTG-inducible promoter (PSPAC) on a multicopy plasmid (Table 1) were grown in Luria broth with (induced) or without (uninduced) IPTG. Samples were taken 1 h after IPTG addition and then assayed for σB-dependent (ctc::lacZ) β-galactosidase activity. The values (Miller units) represent the average of three independent assays.
The strains used in the heightened expression assay have a wild-type copy of rsbT at the sigB operon, as well as the plasmid-encoded IPTG-inducible rsbT allele. As such, there are two plausible mechanisms by which heightened expression of the rsbT alleles could activate σB in this strain. The induced RsbTs could directly activate the RsbU phosphatase or, alternatively, they might compete for the negative regulators of RsbT (i.e., RsbR and RsbS), thereby allowing the wild-type RsbT protein to become free to activate RsbU. To distinguish between these possibilities, the rsbT-expressing plasmids were placed in the ΔrsbS-T strain of B. subtilis and reexamined for the ability of the rsbT variants to activate σB without the possibility of activation by wild-type RsbT or inhibition by RsbS. The cultures were grown, induced for rsbT expression by the addition of IPTG, and analyzed for σB-dependent reporter gene (ctc::lacZ) expression as described above. Unlike the previous experiment, in which several of the rsbT variants could activate σB when expressed in the presence of the wild-type RsbT, no induction of σB was evident when those alleles were expressed in its absence (Table 3). Thus, none of the rsbT variants that were unable to respond to stress appears to be capable of directly activating RsbU.
If the inability of the variant RsbTs to activate RsbU does not inhibit their RsbU binding, the presence of these proteins might interfere with the ability of the wild-type RsbT protein to interact with RsbU and activate σB in response to stress. To explore this possibility, B. subtilis strains expressing a wild-type rsbT allele from the sigB operon, as well as one of the mutant rsbT alleles from the IPTG-inducible promoter on a multicopy plasmid, were examined for σB activation after exposure to ethanol. B. subtilis strains, expressing additional wild-type or variant rsbT from the inducible promoter, had distinct background levels of σB activity, reflecting the inherent activity of the RsbT made in each strain. However, upon exposure to ethanol stress, each of the strains elevated σB-dependent β-galactosidase activity by approximately 30 to 40 Miller units (data not shown). Thus, none of the mutant RsbTs appears to inhibit wild-type RsbT's ability to trigger σB induction in response to stress. Either the mutant RsbTs do not bind to RsbU or RsbU's abundance is sufficiently high so that the binding of the variant RsbTs is insufficient to deny wild-type RsbT access to RsbU. In summary, none of the RsbT variants that accumulate, but fail to induce σB in response to stress, are able to activate RsbU or block wild-type RsbT's access to it. At least some of these (i.e., rsbT5, rsbT7, and rsbT16) do, however, appear to retain the ability to compete for the negative regulators of RsbT and allow the wild-type allele to show activity when the mutant alleles are expressed at elevated levels in a merodiploid strain.
Analysis of RsbT interactions in the yeast dihybrid assay.
Previously, the yeast dihybrid system demonstrated that RsbT is able to interact with itself, RsbR, RsbS, RsbU, and Obg, a ribosome-binding protein needed for stress activation of σB (27, 34). In an attempt to further characterize the six mutant RsbTs that accumulate but fail to activate σB, we used the yeast dihybrid system to examine the binding properties of the RsbT variants.
The rsbT alleles were cloned into plasmid vectors in such a way as to create translational fusions between rsbT and the DNA-binding domain of the GAL4 yeast gene activator protein. To verify that the rsbT::GAL4 fusion proteins formed from each rsbT allele would be able to accumulate at comparable levels in yeast, Western blot analyses were performed on yeast strains carrying each of the rsbT fusions, with an rsbT-specific monoclonal antibody as a probe. A protein of the anticipated size of the RsbT::Gal4BD (30 kDa) was present at similar levels in extracts of yeast strains that expressed either the wild-type rsbT or the rsbT variant fusion proteins (data not shown). Plasmids carrying the rsbT fusions were then transformed into yeast strains expressing the GAL4 activating domain fused to RsbU, RsbR, RsbS, or Obg. The recipient yeast strains lacked an intact GAL4 gene but carried both a gene needed for histidine prototrophy (GAL1UAS-HIS3TATA-HIS3) and an E. coli lacZ gene (GAL1UAS-GAL1TATA-lacZ) expressible from GAL4-activated promoters. Plasmid-containing yeast were selected on the basis of Trp and Leu prototrophy, (markers carried on each of the plasmids) and screened for GAL4-dependent histidine prototrophy and β-galactosidase activity (34). The Gal4 activation data are summarized in Table 4.
TABLE 4.
Activation of lacZ reporter gene by RsbT-Gal4 chimerasa
| AD fusion | Reporter gene activity (Miller units) with BD fusion:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Vector (pAS2-1) | rsbT | rsbT3 | rsbT4 | rsbT5 | rsbT7 | rsbT13 | rsbT16 | |
| Vector (pACT2) | W | 0.19 | W | W | 0.11 | 0.75 | W | W |
| rsbR | 0.05 | 1.35 | W | 0.04 | 0.83 | 1.02 | W | W |
| rsbS | 0.07 | 50.14 | W | 0.05 | 0.14 | 1.27 | W | W |
| rsbT | 0.03 | 0.68 | W | 0.05 | 0.14 | 0.59 | W | W |
| rsbU | 0.05 | 0.50 | W | W | 0.55 | 2.29 | W | W |
| obg | 0.05 | 0.48 | W | W | 0.21 | 1.53 | W | W |
The columns represent yeast clones carrying the Gal4 DNA-binding domain (BD) vector pAS2- 1 either without insert DNA (vector) or with one of the rsbT alleles depicted, translationally fused to the Gal4 DNA-binding domain. The rows represent the Gal4 activation domain (AD) vector pACT2 with or without translational fusions to the RsbT regulators that are listed. Data depict reporter gene (GAL1UAS-GAL1TATA-lacZ) activity in yeast cells cotransformed with both of the plasmids indicated by the rows. W, pairings in which the yeast clones failed to give a blue color after freeze-thawing and exposure to X-Gal. The numbers represent a measurement of lacZ activity in those clones that displayed a blue colony phenotype after exposure to X-Gal. The values represent the average of duplicate assays of three independent clones for each plasmid pair. Aside from a Gal4-dependent lacZ gene, the host yeast strain (Y190) also contains GAL1UAS-HIS3TATA-HIS3, allowing histidine prototrophy if an active GAL4 regulator is formed. All of the blue colonies (i.e., pairings represented by numbers), but none of the white colonies (W), could form colonies on media lacking histidine. X-Gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
Although one of the mutant rsbT alleles (T7) showed an enhanced activity with two of the RsbT binding partners (Obg and RsbU), a common feature of the six mutant rsbT alleles is a reduced activity, with most of the proteins typically bound by wild-type RsbT. This is quite evident in the pairings with RsbS, normally one of the strongest of the interactions between wild-type RsbT and its partners but markedly reduced in all six of the mutant rsbT alleles. This is curious given that a decreased affinity for the RsbS, the principal negative regulator of RsbT, might be expected to enhance RsbT's activity, and yet all of these RsbT variants, including T7, an RsbT with heightened RsbU binding, are inactive.
At least two possibilities can be suggested. One possibility is that RsbT might require an interaction with RsbR and RsbS in order to be properly configured to activate RsbU and that without this interaction RsbU will remain in an inactive state. Such a model is not supported, however, by the observation that RsbT-dependent σB activity is very high in B. subtilis lacking RsbS (21). An alternative possibility is that similar regions of RsbT are required for proper interaction with both RsbS and RsbU. Common sites of contact between alternative binding partners have, in fact, been documented in the partner switching system responsible for the control of the Bacillus transcription factor σF (11, 17). In that instance, the regions of the σF inhibitor (SpoIIAB) that participate in its binding to σF are also involved in its association with the competing release factor, SpoIIAA (11, 17). A similar circumstance would explain why there were no constitutively active mutant RsbTs among our collection. If the same RsbT domains were involved in both its negative control by RsbS and its ability to activate RsbU, highly specific mutations might be needed to disrupt one activity without influencing the other.
The three rsbT alleles, whose products presumably compete for RsbS and allow the wild-type rsbT to display activity (Table 3), are poor RsbS binding partners in the yeast dihybrid assay. This suggests that the interactions that occur between these proteins in B. subtilis may not be mirrored in the yeast system. Chen et al. (12) have recently demonstrated that RsbR must be present if RsbS is to function as an effective inhibitor of RsbT. Perhaps the binding reaction that is observed between RsbS and RsbT in the yeast system only partially reflects the binding that occurs between RsbT and the RsbR/S complex in B. subtilis. If this is true, our “activating” rsbT alleles might still interact with RsbS in B. subtilis but not display significant binding in yeast. Alternatively, the apparent loss of interaction between the RsbT variants and RsbS in the yeast system may be a true reflection of the state of their binding capacities. If, as the yeast dihybrid data also suggest (Table 4) (34), RsbT can interact with itself, RsbT may normally exist in a multimeric form. If this is so, the ability of some of the RsbT variants to activate σB when expressed in the presence of wild-type RsbT, but not when expressed alone, might be a consequence of each of these RsbTs acting in concert as RsbT heterodimers. It is possible that RsbT heterodimers, consisting of an RsbT variant with impaired RsbS binding and wild-type RsbT capable of activating RsbU, could escape RsbS inhibition and activate σB.
Previous studies have shown that the loss of RsbX, the σB regulator that reestablishes negative control over RsbT by the dephosphorylation of RsbS-P, results in constitutively high levels of σB activity in unstressed B. subtilis (4, 9, 29). This implies that even in the absence of obvious stress, B. subtilis RsbT is phosphorylating RsbR/S and being released from the RsbR/S complex. Thus, the binding of RsbT and the RsbR/S complex can be viewed as transient. This likely accounts for the ability of the RsbT variants, unable in themselves to activate RsbU, to compete for the RsbR/S complex and permit the wild-type RsbT to activate RsbU when their synthesis is induced from PSPAC. If the RsbR/S/T complexes were stable, competition by the newly synthesized RsbTs for the complex might be difficult.
The substitution of the groups of charged amino acids along the length of RsbT by alanine and the deletion of RsbT's carboxy terminus revealed several general features of RsbT. Most notable is the importance of carboxy terminus of RsbT on its ability to accumulate in B. subtilis. This is demonstrated by the observation that four (T14, T15, T17 and T18) of the five carboxy-terminal mutations yielded rsbT alleles whose products are not detectable in B. subtilis extracts. The accumulation defect for two of these proteins was less obvious in E. coli, suggesting a Bacillus-specific proteolytic event. It is also noteworthy that four (T9, T10, T11, and T12) of the five substitutions in the central region of the protein had no measurable effect on RsbT's activity. Only when a substitution (T13) extended into a region of kinase homology was RsbT activity affected. Apparently, the presence of charged amino acids in this region does not contribute significantly to RsbT's functions. Finally, in cases in which the substitutions disrupted RsbT's ability to respond to stress while still allowing the mutant allele's products to accumulate (T3, T4, T5, T7, T13, and T16), the effects of the mutations were not limited to a single RsbT property. These mutations altered most of the variant proteins' binding properties in the yeast dihybrid system, as well as affecting both their ability to activate RsbU and their ability to compete for the RsbR/S complex. Presumably, these changes either dramatically altered the proteins' tertiary structure without dramatically affecting their stability or define particular regions of RsbT that are involved in multiple interactions.
Acknowledgments
This study was supported by National Institutes of Health grant GM-48220.
REFERENCES
- 1.Akbar, S., C. M. Kang, T. A. Gaidenko, and C. W. Price. 1997. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol. Microbiol. 24:567-578. [DOI] [PubMed] [Google Scholar]
- 2.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 183:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165-177. [DOI] [PubMed] [Google Scholar]
- 4.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls σB expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, A. K., and W. G. Haldenwang. 1993. Regulation of σB levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis σB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 90:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson, A. K., and W. G. Haldenwang. 1993. The σB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylan, S. A., A. Rutherford, S. M. Thomas, and C. W. Price. 1992. Activation of Bacillus subtilis transcription factor σB by a regulatory pathway responsive to stationary phase signals. J. Bacteriol. 174:3695-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brody, M. S., K. Vijay, and C. W. Price. 2001. Catalytic function of an α/β hydrolase is required for energy stress activation of the σB transcription factor or Bacillus subtilis. J. Bacteriol. 183:6422-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell, E. A., S. Masuda, J. L. Sun, O. Muzzin, C. A. Olson, S. Wang, and S. A. Darst. 2002. Crystal structure of the Bacillus stearothermophilus anti-σ factor SpoIIAB with the sporulation σ factor σF. Cell 108:795-807. [DOI] [PubMed] [Google Scholar]
- 12.Chen, C.-C., R. J. Lewis, R. Harris, M. D. Yudkin, and O. Delumeau. 2003. A supermolecular complex in the environmental stress signalling pathway of Bacillus subtilis. Mol. Microbiol. 49:1657-1669. [DOI] [PubMed] [Google Scholar]
- 13.Delumeau, O., R. J. Lewis, and M. D. Yudkin. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 184:5583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-σ factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufour, A., U. Voelker, A. Voelker, and W. G. Haldenwang. 1996. Relative levels and fractionation properties of Bacillus subtilis σB and its regulators during balanced growth and stress. J. Bacteriol. 178:3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidenko, T. A., X. Yang, Y. M. Lee, and C. W. Price. 1999. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the stress signaling pathway of Bacillus subtilis. J. Mol. Biol. 288:29-39. [DOI] [PubMed] [Google Scholar]
- 17.Garsin, D. A., D. M. Paskowitz, L. Duncan, and R. Losick. 1998. Evidence for common sites of contact between the antisigma factor SpoIIAB and its partners SpoIIAA and the developmental transcription factor σF in Bacillus subtilis. J. Mol. Biol. 284:557-568. [DOI] [PubMed] [Google Scholar]
- 18.Hecker, M., W. Schumann, and U. Voelker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 19.Ju, J., T. Luo, and W. G. Haldenwang. 1998. Forespore expression and processing of the SigE transcription factor in wild-type and mutant Bacillus subtilis. J. Bacteriol. 180:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalman, S., M. L. Duncan, S. M. Thomas, and C. W. Price. 1990. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J. Bacteriol. 172:5575-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, C. M., M. S. Brody, S. Akbar, X. Yang, and C. W. Price. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 178:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, C. M., K. Vijay, and C. W. Price. 1998. Serine kinase activity of a Bacillus subtilis switch protein is required to transducer environmental stress signals but not to activate its target PP2C phosphatase. Mol. Microbiol. 30:189-196. [DOI] [PubMed] [Google Scholar]
- 23.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor of Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Voelker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Scott, J. M., and W. G. Haldenwang. 1999. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor σB. J. Bacteriol. 181:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, J. M., J. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 182:2771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnova, N., J. Scott, U. Voelker, and W. G. Haldenwang. 1998. Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX. J. Bacteriol. 180:3671-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PA5 domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 31.Voelker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Voelker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 32.Voelker, U., A. Dufour, and W. G. Haldenwang. 1995. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of σB. J. Bacteriol. 177:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voelker, U., A. Voelker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-σB antagonist, RsbV, by stress or starvation-induced phosphatase activities. J. Bacteriol. 178:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voelker, U., A. Voelker, and W. G. Haldenwang. 1996. The yeast two-hybrid system detects interactions between Bacillus subtilis σB regulators. J. Bacteriol. 178:7020-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voelker, U., A. Voelker, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 38.Yasbin, R. E., G. A. Wilson, and F. E. Young. 1973. Transformation and transfection of lysogenic strains of Bacillus subtilis 168. J. Bacteriol. 113:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, S., and W. G. Haldenwang. 2003. RelA is a component of the nutritional stress activation pathway of the Bacillus subtilis transcription factor σB. J. Bacteriol. 185:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, S., J. M. Scott, and W. G. Haldenwang. 2001. Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor σB. J. Bacteriol. 183:2316-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]