Abstract
Background: Use of hormone replacement therapy (HRT) may increase the risk of adult-onset asthma in women. Various in vitro studies have reported that estradiol stimulates human mast cell lines causing release of allergic mediators which was not observed in estrogen receptor-α (ER-α) knockout mice. Thus, estrogen might be a key element in occurrence of asthma. In the present study, we proposed to determine the role of ER-α in an experimental model of bronchial asthma. Methods: Trypsin and egg albumin induced chronic model of asthma were used. On the 28th day, various parameters such as pO2 level, serum bicarbonate level, tidal volume, respiratory rate, air flow rate, differential white blood cells count in the bronchoalveolar lavage (BAL) fluid and serum cholesterol level were measured as well as lung histopathological examination and uterine weight measurement were carried out. Results: Estradiol treatment resulted in lower pO2 level, tidal volume and air flow rate. Also, serum bicarbonate level, respiratory rate and eosinophil rate and eosinophil count in BAL fluid were higher as compared to asthmatic control group. These effects were not observed in methyl-piperidino-pyrazole (MPP) co-treated group. Histopathological data suggested that the destruction of alveolar and muscular layers was more prominent in estradiol-treated group than asthmatic control and MPP co-treated groups. Estradiol-treated group showed lower total serum cholesterol levels and higher uterine weight as compared to asthmatic control group which was not observed in MPP co-treated group; indicating antagonism of estradiol by MPP at ER-α receptor. Conclusion: Estrogen seems to have a strong promoting effect on pathogenesis of bronchial asthma via ER-α receptors.
Key Words: Estrogen receptor Alpha (ER-α), Estradiol, Asthma
INTRODUCTION
Asthma is defined as a chronic inflammatory disorder of the airway, characterized by reversible airflow obstruction causing cough, wheeze, chest tightness and shortness of breath. Inflammation of the bronchial wall involving eosinophils, mast cells and lymphocytes together with the cytokine and inflammatory products of these cells induces hyper-responsiveness of the bronchi so that they narrow more readily in response to a wide range of stimuli [1]. The prevalence and morbidity of asthma and other allergic diseases have increased dramatically during the last 30 years, particularly in industrial countries [2]. Asthma is more common in males during infancy, childhood, and preadolescence. From late adolescence to middle age, females have a higher prevalence and morbidity from asthma [3]. Use of hormone replacement therapy (HRT) may increase the risk of adult-onset asthma in women [4]. Super-physiological concentrations of estradiol have been shown to induce mast cell degranulation [5, 6]. Further, preincubating mast cells with physiological concentrations of E2 has been shown to increase the subsequent histamine release induced by cross-linking surface-bound IgE with antibodies [7, 8]. The 17-β-estradiol (17-β-E2) stimulates human mast cell lines causing release of beta-hexosaminidase (a marker for the granules containing preformed allergic mediators) and also enhances the synthesis and release of leukotriene C4. This effect of estradiol was not observed in estrogen receptor-α (ER-α) knockout mice [3, 9]; therefore, it can be said that estrogen might be a key element in occurrence of asthma. In the present study, we proposed to study the effect of estradiol in presence and absence of methyl-piperidino-pyrazole (MPP), a selective ER-α antagonist, in egg albumin and trypsin-induced chronic asthma in mice.
MATERIALS AND METHODS
Materials. The 17-β-E2 and MPP were purchased from Sun Pharma (Vadodara, India) and Sigma Chemicals (USA), respectively. Trypsin and egg albumin were obtained from Rakesh Chemicals (Ahmedabad, India). Total Cholesterol kit was procured from Span Diagnostics (Vadodara, India) while Estrogen kit (Immulite 1000-LKE21) was obtained from Siemens Healthcare Diagnostics (Deerfield, USA).
Animals. Healthy Swiss Albino mice of both sexes, weighing 30-50 g, were used in the present study. The animals, procured from Zydus Research Centre, Ahmedabad, India, were housed at 18 to 29C, with 12 h light-dark cycles, in polypropylene cages with free access to food and water ad libitum during the course of the study. The protocol (KBIPER/08/122) was approved by Committee for the Purpose of Control and Supervision of Experiments on Animals before carrying out the project. A combination of trypsin and egg albumin was used to induce asthmatic status in mice [10-12]. The animals were divided into 4 groups of 12 animals (6 males and 6 females each). They were exposed to aerosol of trypsin (1 mg/ml and 1 ml/min.) once daily for 5 min, followed by a rest of 2 h and then exposed to egg albumin (1% w/v solution and 1 ml/min.). This procedure was repeated for 10 days and later, egg albumin aerosol was discontinued whereas trypsin exposure was continued till the 21st day. On the 21st day after last exposure to trypsin, the animals were examined for parameters mentioned below later. On day 28, only egg albumin challenge was given. Group I animals did not receive any treatment except saline and served as normal control. Group II animals were exposed to egg albumin, but did not receive any drug treatment. They served as asthmatic control animals. In Group III and IV animals, drug treatment was initiated one week before and continued till the 28th day of the initiation of trypsin and egg albumin administration. Group III animals received 17-β-E2 (100 μg/25 g, i.p.) whereas Group IV animals received both 17-β-E2 (100 μg/25 g, i.p.) and MPP (75 μg/25g, i.p.) as drug treatment. The female mice were given estradiol (100 μg/25 g, i.p.) 24 hours before starting the study to bring them in estrous phase. After 24 hours, the female mice were checked for estrous cycle phase by vaginal smear preparation and were dosed with the drug in estrous phase. On day 1 before any exposure (basal value), on day 21 after trypsin exposure and on day 28 after egg albumin challenge, the following parameters were measured for each animal:
I, pO2 level; II, serum bicarbonate level; III, tidal volume; IV, respiratory rate; V, air flow rate; VI, serum estrogen level (reagent kit) and VII, serum cholesterol level (reagent kit)
On day 28, in addition to the above parameters, the following parameters were also measured:
I, bronchoalveolar lavage (BAL) fluid study; II, uterine weight and III, histopathology of lungs.
Measurement of pO 2 . The measurement of arterial O2 tension (pO2) was performed done with the help of Pulse Oxymeter instrument according to the methods described by Apps et al. [13] and Fabbri et al. [14].
Measurement of serum bicarbonate level . The method used in the present study to measure serum bicarbonate level was slightly modified from that described by Godkar [15]. About 1-2 ml of blood was collected from each animal under anesthesia after 1 h of the exposure to egg albumin. The serum was separated from blood by minimum exposure to air and stored in a sealed tube till bicarbonate level was estimated. For bicarbonate level measurement, 10 ml of 1 g/dl saline was pipetted out in 100 ml beaker. To this, 0.1 ml of the serum and 2 drops of phenol red indicator were added and mixed well. In the above mixture, NaOH (0.01 N) was added drop wise till the end point was achieved (7.35 pH or color changed from yellow to pink). The volume of NaOH required was noted down and considered as control reading (X ml). In another set, 9.0 ml of 1 g/dl saline and 1 ml HCL (0.01 N) were added. The above procedure was repeated and volume of NaOH required was noted (Y ml).
Now, Burette Reading R = Y ml – X ml and serum bicarbonate level (mEq/l) = (1-R) × 100.
Measurement of tidal volume, respiratory rate and airflow rate. The measurement of tidal volume, respiratory rate and airflow rate were done with the help of Respiratory volume transducer that were used with strain gauge coupler and student physiograph. The strain gauge coupler was calibrated with the help of standard 0.02 cc volume calibrator [16, 17].
Serum estrogen level. Serum estrogen level was measured by chemiluminescence ELISA. The assay system utilizes one anti-estrogen antibody for solid phase (microtiter wells) immobilization and another mouse monoclonal anti-estrogen antibody in the antibody-enzyme (horseradish peroxidase) conjugate solution. Estradiol molecules in the test sample were sandwiched between the solid phase and enzyme-linked antibodies. After 60 minute incubation at room temperature, the wells were washed with water to remove unbound labeled antibodies. A solution of chemiluminescent substrate is then added and read relative light units in a luminometer. The intensity of the emitting light is proportional to the amount of enzyme present and is directly related to the amount of estradiol in the sample. By reference to a series of estradiol standards assayed in the same way, the concentration of estradiol in the unknown sample is quantified.
Serum cholesterol level. Serum cholesterol level was measured by colorimetric technique [18]. Cholesterol esterase hydrolyses cholesteryl ester into cholesterol. Cholesterol is then oxidized by cholesterol oxidase to yield H2O2. Peroxidase reacts with the hydrogen peroxide to initiate the coupling reaction between the 4-aminoantipyrine and N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline sodium salt, forming a blue color dye which can be detected by spectrophotometry at λ = 600 nm.
BAL fluid study: differential leukocyte count. On the 28th day, after 3 hours of egg albumin challenge or just prior to animal death, whichever were earlier, the tracheobronchial tree was lavaged with 1 ml of saline [19]. The fluid was collected and centrifuged at 26.88 g or 5 min. The supernatant was discarded and pellet was resuspended in 0.5 ml saline. A thin film of 0.05 ml suspended in saline was made on clean grease free slide and fixed with methyl alcohol for 3-5 min and dried. Then, few drops of Geimsa stain in buffered saline (pH 6.8) were added to it and kept for 15 min or more. It was washed off with tap water and dried. The number of each type of leukocytes in 0.5 ml fluid was determined under the microscope at 450× magnification. [20].
Uterine weight. On the 28th day, the animals were sacrificed and uterus was dissected out along with uterine horn and kept in saline till weighed.
Histopathology of lungs. On the 28th day, the animals were sacrificed and their lungs were dissected out. The procedure used for histopathological study was fixation of the tissue with formalin, embedding in paraffin blocks, sectioning with microtome (0.7 µ thicknesses) and finally staining by hematoxylin and eosin stain technique [21].
Statistical analysis. The values are expressed as mean SEM. Statistical significance of difference in parameters amongst groups was determined by one-way ANOVA followed by Tukey’s multiple range test. P<0.05 was considered to be significant.
RESULTS
Serum estrogen level. On day 28, serum estrogen level was significantly higher in estradiol group (male: 6991.50 + 507.163 pg/ml and female: 7543.50 + 430.838 pg/ml) as compared to normal (male: 164.00 + 17.203 pg/ml and female: 181.33 + 24.585pg/ml) and asthmatic control groups (male: 272.50 + 23.564 pg/ml and female: 263.33 + 26.484 pg/ml) which was not observed in MPP co-treated group (male: 3072.66 + 59.559 pg/ml and female: 2512.17 + 374.941 pg/ml).
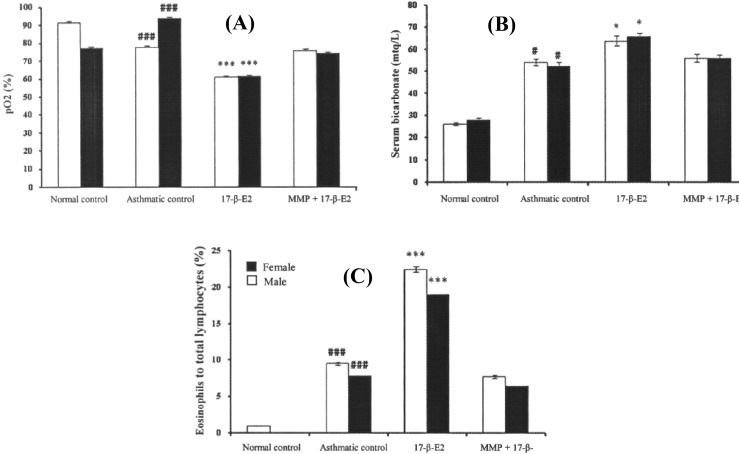
pO 2 level and serum bicarbonate level. Challenging of animals (both males and females mice) with egg albumin on day 28 of the study showed a significant lower pO2 level and a higher serum bicarbonate level in asthmatic control group as compared to normal control group. In addition, a significant lower pO2 and a higher level were observed in estradiol-treated group as compared to asthmatic control group which was not observed in MPP-estradiol co-treated group (Fig. 1A and 1B).
Fig. 1.
Effect of estradiol and MPP + estradiol co-treatment on (A) pO2 level, (B) serum bicarbonate level and (C) percent of eosinophils to total lymphocytes in BAL fluid in male and female mice. Each bar represents mean ± SEM (n = 6). Statistical analysis by One-way ANOVA was followed by Tukey’s multiple range test indicated (A) significant lower in pO2 level (B) significant higher serum bicarbonate level and (C) significant higher eosinophil count in BAL fluid. ### P<0.001 versus normal control group and ***P<0.001 versus asthmatic control group, #P<0.05 versus normal control group and *P<0.05 versus asthmatic control group.
Tidal volume, respiratory rate and airflow rate. A significant lower tidal volume and air flow rate were observed in asthmatic control group as compared to normal control group after egg albumin challenge. Further, tidal volume and air flow rate were lower in estradiol-treated group as compared to asthmatic control group which was not observed in MPP- estradiol co-treated group. In contrast to tidal volume and air flow rate, a significant higher respiratory rate was observed in estradiol-treated group as compared to asthmatic control group which was not observed in MPP-estradiol co-treated group (Table 1).
Table 1.
Effect of estradiol and MPP + estradiol co-treatment on tidal volume, respiratory rate and airflow rate of male and female mice
| Parameter |
Normal control
|
|
Asthmatic control
|
|
Estradiol treated
|
|
MPP + estradiol
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| male | female | male | female | male | female | male | female | ||||
| Tidal volume (ml) | 0.19 ± 0.003 | 0.17 ± 0.007 | 0.13 ± 0.002### | 0.12 ± 0.005### | 0.07 ± 0.002*** | 0.08 ± 0.005*** | 0.12 ± 0.004 | 0.12 ± 0.005 | |||
| Respiratory rate (breaths/min) | 220.0 ± 3.43 | 222.7 ± 2.86 | 259.7 ± 2.89### | 255.3 ± 1.43### | 296.7 ± 3.57*** | 297.0 ± 3.53*** | 271.3 ± 2.86 | 264.3 ± 3.67 | |||
| Airflow rate (ml/min) | 40.90 ± 0.242 | 37.59 ± 1.109 | 33.75 ± 0.599# | 31.92 ± 1.301# | 21.29 ± 0.815* | 23.22 ± 1.600* | 32.09 ± 1.132 | 32.12 ± 0.850 | |||
Values in table represents mean ± SEM (n = 6). Statistical analysis by One-way ANOVA followed by Tukey’s multiple range test indicated significant lower tidal volume (### P<0.001 versus normal control group and ***P<0.001 versus asthmatic control group), air flow rate (#P<0.05 versus normal control group and *P<0.05 versus asthmatic control group) and significant higher respiratory rate (### P<0.001 versus normal control group and ***P<0.001 versus asthmatic control group.
BAL fluid study. A significant higher percent of eosinophil to total lymphocyte was observed in BAL fluid of asthmatic control group as compared to normal control group. Estradiol-treated group showed significantly higher percent of eosinophil to total lymphocyte count in BAL fluid as compared to asthmatic control group. However, this change was not observed in MPP + estradiol co-treated animals (Fig. 1C).
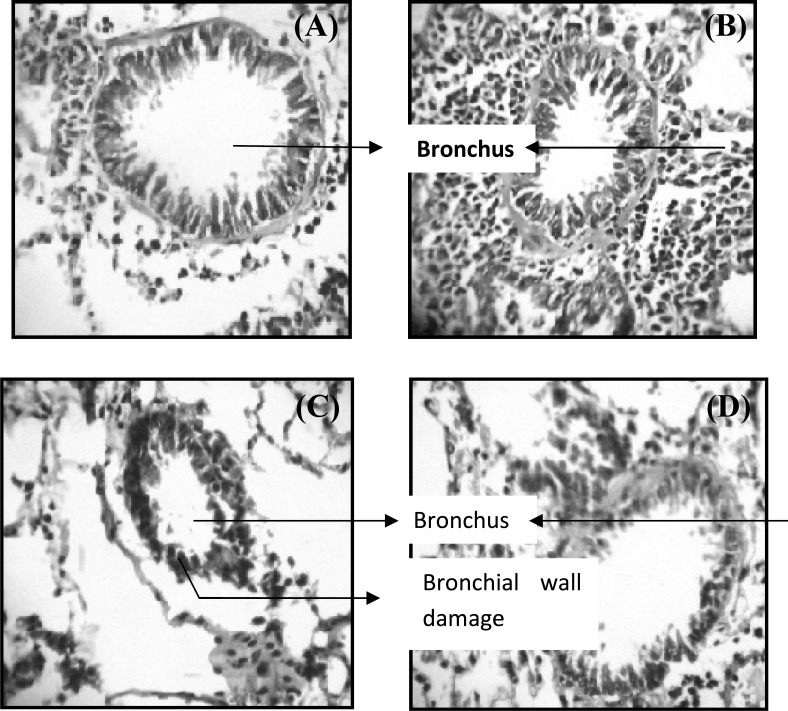
Histopathology of lungs. Normal control animals showed an intact bronchial structure (Fig. 2A), whereas trypsin and egg albumin-sensitized animals showed inflammation with minimal destruction in bronchial wall lining (Fig. 2B). Estradiol-treated animals showed a marked destruction in bronchial wall lining (Fig. 2C), whereas MPP + estradiol co-treated animals showed a moderate damage to bronchial wall as compared to estradiol-treated animals (Fig. 2D).
Fig. 2.
Histopathology of lung obtained from mice exposed to chronic exposure to trypsin and egg albumin and concomitantly treated with either estradiol alone or in combination with MPP. (A) normal structure of bronchus in normal control animals; (B) inflammation in bronchi on chronic exposure to trypsin and egg albumin; (C) significant damage to bronchial wall structure due to additional treatment of estradiol and (D) much lesser damage to bronchial walls in MPP + estradiol-treated animals (magnification 400×, hematoxylin stain).
Serum cholesterol level and uterine weight. A significant lower total cholesterol level was found only in estradiol-treated group as compared to normal and asthmatic control while no marked change had been found in MPP and estradiol co-treated group, indicating antagonism of estradiol by MPP. In estradiol-treated mice, an increase in uterine weight was found that this increase was significantly lesser in mice which received additional MPP treatment (Table 2).
Table 2.
Effect of estradiol and MPP + estradiol co-treatment on serum cholesterol level and uterine weight of male and female mice
| Parameter |
Normal control
|
|
Asthmatic control
|
|
Estradiol treated
|
|
MPP + estradiol
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| male | female | male | female | male | female | male | female | ||||
| Serum Cholesterol (g/dL) | 115.5 ± 3.555 | 111.41 ± 2.963 | 119.5 ± 1.963 | 106.34 ± 5.753 | 94.79 ± 2.318# | 83.19 ± 2.386# | 113.57 ± 2.501 | 101.59 ± 4.980 | |||
| Uterine weight (g) | ------ | 0.24 ± 0.015 | -------- | 0.27 ± 0.015 | --------- | 0.47 ± 0.034### | ---------- | 0.38± 0.019 | |||
Values in the table represents mean ± SEM (n = 6). Statistical analysis by One-way ANOVA followed by Tukey’s multiple range test indicated significant lower total cholesterol level (#P<0.05 versus normal control group and asthmatic control group) and significant higher uterine weight (###P<0.001 versus normal control group and asthmatic control group).
DISCUSSION
Asthma and other allergic diseases of the airways are up to three times more common in women than in men during early to middle adulthood [22-24]. A number of clinical and epidemiological studies suggest that female hormones contribute to these differences. A recent study found that women taking HRT had a higher risk of new onset asthma [25].
Further, 30-40% of women who have asthma have experienced worsening of their symptoms during the premenstrual phase, when estrogen and progesterone concentrations are changing rapidly [26]. Moreover, a recent study showed that ER polymorphisms are associated with airway hyper-responsiveness and lung function decline, particularly in females with asthma [27]. Mast cells expressing ER and estradiol have been shown to induce mast cell degranulation [6, 9]. Therefore, in the present study, we try to determine the role of ER-α in an experimental model of bronchial asthma. Trypsin and egg albumin induce many of the clinical features of bronchial asthma such as antigen challenge induced bronchoconstriction, airway inflammations, bronchial eosinophilia, airway remodeling and bronchial hyper-sensitivity [10-12].
In asthmatic patients, analysis of blood gases reveals a severe hypoxemia with arterial oxygen (pO2) lower than 60 mmHg, hypocapnia and respiratory alkalosis [28]. In the present study, the sensitized animal when challenged with trypsin and egg albumin, showed a lower serum pO2 level similar to that of observed in patients suffering from asthma. As severity of airflow obstruction increases, pCO2 first normalizes and subsequently increases. Increased pCO2 level in serum will eventually result in increased bicarbonate levels because carbon dioxide in blood is transported as bicarbonates. Estradiol-treated animals showed further lower pO2 and elevated serum bicarbonate as compared to asthmatic control group. This effect of estradiol was not observed in animals co-treated with MPP, suggesting antagonism of estradiol effects.
Next important parameters for asthma include the respiratory functions like tidal volume, respiratory rate and airflow rate. Tidal volume is the volume of air inspired or expired per breath. In asthma, which is an obstructive disease, there is a difficulty in expiration, and hence the volume of air expired is decreases. In addition, there is shallow and rapid breathing thus decreasing the tidal volume and simultaneously increasing the respiratory rate. The lungs do not provide adequate respiratory exchange due to constricted air flow volume and the levels of oxygen in the blood begin to fall [29]. As a consequence of this, air flow rate, which is directly proportional to tidal volume and respiratory rate, also decreases. In the present study, a higher respiratory rate and lower tidal volume and airflow rate were observed in estradiol-treated group as compared to asthmatic control group, which was not observed in MPP co-treated group.
Estrogen treatment and subsequent higher serum estrogen levels show a significant reduction in airway function. Asthma is always associated with strong inflammatory component which gives a chronic status to this disease. In an attempt to determine whether the inflammatory process in the lungs and respiratory tract is affected by estradiol, we did both BAL fluid study and histological examination of lungs. The presence of peripheral blood eosinophilia and activated eosinophils in the chronic inflammatory infiltrate of the airways is characteristic of both allergic and non-allergic asthma. In asthmatic patients, after transendothelial migration, eosinophils adhere to bronchial epithelium, where they degranulate and release eosinophil cation protein, major basic protein, eosinophil peroxidase and superoxide causing damage to epithelium. This can be observed by a higher eosinophil count [30, 31]. We observed significantly higher percent of eosinophil to total lymphocyte count in BAL fluid in trypsin and egg albumin-treated mice which were still higher in estradiol-treated mice. This was further confirmed by histological examination of lungs obtained from these animals. Histopathological examination of lung tissue of asthmatic control group showed a marked inflammation of bronchus. Whereas estradiol-treated group shows more severe destruction of the bronchial wall as compared to asthmatic control and MPP + estradiol co-treated animals.
Estradiol decreases total cholesterol level and increases in uterine weight through ER-α [32, 33]. MPP, a selective ER-α antagonist, should block these effects. Our results are in accordance with this suggestion that the dose of MPP used for in vivo antagonism was quite satisfactory for our experimental study.
From the above findings, it can be said that estrogen might be having a role in asthma by acting through ER-α as its actions are antagonized by a selective ER-α antagonist, MPP.
In addition, estrogen has a strong promoting effect on pathogenesis of bronchial asthma and ER-α receptor is likely to be involved in this effect. This supports the various clinical evidences showing increased incidences of asthma in HRT-treated women.
References
- 1.Ruchi S G, Kevin B W. National Asthma Education and Prevention Program, Expert panel report 2, Guidelines for the Diagnosis and Management of Asthma US Department of Health and Human Services. Pediatrics. 1997;123:S193–S198. [Google Scholar]
- 2.Burr M L, Wat D, Evans C, Dunstan F D, Doull I J. Asthma prevalence in 1973, 1988 and 2003. Thorax . 2006;61(4):296–299. doi: 10.1136/thx.2005.045682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narita S, Goldblum R, Watson C, Brooks E G, Estes D M, Curran E M, Midoro-Horiuti T. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ Health Perspect . 2007;115(1):48–52. doi: 10.1289/ehp.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe K D. Hormone replacement: a risk factor for asthma? 2000. Pulmonaryreviews com .
- 5.Spanos C, Mansoury M, Letourneau R, Minogiannis P, Greenwood J, Siri P, Sant G R, Theoharides T C. Carbachol-induced bladder mast cell activation: augmentation by estradiol and implications for interstitial cystitis. Urology. 1996;48(5):809–816. doi: 10.1016/S0090-4295(96)00239-7. [DOI] [PubMed] [Google Scholar]
- 6.Vliagoftis H, Dimitriadou V, Boucher W, Rozniecki J J, Correia I, Raam S, Theoharides T C. Estradiol augments while tamoxifen inhibits rat mast cell secretion. Int Arch Allergy Immunol. 1992;98(4):398–409. doi: 10.1159/000236217. [DOI] [PubMed] [Google Scholar]
- 7.Cocchiara R, Albeggiani G, Di Trapani G, Azzolina A, Lampiasi N, Rizzo F, Diotallevi L, Gianaroli L, Geraci D. Oestradiol enhances in vitro the histamine release induced by embryonic histamine-releasing factor (EHRF) from uterine mast cells. Hum Reprod. 1992;7(8):1036–1041. doi: 10.1093/oxfordjournals.humrep.a137790. [DOI] [PubMed] [Google Scholar]
- 8.Cocchiara R, Albeggiani G, Di Trapani G, Azzolina A, Lampiasi N, Rizzo F, Geraci D. Modulation of rat peritoneal mast cell and human basophil histamine release by estrogens. Int Arch Allergy Appl Immunol. 1990;93(2-3):192–197. doi: 10.1159/000235300. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X J, McKerr G, Dong Z, Higgins C A, Carson J, Yang Z Q, Hannigan B M. Expression of oestrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages or other immune cells in human upper airways. Thorax. 2001;56(3):205–211. doi: 10.1136/thorax.56.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidlin F, Amadesi S, Dabbagh K, Lewis D E, Knott P, Bunnett N W, Gater P R, Geppetti P, Bertrand C, Stevens M E. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169(9):5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 11.Ebeling C, Forsythe P, Ng J, Gordon J R, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115(3):623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Kim S H, Park E J, Kang C Y, Cho S H, Kim S. Anti-allergic effects of PG102, a water-soluble extract prepared from Actinidia arguta, in a murine ovalbumin-induced asthma model. Clin Exp Allergy. 2009;39(2):280–289. doi: 10.1111/j.1365-2222.2008.03124.x. [DOI] [PubMed] [Google Scholar]
- 13.Apps M C P. A guide to lung function test. Br J Hosp Med. 1992;48(7):396–401. [PubMed] [Google Scholar]
- 14.Fabbri L, Caramori G, Maestrelli P. In: Definition, clinical features, investigations and differential diagnosis of asthma In: Allergy and Allergic Diseases. Kay A B, editor. London: National Heart and Lung Institute; 1997. pp. 1347–1359. [Google Scholar]
- 15.Godkar P B. Textbook of Medical Laboratory Technology. 1st ed. New Delhi, India: Bhalani Publishing House; 1996. Acid-base balance; pp. 252–257. [Google Scholar]
- 16.Khandpur R. Handbook of Biomedical Instrument . 1st ed. New Delhi, India: Tata McGraw-Hill Publishing Company Ltd; 1996. Pulmonary function analyzer; pp. 308–333. [Google Scholar]
- 17.Guyton A, Hall J. Textbook of Medical Physiology. 11th ed. Pennsylvenia, USA: Saunder Publication; 2006. Respiration; pp. 471–532. [Google Scholar]
- 18.Herbert K. Lipids. In: Kaplan, L A, Pesce, A J, editors. Clinical Chemistry: Theory, Analysis and Co-relation . USA: St Louis, C V Mosby; 1984. pp. 1182–1230. [Google Scholar]
- 19.Olivares N, Leon A, Lopez A, Puig A, Cadiz A, Falero G, Martınez M, Sarmiento M E, Farinas M, Infante J F, Sierra G, Solıs R L, Acosta A. The effect of the administration of human gamma globulins in a model of BCG infection in mice. Tuberculosis. 2006;86(3-4):268–272. doi: 10.1016/j.tube.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Vogel H. Drug discovery and evaluation Pharmacological assays. 2nd ed. New York, USA: Springer-Verlag, Berlin Heidelberg; 2002. pp. 351–383. [Google Scholar]
- 21.Garg K, Bhal I, Kaul M. A Textbook of Histology. 2nd ed. New Delhi, India: CBS Publishers and Distributers; 1996. pp. 152–156. [Google Scholar]
- 22.De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110(2):228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 23.Mannino D M, Homa D M, Akinbami L J, Moorman J E, Gwynn C, Redd S C. Surveillance for asthma—United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 24.Schatz M, Camargo C A Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91(6):553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- 25.Barr R G, Wentowski C C, Grodstein F, Somers S C, Stampfer M J, Schwartz J, Speizer F E, Camargo C A Jr. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med. 2004;164(4):379–386. doi: 10.1001/archinte.164.4.379. [DOI] [PubMed] [Google Scholar]
- 26.Vrieze A, Postma D S, Kerstjens H A. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol . 112(2):271–282. doi: 10.1067/mai.2003.1676. [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra A, Howard T D, Vonk J M, Ampleford E J, Lange L A, Bleecker E R, Meyers D A, Postma D S. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol. 2006;117(3):604–611. doi: 10.1016/j.jaci.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Critical Care. 2002;6(1):30–44. doi: 10.1186/cc1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venegas J G, Winkler T, Musch G, Marcos F, Melo V, Layfield D, Nora T, Fischman A J, Callahan R J, Giacomo B, Scott Harris R. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434(7034):777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 30.Milos F, Snezana C. The Roll of Eosinophils in Asthma. Med Biol . 2002;8(1):6–10. [Google Scholar]
- 31.Wardlaw A J. Eosinophils trafficking in asthma. Clin Med. 2001;(2):214–218. doi: 10.7861/clinmedicine.1-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida S, Hutz M H. Estrogen receptor 1 gene polymorphisms in premenopausal women: interaction between genotype and smoking on lipid levels. Braz J Med Biol Res. 2008;41(10):872–876. doi: 10.1590/s0100-879x2008001000007. [DOI] [PubMed] [Google Scholar]
- 33.Davis A M, Ellersieck M R, Grimm K M, Rosenfeld C S. The effects of the selective estrogen receptor modulators, methyl-piperidino-pyrazole (MPP), and raloxifene in normal and cancerous endometrial cell lines and in the murine uterus. Mol Reprod Dev. 2006;73(8):1034–1044. doi: 10.1002/mrd.20520. [DOI] [PubMed] [Google Scholar]