Abstract
Background: Cholesterol oxidase (CHO) has various clinical and industrial applications. Recently, microbial CHO have received a great attention for their wide usage in medicine. Here, taxonomic characterizations of isolated strain from soil, optimization of the conditions for CHO production and biochemical characterizations of produced CHO enzyme were described. Finally, CHO gene was cloned into a cloning vector. Methods: Various samples were collected and cultivated in a screening medium consisting of cholesterol. For isolation of CHO-producing bacteria, well-grown colonies were inoculated into an optimized medium. Different biochemical and microbiological tests were performed on isolated bacteria to identify their properties. For phylogenic analysis, a partial sequence of l6s rRNA was amplified by PCR using universally conserved primers. A modified method was applied for determination of CHO activity. Then, extracellular CHO activity was assessed under different temperature, pH and cholesterol concentration conditions. Finally, CHO gene was amplified by PCR and cloned into STV28. Results: According to the morphological, cultural and biochemical tests, the isolated bacterium was identified as Rhodococcus sp. strain 501 and deposited in GenBank with accession number FN298676. Results showed that optimum temperature and pH for CHO activity were 35°C and 7.5, respectively. Alignment of nucleotide sequence of CHO gene showed 99% homology with other bacterial CHO genes. Conclusion: Rhodococcus sp. strain 501 produced significant levels of extracellular CHO in an optimized medium for a short period. CHO gene was cloned into cloning vector that can be a valuable tool for better identification and further studies on gene expression.
Key Words: Rhodococcus, Cholesterol oxidase, Soil
INTRODUCTION
Cholesterol oxidase (CHO) is an enzyme which catalyzes the oxidation of cholesterol and converts 5-cholesten-3β-ol into 4-cholesten-3-one [1]. CHO enzyme has many applications in medicine [2], agriculture, industry, and pharmaceutical purposes [3]. For instance, it can be used for production of diagnostic kits to detect blood cholesterol [4], biological insecticide [5] and precursors for steroid hormones [6]. Many bacteria can produce this enzyme including members of the genera Arthrobacter, Brevibacterium, Pseudomonas, Nocardia, Rhodococcus, Streptomyces, Corynebacterium and Shizophyllum [7]. This enzyme can be produced from a bacterium in 3 forms: intracellular, extracellular and membrane-bound. Due to the wide spectrum applications of CHO, screening and isolation of bacterial strains producing extracellular form of CHO are of great importance [8]. Many microorganisms have been determined to produce extracellular CHO including Rhodococcus equi, Rhodococcus erythropolis, Streptomyces sp. strain SA-COO, Arthrobacter simplex, Brevibacterium sterolicum, Streptomyces lividans, Shizophylum commune etc. [9, 10]. In the present study, Rhodococcus sp. was isolated from soil and its biochemical and molecular characterizations were elucidated. Then, optimization of temperature and pH conditions for CHO production was performed. The type of CHO enzyme produced by Rhodococcus sp. was determined using an enzyme activity assay on supernatant of bacterial culture. Finally, we attempted to instruct a recombinant plasmid containing CHO gene from an isolated strain for further purposes [11].
MATERIALS AND METHODS
Isolation of microorganisms. A total of 187 samples were gathered from soil of Varamin tannery, and urban composts, dairy, butter, and traditional soap-producing factories of Borujerd (Iran). In order to isolate microorganisms, 1 g of each sample was suspended in 100 ml of distilled water. The suspension was shaked strongly for 30 min. A volume of 100 µl of supernatant was inoculated in medium (medium A) containing cholesterol as the sole carbon source. A medium contained (g/l): agar, 1.5%; K2HPO4, 0.25; NH4NO3, 17; MgSO4.H2O 0.25%; FeSO4, 0.001; NaCl, 0.005; cholesterol, 0.1% and Tween 80, 0.5 ml. The pH of medium was adjusted to 7.0. The inoculated plates were incubated at 30C for 7-12 days. After incubation period was completed, abscission colonies were appeared on the plate surface. For fast growing and generating, larger colonies were subcultured in secondary medium (medium B) containing cholesterol as the only source of carbon as well as yeast extract [8]. This medium contained yeast extract, 0.3 g; (NH4)2HPO4, 0.1 g; cholesterol, 0.15; Tween 80, 0.05 ml; agar, 1.5% and distilled water, 100 ml. Each colony on medium A was cultured in medium B and incubated at 30C for 24 h. Then, larger colonies generated on medium B were used for further identification and differential tests. Identification of isolated microorganisms was performed by microbiological examination and biochemical tests [8, 9].
Colony staining method. To confirm CHO-producing strain, colony staining method was done on the grown colonies. The protocol was as follows: filter papers were dipped into a solution containing 0.5% cholesterol; 1.7% 4-aminoantipyrin; 6% phenol and 3000U/l horseradish peroxidase (HRP) in 100 mM potassium buffer phosphate (pH 7.0). Thereafter, soaked filter was located on grown colonies on the plate and incubated at 30C for 24 h. CHO activity of tested colonies was confirmed by measuring the time required for the development of red color due to the formation of quinoneimine dye [9].
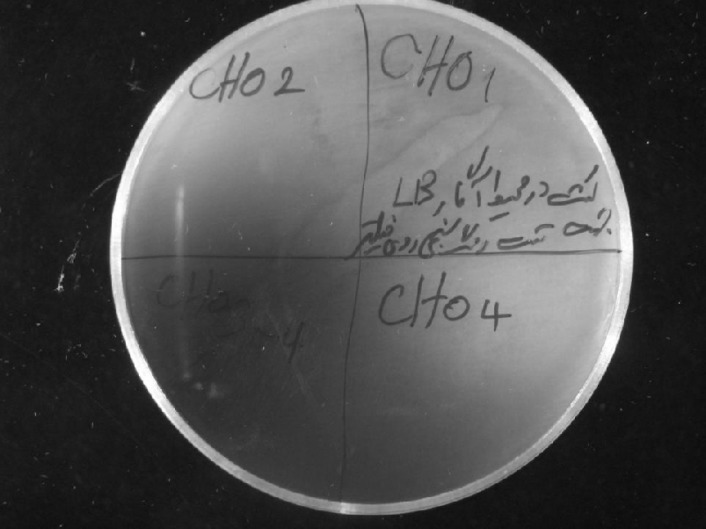
CHO indicator plates. CHO enzyme is able to convert cholesterol into cholest-4-en-3-one and hydrogen peroxide. CHO-producing colonies were selected on suitable indicator plates. These plates were prepared by adding 1.0 g cholesterol, 1.0 g Triton X-100, 0.1 g o-dianisidine and 1000 U/l peroxidase to 1 liter of LB agar medium [12]. Bacterial colonies were cultured on these plates and incubated at 30C. Cholesterol penetrates into bacterial cells where it can be converted into hydrogen peroxide by CHO. Reagents that exist in the medium react with hydrogen peroxide (H2O2) to form azo compound which turns the color of medium intense brown [12].
Assessment of enzyme activity. CHO activity of isolated bacteria was detected by hydrogen peroxide generated during cholesterol oxidation process and was measured by the method of Sasaki et al. [13]. In this reaction, hydrogen peroxide was coupled with 4-aminoantipyrine and phenol by peroxides to produce quinoneimine dye with maximum absorption in 500 nm. Dissolved cholesterol in non-ionic detergent Triton X-100 was used as substrate of the reaction. The reaction mixture was consisted of 3 µmol of cholesterol in 1.0 ml of 1% Triton X-100, 300 µmol of phosphate buffer (disodium phosphate-monopotassium phosphate), pH 7.0, 0.1 ml of enzyme solution, 1.2 µmol of 4-aminoantipyrine, 21 µmol of phenol and 20 U of HRP in a final volume of 3 ml. Reaction was performed at 37C for 10 min with shaking during incubation period. This reaction was terminated by heating at 100C for 3 min. One unite of enzyme was defined as the amount of enzyme that forms 1 μmol of H2O2 per minute at 37C. To evaluate the optimum pH for the enzyme activity, Sasaki's method [13] was done as follows: The enzyme solution was incubated from pH 4.5 to 9 for 24 h. Enzyme activity was measured under standard condition. Acetate buffer was used for pH 4.5 to 5.5 and phosphate buffer for pH 6 to 7.5 and Tris-HCl for pH 8 to 9. In order to determine the effect of temperature on enzyme activity and find the optimum temperature, Sasaki's method [13] was performed on enzyme solution under standard condition except for reaction temperature. The enzyme solution was incubated at 25, 30, 35, 40, 45 and 50C for 24 h. [7, 13]. To determine the cell-bound and extracellular CHO activity, bacterial cultures were centrifuged in 10,000 ×g for 5 min. Supernatant was used as an extracellular enzyme source. In order to find the cell-bound CHO, cellular pellet was washed twice with 0.1 M phosphate buffer (pH 7.2). After resuspending cellular pellet in the same buffer containing 0.1% of Triton X-100, the supernatant was incubated at 30C for 30 min, centrifuged in 10,000 ×g and used for detecting cell-bound CHO [8].
Drawing standard curve for substrate concentrations. In this step, measurement of enzyme activity was done with commercial CHO purchased from Sigma (Germany). First, different concentrations of CHO from 1-50 µmol were prepared to obtain an appropriate concentration of cholesterol (as substrate) for the reaction. Then, standard curves were drawn for various concentrations [11, 13].
Molecular biology procedures:
Bacterial genomic DNA extraction. Chromosomal DNA was prepared using a modified protocol which was previously described [14]. Bacteria from 5 ml aliquots of a stationary phase broth culture were cultured in LB broth medium containing NaCl, 5 g/l; Bacto yeast extract, 5 g/l; Bacto Tryptone, 10 g/l (pH 7.2) and then precipitated by centrifugation at 6000 ×g for 20 min. DNA was extracted using bacterial DNA extraction kit (Metabion, Germany) according to the manufacturer's instructions.
Phylogenic analysis of isolated strain. For final assessment of isolated bacteria, a partial sequence of 16s rRNA was amplified by PCR using two sets of primers (Reverse 1492: 5'-TACGGYTACCTTGT TACGACT-3' and Forward 27: 5'- AGAGTTTGA TCMTGGCTC-3). These primers were designed based on universally conserved sequences. Reaction mixture for PCR amplification was prepared as follows: 2 µl DNA template, 1 U of Taq DNA polymerase (Fermentas, Russia), 1.5 mM of MgCl2, 2.5 µl of PCR buffer 10×, 0.4 pmol of each primers, 0.3 mM of dNTP Mix and 16.8 µl of double distilled water in a final volume of 25 µl. Thermal cycling PCR program was as follows: initial denaturation of DNA at 95C for 5 min, then denaturation at 94C for 45 s, annealing at 58C for 45 s, extension at 72C for 90 s in 30 cycles. Final extension was performed at 72C for 10 min and then the reaction mixture was held on 10C till analysis. PCR product was analyzed by electrophoresis on 0.8% agarose gel. After purification of PCR product from gel agarose by gel extraction kit (Bioneer, Germany), purified DNA was sequenced by Genfanavaran Co. (Iran). Resulting sequence was then compared with the non-redundant sequence database in NCBI.
Nucleotide sequence accession number. The nucleotide sequence of 16s rRNA was deposited in NCBI database with accession number FN298676.
Primer designing and PCR condition for amplification of CHO gene. Nucleotide sequence of identified bacteria was aligned with the other known or putative CHO gene sequences available in databases to identify similar sequences. On the basis of highly similar sequences, a pair of primers specific for isolated bacteria was designed with a cleavage site for PstI restriction endonuclease enzyme located in 5' end of each primer. The primer sequences are as follows:
CHO Forward:
5'-ATACTGCAGATGACCGATAGCCGGGCGAACA-3'
CHO Reverse:
5'-ATAAAGCTTTCACTGGATGTCGGACGAGATG-3'
Reaction mixture for PCR amplification consisted of 3 µl DNA template, 1 U of Taq DNA polymerase (Fermentas, Russia), 1.5 mM of MgCl2, 2.5 µl of PCR buffer 10×, 0.5 pmol of each primers, 0.4 mM of dNTP Mix and 15 µl of double distilled water in a final volume of 25 µl. DNA was denatured at 95C for 5 min and then subjected to 30 cycles under the following conditions: denaturation at 94C for 45 s, annealing at 58C for 45 s, and extension at 72C for 90 s. Final extension was done at 72C for 10 min. a volume of 4 µl of resulting PCR products (about 1600 bp) was analyzed by electrophoresis on 0.8% gel agarose. In order to confirm the existence of CHO gene, PCR products were purified on agarose gel using Qiagen gel extraction kit (Qiagen, Iran) and then were sequenced. The resulting sequence was compared with the non-redundant sequence databases at NCBI using BLAST program.
Cloning of CHO gene in STV28 cloning vector. The purified fragment and STV28 cloning vector were double digested by PstI and HindIII (Fermentas, Russia) at 37C overnight. Then, ligation was achieved by T4DNA ligase at 16C overnight. The resulting recombinant plasmid was named 502 and transformed into E. coli strain DH5α competent cells by heat shock method [14]. The transformants were spread onto LB agar supplemented with 120 µg chloramphenicol at 37C overnight. A number of grown colonies on the plate were randomly selected and DNA extraction was performed using plasmid DNA extraction Kit (Roche, Germany). Then, presence of CHO gene in recombinant plasmid was checked by PCR and also digested with PstI and HindIII restriction enzymes. After primary confirmation with these methods, purified DNA was verified by DNA sequencing and analyzed using BLAST program.
Nucleotide sequence registration in Gene Bank. The nucleotide sequence of CHO gene has been deposited in NCBI database with accession number FN421337.
RESULTS
A total of 187 samples from various sources were cultured in a mineral medium which was suitable for growing of CHO producer bacteria. Only two strains, isolated as CHO producer enzyme, grew and produced enzyme into separated plates. In the primary screening experiences, 187 samples were cultured in a medium containing cholesterol as the sole carbon source. Only two strains (strains 501 and 502 with accession numbers FN298676 and FN430570, respectively) were isolated as CHO producer bacteria. Then, the strain was examined for CHO production and the result showed that the strain 501 had the higher ability for CHO production in comparison with the other one. Therefore, strain 501 which was a Gram-positive coccobacilli was selected for further studies.
The results obtained from biochemical and physiological properties of this strain are shown in Table 1. For better detection and isolation from other bacteria, strain 501 grew on blood agar and formed mucoid colonies. The isolate did not grow on Mc Conkey agar because it could not use lactose, but grew on blood agar and formed mucoid colonies.
According to the morphological, biochemical and morphologic results, this strain belongs to the Rhodococcus genus [6]. Regarding to the confirmation of the results by phylogenic analysis of 16s rRNA sequence, about 99% homology was found in 16s rRNA sequence with other producers of CHO (Arthrobacter sp. F2 Gene Bank: AY963570.1, Brevibacterium sp. DGCDC-82 DQ345780.1, Rhodococcus equi corU1: AJ242746.1, Brevibacterium sterolicum CHOBD00712.1, Rhodococcus sp. 501 FN421337) (Fig. 1). In first step, the isolate produces CHO as above mentioned and colony staining method was carried out to confirm that the isolate produce CHO enzyme. Mechanism of the reaction is because of the formation of quinoneimine dye (Fig. 2). In the next step, CHO indicator plates were used for further confirmation of CHO production by the isolated bacteria. Formation of AZO compound changed the medium color into intense brown (Fig. 3). By the method of Sasaki et al. [13], extracellular and cell-bound CHO activity was performed and the results showed that extracellular enzyme expresses more than cell-bound. In order to determine the molecular mass of CHO produced by strain 501, the samples were applied on 10% denaturing poly acrylamide gel (SDS-PAGE) and stained with Coomassie brilliant Blue R-250. A molecular weight size marker was used to determine a protein band with 55 kDa molecular weight (Fig. 4) Activity of CHO enzyme was evaluated using commercial CHO enzyme (Fig. 5).
Fig. 1.
Comparison of nucleotide sequence of CHO gene from Rhodococcus sp. 501 with that of CHO gene from other bacteria
Fig. 2.
Colony staining method. Development of red color due to the formation of quinoneimine dye is an evidence for CHO activity
Fig. 3.
Formation of AZO component because of CHO production by isolated bacteria
Fig. 4.
Determination of CHO molecular weight and its purity by SDS-PAGE. Lane 1, cholesterol oxidase from Rhodococcus sp. PTCC 1633 (control positive); lane 2, cholesterol oxidase produced by Rhodococcus sp. 501; lane 3, page ruler unstained protein ladder and lane 4, negative control.
Fig. 5.
Standard curve of cholesterol concentration
Table 1.
Biochemical and microbiological properties of isolated bacterium.
| Test | Result | Test | Result |
|---|---|---|---|
| Gram reaction | P | MR | N |
| haemolisis | N | VP | N |
| CAMP test | P | Motility | N |
| catalase | P | urease | N |
| oxidase | P | Nitrate reduction | N |
| glucose | N | OF | N |
| lactose | N | Produce of Indole | N |
| sucrose | N | pyruvate | P |
| xilose | N | motility | P |
| Blood agar culture |
Mucoid colonies | DNase | N |
| Mc Conkey agar culture |
No growth | H2S | N |
| Spore | absent | coagulase | N |
N, negative; P, positive; MR, methyl red; VP, Voges-Proskauer test; OF, oxidation-fermentation
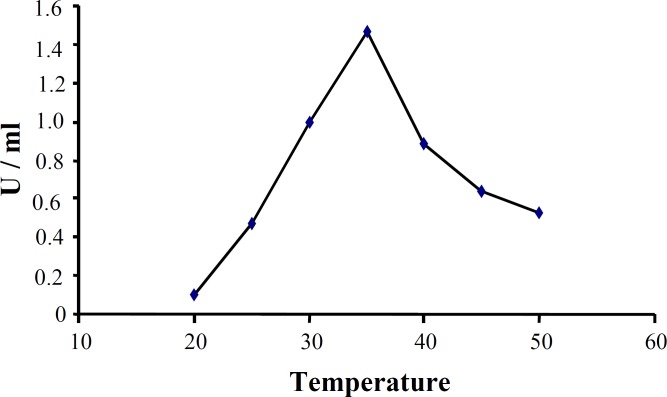
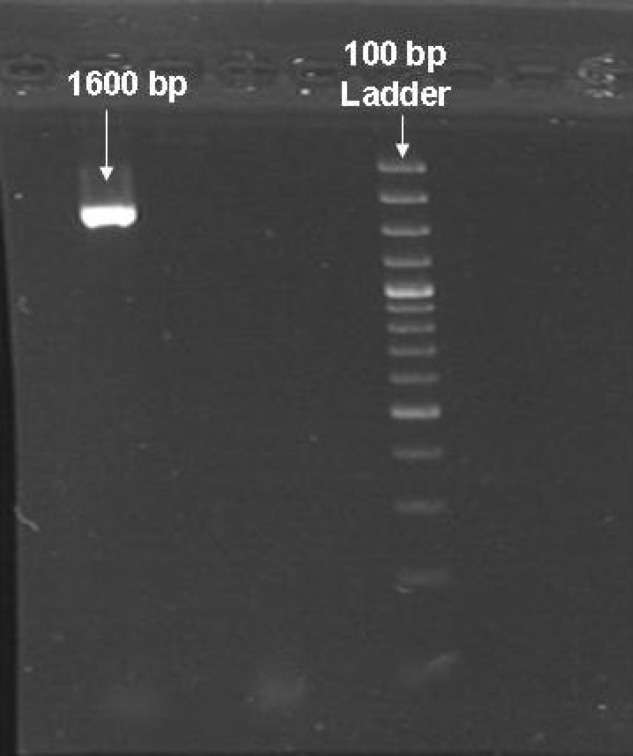
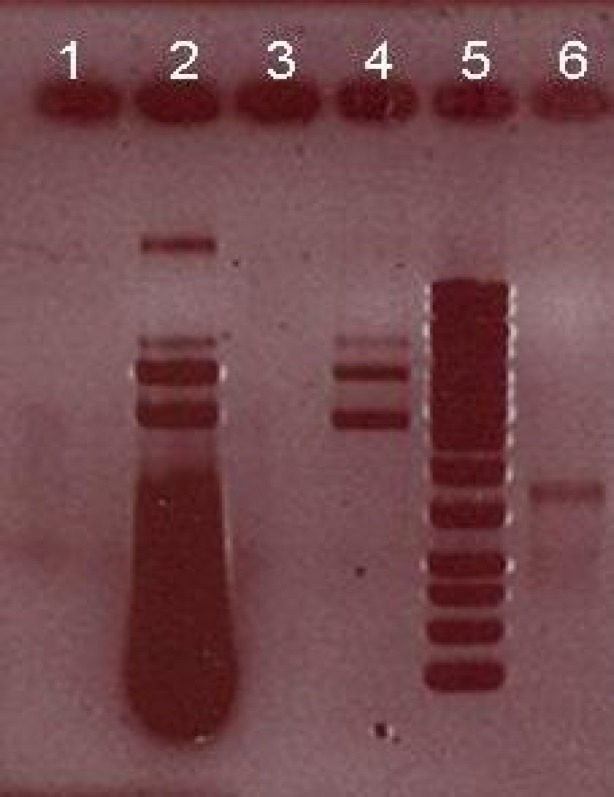
The effect of various pH on activity and stability of extracellular CHO enzyme was assessed under a standard condition except for pH. Figure 6 shows that the best pH for cellular CHO enzyme produced by native isolated strain was pH 7.5. The effect of different temperatures on enzyme activity showed that under a standard condition, CHO had a good activity between 20 and 50C with optimum activity at 35C (Fig. 7). CHO gene was amplified using a pair of primers specific for Rhodococcus sp. 501. PCR products were analyzed by 0.8% gel electrophoresis (Fig. 8). After purification of PCR product from the gel, both fragment and STV28 cloning vectors were doubled digested with HindIII and PstI. Then, they were ligated with T4 DNA ligase and transformed into E. coli DH5α. Afterwards, plasmid DNA extraction from selected colonies was performed and the presence of recombinant plasmid was primary confirmed by enzyme digestion and PCR methods (Fig. 9). DNA sequencing of recombinant plasmid showed that cloning of CHO gene in STV28 cloning vector was successful.
Fig. 6.
Effect of pH on CHO activity. Enzyme activity was measured under standard condition except for reaction pH. The best pH for CHO enzyme is produced by native isolated strain is pH 7.5.
Fig. 7.
Effect of temperature on CHO activity. Enzyme activity was measured under standard condition except for reaction temperature. Optimum activity was seen at 35 C
Fig. 8.
Analysis of PCR product on 0.8% gel electrophoresis. Single band in 1600 bp represents for CHO gene
Fig 9.
Gel electrophoresis analysis of CHO gene and cloning vector after double digestion by HindIII and PstI. Lane 2, recombinant plasmid containing CHO gene + STV28 (7300 bp); lane 4, undigested STV28 plasmid as control (5700 bp); lane 5, 1 kb ladder; lane 6, digested CHO gene with HindIII and PstI (1600 bp).
DISCUSSION
CHO is the first enzyme in cholesterol degradation pathway. This enzyme is produced by several microorganisms which are found in various environments. The first CHO enzyme was isolated from Nocardia erythropolis and its effect on cholesterol oxidation was demonstrated. In a study, Schatz et al.[10] isolated mycobacterium from soil and Statdman [10] could obtain 4-cholest-en-3one by incubating the cell-free extract of the enzyme from this Mycobacterium [10]. Over 276 bacteria and 132 actinomycete strains have CHO activity. Some of these bacteria secrete intracellular enzyme and many of them have the ability to produce extracellular form of CHO [10].
Rhodococcus strains such as Rhodococcus equi (ATCC6939) isolated from butter produced an extracellular enzyme with significant CHO activity and based on reports, this species shows the highest level of CHO activity. Sojo et al. [15] demonstrated that Rhodococcus erythropolis, under appropriate conditions, can show a significant CHO activity when grown in a mineral medium containing cholesterol as a sole of carbon [15].
A strain of Rhodococcus sp. isolated from soil can express CHO in both extracellular and membrane bound forms. Kreit et al. [16] reported that a high amount of CHO enzyme produced by Rhodococcus is extracellular form and only a low amount of CHO is membrane bound or intracellular type.
In this study, we describe the isolation of Rhodococcus sp. 501 from soil samples around a tanneries factory. This native strain can use cholesterol as the sole source of carbon. In addition, there are several methods for isolation of CHO-producing microorganisms. For primary isolation of CHO-producing bacteria, a medium which was described by Watanabe et al. [17] was used. In comparison with other methods used in previous studies, this method was simpler and all of the required materials for preparation of the medium were in access [8]. Grown colonies on medium A were cultured in medium B. Due to the presence of yeast extract in medium B, grown colonies were larger. This criterion was helpful for better identification of colonies [18]. In fact, medium B is necessary for primary detection of CHO-producing bacteria, because cholesterol is the only source of carbon.
Microbiological and biochemical tests showed that this Gram-positive bacterium belongs to Rhodococcus genus, which can produce CHO enzyme with high level and has the ability to degrade into 4-cholest-3-on [13]. Then, measurement of CHO production was performed. According to our results, this strain is able to produce extracellular forms of CHO. In order to compare extracellular and membrane-bound types of CHO, Sasaki et al. [13] method was used which finally revealed that the activity of extracellular form is higher than membrane bound type (data not shown). This finding was in accordance with previous studies [10].
Several studies have used various methods to confirm the production of CHO enzyme by different bacteria [12]. In the present study, colony staining method and CHO indicator plates were used for this purpose.
A protein band on SDS-PAGE was an evidence for the presence of CHO enzyme produced by strain 501. Rhodococcus sp. PTCC 1633 was used as a control positive [11]. Molecular weight of this enzyme was estimated at 55 kDa. The CHO molecular weight is similar to that produced by Brevibacterium [17, 19], Rhodococcus erythropolis [13], Rhodococcus equi [18] and Rhodococcus sp. PTCC 1633 [19]. It must be noted that molecular weight of CHO protein produced by other bacterial strains has a range of 30-61 kDa. However, Sojo et al. [15] have estimated molecular weight of CHO at 55 and 56 kDa from Rhodococcus erythropolis and 56 kDa from Rhodococcus equi no. 23 [10, 7].
In 1982, Cheetham et al. [20] noted that optimum pH for CHO derived from Nocardia and Rhodococcus is 7.5. In this study, investigation of pH and temperature effects on CHO activity showed that optimum pH for activity of the enzyme is 7.0, which is similar to that for Cheetham [20] and Watanabe [17] studies. Moreover, CHO produced by Rhodococcus sp. 501 has an activity in a broad range of pH [3-6, 8, 9].
According to Yazdi et al. [7] study, CHO enzyme is active in 4-50C, but our results showed that CHO has activity at 15-60C with complete activity at 35C.
In the next step, 16s rRNA phylogenic analysis was carried out to confirm isolated bacterium. Nucleotide sequences alignment showed 99% homology with other CHO producer bacteria [11].
For better identification, the gene encoding CHO enzyme from Rhodococcus sp. 501 was amplified with PCR method using specific primers. Purified PCR product was cloned into STV28 cloning vector. Nucleotide sequence of the cloned gene was approved and deposited in Gene Bank after sequencing. Although CHO enzyme produced from bacteria has many advantages, it seems that production of this enzyme in a large scale for industrial usage is expensive and time consuming. Recently many studies have been focused on cloning CHO genes of different bacteria into a suitable expression vectors and proper hosts [1, 11, 21, 22]. Further studies are required for cloning this gene into expression vector and evaluating recombinant enzyme for industrial applications.
ACKNOWLEDGEMENTS
This work was performed in National Institute for Genetic Engineering and Biotechnology (NIGEB) in Iran. The authors thank Dr. Siadat and Dr. Aghasadeghi for donation of plasmid (vector) and bacterial strains (host), respectively. This research was financially supported by Lorestan University of Medical Sciences (Lorestan, Iran).
References
- 1.Murooka Y, Ishizaki T, Nimi O, Maekawa N. Cloning and expression of a Streptomyces cholesterol oxidase gene in Streptomyces lividans with plasmid pIJ 702. J Appl Environ Microbiol. 1986;52(6):1382–1385. doi: 10.1128/aem.52.6.1382-1385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allain C, Poon L S, Chan C S G, Richmond W, Fu P C. Enzymatic determination of total serum cholesterol. J Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 3.Ahmad S, Garg S K, Johri B N. Biotransformation of sterols: selective cleavage of the side chain. J Biotechnol Adv. 1992;10(1):61–67. doi: 10.1016/0734-9750(92)91351-e. [DOI] [PubMed] [Google Scholar]
- 4.Noma A, Nakayama K. Comparative studies on cholesterol oxidases from different sources. J Clin Chim Acta . 1976;73(3):487–496. doi: 10.1016/0009-8981(76)90152-2. [DOI] [PubMed] [Google Scholar]
- 5.Purcell J P, Greenplate J T, Jennings M G, Ryerse J S, Pershing J C, Sims S R, Prinsen M J, Corbin D R, Tran M, Sammons R D. Cholesterol oxidase: a potent insecticidal protein active against boll weevil larvae. J Biochem Biophys Res Commun. 1993;196(3):1406–1413. doi: 10.1006/bbrc.1993.2409. [DOI] [PubMed] [Google Scholar]
- 6.Bell K S, Philp J C, Aw D W J, Christofi N. A review of the genus Rhodococcus. J Appl Microbiol. 1998;85(2):195–210. doi: 10.1046/j.1365-2672.1998.00525.x. [DOI] [PubMed] [Google Scholar]
- 7.Yazdi M, Yazdi Z, Zarrini G H, Ghasemian A. Purification and characterization of extra-cellular cholesterol oxidase from Rhodococcus sp PTCC 1633. J Biotechnol. 2008;7(4):751–75. [Google Scholar]
- 8.Yazdi M T, Malekzadeh F, Zarrini G H, Faramarzi M A, Kamranpour N, Khaleghparast S H. Production of cholesterol oxidase by a newly isolated Rhodococcus sp. World J Microbiol Biotechnol . 2001;17(7):731–737. [Google Scholar]
- 9.Lee S Y, Rhee H I, Tae W C, Shin J C, Park B K. Purification and characterization of cholesterol oxidase from Pseudomonas sp and taxonomic study of the strain. J Appl Microbiol Biotechnol. 1989;31(5-6):542–546. [Google Scholar]
- 10.Mac Lachlan J, Wotherspoon A T L, Ansell R O, Brooks C J W. Cholesterol oxidase: sources, physical properties and analytical applications. J Steroid Biochem Mol Biol. 2000;72(5):169–195. doi: 10.1016/s0960-0760(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 11.Ghasemian A, Tabatabaei Y M, Sepehrizadeh Z, Tabatabaei Y Z, Zarrini G H. Overexpression, one-step purification, and characterization of a type II cholesterol oxidase from a local isolate Rhodococcus sp PTCC 1633. World J Microbiol Biotechnol. 2009;25(5):773–779. [Google Scholar]
- 12.Nishiya Y, Harada N, Teshima S, Yamashita M, Fujii I, Hirayama N, Murooka Y. Improvement of thermal stability of Streptomyces cholesterol oxidase by random mutagenesis and a structural interpretation. J Pro Eng. 1997;10(3):231–235. doi: 10.1093/protein/10.3.231. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki I, Goto H, Yamamoto R, Tanaka H, Takami K I, Yamashita K J, Horio T. Hydrophobic ionic chromatography: its application to microbial glucose oxidase, hyaluronidase, cholesterol oxidase and cholesterol esterase. J Biochem. 1982;91(5):1555–1561. doi: 10.1093/oxfordjournals.jbchem.a133846. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J. Molecular cloning a laboratory manual. Aurora, CO, USA : Cold Spring harbor Laboratory Press Dream Books Co; 1989. [Google Scholar]
- 15.Sojo M, Bru R, Loppez M D, Garcia C F, Arguelles J C. Cell-linked and extracellular cholesterol oxidase activities from Rhodococcus erythropolis isolation and physiological characterization. JAppl Microbiol Biotechnol. 1997;47(5):583–589. doi: 10.1007/s002530050977. [DOI] [PubMed] [Google Scholar]
- 16.Kreit J, Lefebvre G, Germain P. Membrane-bound cholesterol oxidase from Rhodococcus sp cells Production and extraction. J Biotechnol. 1994;33(3):271–282. [Google Scholar]
- 17.Watanabe K, Aihara H, Nakagawa Y, Nakamura R, Sasaki T. Properties of the purified extracellular cholesterol oxidase from Rhodococcus equi No 23. J Agr Food Chem. 1989;37(4):1178–1182. [Google Scholar]
- 18.Navas J, Gonzalez-Zorn B, Ladron N, Garrido P, Vazquez-Boland J A. Identification and mutagenesis by allelic exchange of choE encoding a cholesterol oxidase from the intracellular pathogen Rhodococcus equi. J Bacteriol . 2001;183(16):4796–4805. doi: 10.1128/JB.183.16.4796-4805.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujishiro K, Uchida H, Shimokava K, Nakano M, Sano F, Ohta T, Nakahara N, Aisak K, Uwajima T. Purification and properties of a new Brevibacterium sterolicum cholesterol oxidase produced by E coli MM294/pnH10. J FEMS Microbiol Lett . 2002;215(2):243–248. doi: 10.1111/j.1574-6968.2002.tb11397.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheetham P S, Dunnill P, Lilly M D. The characterization and interconversion of three forms of cholesterol oxidase extracted from Nocardiarhodochrous. JBiochem . 1982;201(3):512–521. doi: 10.1042/bj2010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujishiro K, Ohta T, Hasegawa M, Yamaguchi K, Mizukami T, Uwajima T. Isolation and identification of the gene of cholesterol oxidase from Brevibacterium sterolicum ATCC21387, a widely used enzyme in clinical analysis. J Biochem Biophys. 1990;172(2):721–727. doi: 10.1016/0006-291x(90)90734-5. [DOI] [PubMed] [Google Scholar]
- 22.Sampson N S, Chen X. Increased expression of Brevibacterium sterolicum cholesterol oxidase in Escherichia coli by genetic modification. J Protein Exp Pur. 1998;12(3):347–352. doi: 10.1006/prep.1997.0855. [DOI] [PubMed] [Google Scholar]