Abstract
Background: The involvement of water-soluble carotenoids, crocins, as the main and active components of Crocus sativus L. extract in learning and memory processes has been proposed. In the present study, the effect of crocins on sporadic Alzheimer's disease induced by intracerebroventricular (icv) streptozocin (STZ) in male rats was investigated. Methods: Male adult Wistar rats (n = 90 and 260-290 g) were divided into 1, control; 2 and 3, crocins (15 and 30 mg/kg); 4, STZ; 5 and 6, STZ + crocins (15 and 30 mg/kg) groups. In Alzheimer's disease groups, rats were injected with STZ-icv bilaterally (3 mg/kg) in first day and 3 days later, a similar STZ-icv application was repeated. In STZ + crocin animal groups, crocin was applied in doses of 15 and 30 mg/kg, i.p., one day pre-surgery and continued for three weeks. Prescription of crocin in each dose was repeated once for two days. However, the learning and memory performance was assessed using passive avoidance paradigm, and for spatial cognition evaluation, Y-maze task was used. Results: It was found out that crocin (30 mg/kg)-treated STZ-injected rats show higher correct choices and lower errors in Y-maze than vehicle-treated STZ-injected rats. In addition, crocin in the mentioned dose could significantly attenuated learning and memory impairment in treated STZ-injected group in passive avoidance test. Conclusion: Therefore, these results demonstrate the effectiveness of crocin (30 mg/kg) in antagonizing the cognitive deficits caused by STZ-icv in rats and its potential in the treatment of neurodegenerative diseases such as Alzheimer's disease.
Key Words: Streptozocin, Learning, Memory
INTRODUCTION
It is a well-established fact that intracerebro-ventricular (icv) injection of streptozocin (STZ) is characterized by a progressive deterioration of learning, memory, and cerebral glucose and energy metabolism. However, intracerebro-ventriculary application of STZ could provide an appropriate and relevant experimental model of sporadic Alzheimer's disease (SAD) [1]. Alzheimer's disease is a progressive and irreversible neuropsychiatric disorder characterized by neuronal degeneration, loss of memory and behavioral disturbances [2]. It must be considered that oxidative stress is the prime candidate for neuronal degeneration which leads to neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases [3]. However, degeneration of cholinergic neurons by free radicals, which yield to reduction of acetylcholine (Ach) concentration in hippocampus, is the main factor for SAD [4].
Crocus sativus L. is a plant cultivated in various parts of the world. There is also experimental evidence that saffron and its components are involved in cognition [5]. It has been shown that administration either of extracts of C. sativus L. or of its constituent crocin reduce ethanol-induced memory impairment in the passive avoidance in mouse [6]. Recently, we have demonstrated that saffron extract counteracted recognition memory deficits in a STZ-icv model of Alzheimer's disease in rats [7]. Flavonoids and especially crocins are the main components of saffron stigmas [8], which the latter could efficiently have antagonized memory deficits in a normal rat [9]. However, with respect to beneficial effect of free radical scavengers and antioxidant components in cognitive impairment [10], the active C. sativus L. which constitutes crocins as a potent antioxidant could yield putative positive benefit in altering, reversing or forestalling the neuronal-behavioral decrements [11]. With respect to the mentioned reports, the aim of the present study was to investigate the role of crocin in antagonizing extinction of recognition memory in a STZ-induced model of SAD in rats using passive avoidance and Y-maze tasks.
MATERIALS AND METHODS
STZ and crocin were prepared from Sigma Chemicals Co. (St. Louis, MO, USA). Ketamine (10%) and xylazine (2%) were purchased from Alfasan Co. (Holland). Artificial cerebro-spinal fluid (ACSF) constituents were obtained from Merck Co. (Germany).
Animals. Male adult Wistar rats (n = 90 and 260-290 g) were procured from Pasteur Institute of Iran (Tehran). At the start of the experiment, the animals were housed three to four per cage in a temperature-controlled colony room under a 12 h light/dark cycle. Then, they were given free access to water and kept at 80-85% of their free-feeding body weight throughout the experiment. All behavioral experiments were carried out between 11 a.m. and 4 p.m. This study was conducted in accordance with the policies set forth in the Guide for the Care and Use of Laboratory Animals (NIH) and those in the Research Council of Shahed University of Medical Sciences (Tehran, Iran).
Experimental procedure. Rats were randomly divided into the following groups: 1, sham-control group, that received bilateral icv injection of ACSF (10 µl on each side) as STZ solvent; 2 and 3, crocin-treated control groups that received crocin in doses of 15 and 30 mg/kg, i.p.; 4, STZ-injected group (STZ) that received icv injection of STZ; 5 and 6, crocin-treated STZ groups (STZ + crocin) that received crocin in doses of 15 and 30 mg/kg, i.p. Application of each crocin dose was repeated once for two days during total treatment period (3 weeks). STZ and crocin-treated STZ groups were given a bilateral icv injection of STZ in dose of 3 mg/kg (Sigma, St. Louis, USA). STZ was freshly dissolved in cold ACSF at a volume of 10 µl on each side and the injection was repeated on day 3 [12]. In the sham-control group, only ACSF (120 mM NaCl, 3 mM KCl, 1.15 mM CaCl2, 0.8 mM MgCl2, 27 mM NaHCO3 and 0.33 mM NaH2PO4, adjusted to pH 7.2) (Merck Chemical, Germany) was icv injected. For stereotaxic surgery, rats were anesthetized with a combination of ketamine and xylazine (100 and 5 mg/kg, i.p., respectively) and then they were placed in a stereotaxic apparatus (Stoelting, USA). The scalp was cleaned with iodine solution, incised on the midline and a burr hole was drilled through the skull 0.8 mm post bregma, 1.4 mm lateral from midsagittal line and 3.4 mm below the dura according to the Pxinos and Watson stereotaxic atlas [13].
Behavioral tests:
Spatial Y-maze memory. Experimental apparatus for Y-maze consisted of a black-painted maze made of Plexiglas. Each three arms of the Y-maze olabeled with A, B and C was 40 cm long, 30 cm high and 15 cm wide [14, 15] and positioned at an equal angle and converged in an equilateral triangular central area with 15 cm at its longest axis. Each rat, native to the maze, was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The sequence of each arm entry was recorded manually (i.e., ACBABA CACBCACAC etc.). A spontaneous alternation behavior, which is regarded as a measure of spatial memory, was defined as the entry into all three arms on consecutive choices in overlapping triplet sets (i.e., ACB, ABA, CAC, BCA, CAC) [15]. The percent of spontaneous alternation behavior was calculated as the ratio of actual to possible alternations.
Percent alternation = [actual alternation (i.e., ACB, BCA = 6)/Maximum Alternation (i.e., ACBABACACBCACAC = 15 – 2 = 13)] × 100 = (6/13) ×100 = 46.15 %.
Total Number of arms entered minus 2 [15]. Each test was done once on each animal.
Single-trial passive avoidance test. The apparatus (BPT Co., Tehran) consisted of an illuminated chamber connected to a dark chamber by a guillotine door. Electric shocks were delivered to the grid floor by an isolated stimulator. On the first and second days of testing, each rat was placed on the apparatus and left for 5 min to habituate to the apparatus and on the third day, an acquisition trial was performed. The rats were individually placed in the illuminated chamber. After a habituation period of 2 min, the guillotine door was opened and after the rat entering the dark chamber, the door was closed and an inescapable scrambled electric shock (1 mA, 1 s once) was delivered. In this trial, the initial latency of entrance into the dark chamber was recorded and the rats with initial latency greater than 60 s were excluded from the study. Twenty-four hours later, each rat was placed in the illuminated chamber for retention trial. The interval between the placement in the illuminated chamber and the entry into the dark chamber was measured as step-through latency (STL, up to a maximum of 150 s). This test was conducted after 3 weeks post-surgery.
Psychomotor coordination (PMC) index. This test was done by Rota-Rod Treadmill with shock facility apparatus (Harvard model 865) after Y-maze task. First, animals were trained by placing them on a rolling bar and had been to walk on it. Then, the animals were conducted to Rota-Rod test with these characteristics: initial speed = 4 rpm, final speed = 30 rpm, initial to final speed time = 4 min, shock intensity = 1.1 mA, shock duration = 0.2-0.8 s, experimental length time = 5 min and interval between experiments = 2 min. Rota-Rod test was repeated 5 times for each rat and the mean stay time on the rod per trial was taken as a PMC index [14]. Also, the mean number of fallings for each animal was recorded. Each animal was experimented only one period in this test.
Statistical analysis. All results were expressed as mean SEM. For the passive avoidance test, the non-parametric Kruskal-Wallis test was used which, if significant, was followed by Mann-Whitney U test for pair-wise comparisons. Data for the Y-maze task were evaluated by Wilcoxon’s rank sum test. However, ANOVA and followed post hoc Tukey’s tests were used for analysis of weight changes. In all calculations, a difference at P<0.05 was regarded as significant.
RESULTS
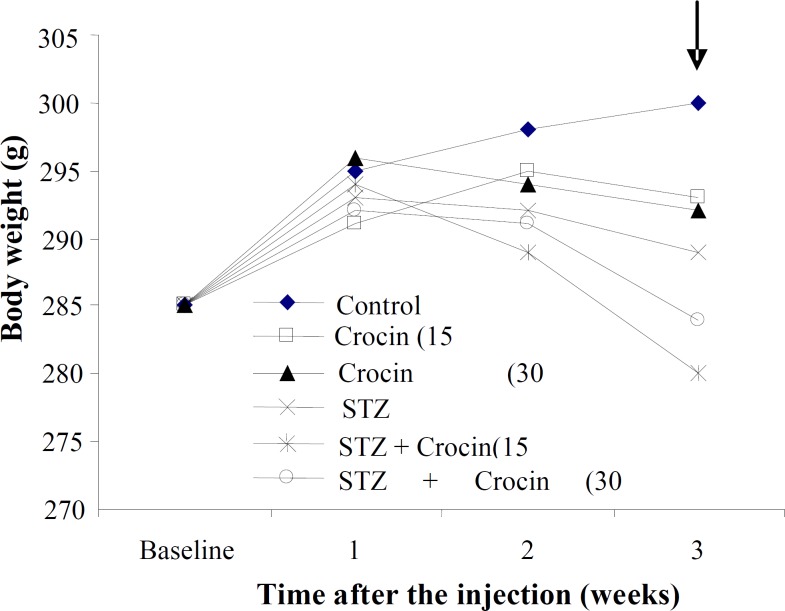
Effects of STZ and crocin on body weight. Rats injected with STZ-icv showed a significant reduction in body weight compared to those of controls (Fig. 1). Treatment of STZ-icv animals with crocin could yield more decrease in body weight than other animal groups. However, the comparison of the animal weights 3 weeks after experiment shows a more weight loss in crocin treatment STZ-icv rats (68%) than STZ (15%) group.
Fig. 1.
Effect of STZ and crocin on body weight. Baseline weight was measured on the day of the first STZ injection. Arrows indicate the significant difference among groups
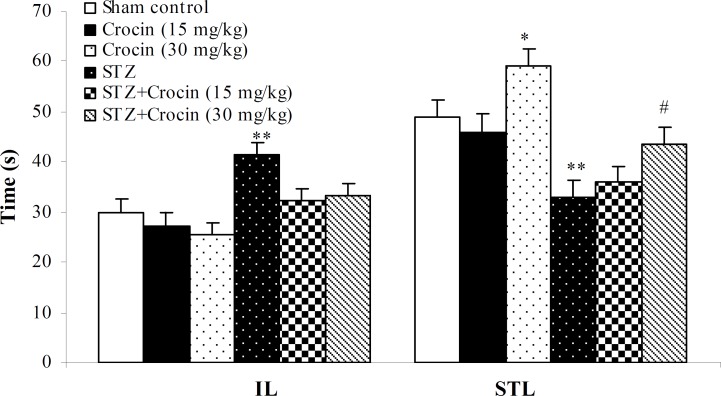
Effect of crocin on memory retention deficit in passive avoidance test. As shown in Figure 2, the initial latency in STZ group (41.55 ± 2.33) was significantly greater than sham control animals (29.9 ± 2.65, P<0.01). STL in crocin group (30 mg/kg, 58.98 ± 3.48) increased markedly than control animals (48.8 ± 3.65, P<0.05). However, a higher dose of crocin (30 mg/kg) could significantly antagonized memory deficits induced by STZ-icv i.e. the STL time in STZ group (33.1 ± 3.2) reach to 43.5 ± 3.48 s in STZ + crocin (30 mg/kg) group with respect to control animals (48.8 ± 3.65, P<0.05).
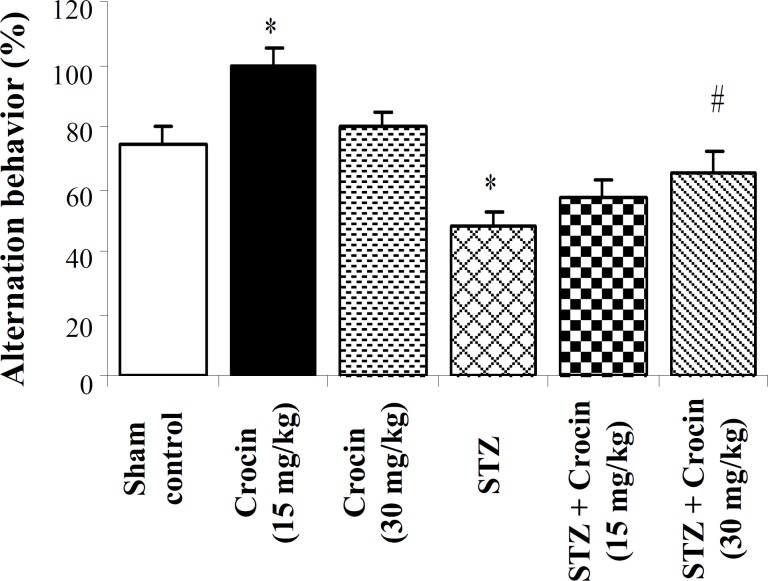
Effect of crocin on spatial cognition deficit in Y-maze task. Although application of STZ-icv could markedly decrease the score of alternation behavior from 73.9 ± 6.28 to 48.21 ± 4.81in control animals, the treatment of STZ-icv rats with crocin in dose of 15 mg/kg could significantly increase (99.98 ± 4.89)
Fig. 2.
The effect of crocin (15 and 30 mg/kg) on passive avoidance performance after icv injection of STZ in rats as indicated by initial (IL) and step-through latencies (STL) after 3 weeks. Values are expressed as means ± S.E.M. * P<0.05, ** P<0.01 (in comparison with control group), # P<0.05 (STZ + crocin vs. STZ).
it (Fig. 3). However, if the effect of crocin in dose of 30 mg/kg on alternation behavior is not significant, its mild alleviation effect on STZ-induced spatial cognition deficits must be mid (Fig. 3).
Fig. 3.
Percentage of Alternation behavior in Y-maze task on the third week after treatment (mean ± SEM). * and # P<0.05 as compared to control and STZ groups respectively
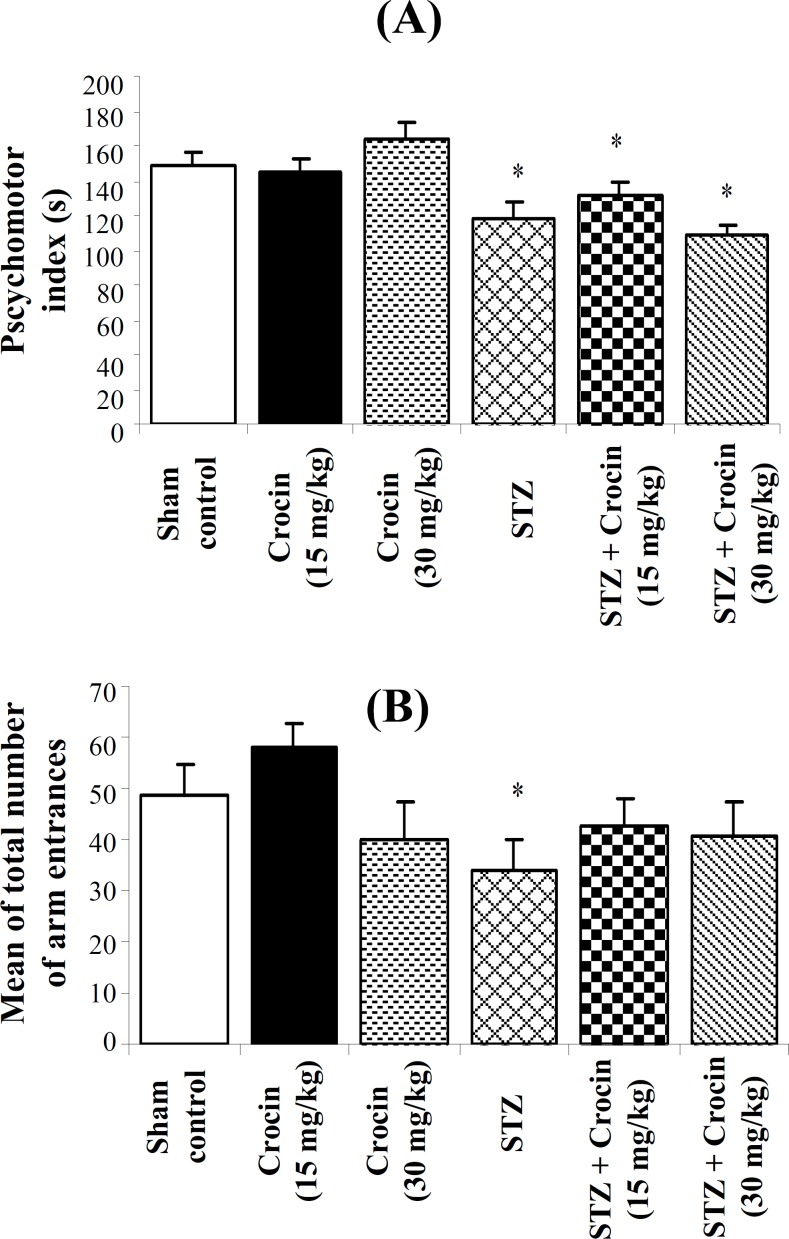
PMC index. Figure 4A shows the results of PMC test by the Rota-Rod Treadmill apparatus. The mean PMC indices of experiments for STZ, STZ + crocin (15 mg/kg) and STZ + crocin (30 mg/kg) groups were obtained 118 ± 9.56, 132 ± 7.82 and 108 ± 6.25, respectively which were significantly lower than control animals (148 ± 8.78, P<0.05). Also, the number of animals falling (Fig. 4B) in STZ (98 ± 9.81) and STZ + crocin (30 mg/kg, 95 ± 6.25) groups were markedly greater than control ones (68 ± 6.75).
Fig. 4.
(A) Psychomotor coordination by Rota-Rod Treadmill apparatus (mean ± SEM). (B) Number of fallings from Rota-Rod rolling bar (mean ± SEM). *P<0.05 as compared to control.
DISCUSSION
The results of the present study demonstrated that treatment of STZ-icv rats with crocin (30 mg/kg) for 3 weeks could significantly ameliorate cognition deficits. In addition, the efficacy of crocin in antagonizing extinction of recognition memory in the normal rats has been yield. In conformity with this, the results from Y-maze task for the first time showed that STZ-icv animals also exhibit a higher score of errors and lower correct choices, indicating an abnormality in spatial cognitive processes. However, in normal and STZ-icv rats, crocin in doses of 15 and 30 mg/kg, respectively could improve the percent of alternation behavior tasks. On the basis of the obtained results, it is suggested that impairment in passive avoidance behavior may reflect poorer acquisition and/or retention of memory after icv injection of STZ. The results from the Y-maze task may also indicate a spatial cognition deficit in STZ-icv rats.
In this study, treatment of STZ-icv rats with high dose of crocin (30 mg/kg), starting 1 day before surgery for three weeks, caused a significant improvement in learning, memory, and spatial cognitive skills. Other reports show that SAD is characterized by alterations at the level of various neurotransmitters and related markers and receptors [16, 17]. Hence, cholinergic system is the most severely affected body system in SAD [18], so it has been suggested that elevation of the Ach level might be helpful in attempts to improve the symptoms of cognitive deficits in Alzheimer's disease [19].
However, it was shown that crocins (30 mg/kg and to some extent 15 mg/kg) significantly reduce reference memory errors produced by scopolamine [9]. This report potentiates the direct or indirect hypotheses of cholinergic mimicking action of the crocin in higher doses. Meanwhile, regarding to ambiguous mechanism(s) underling role of crocin on memory, the induction of hippocampal long-term potentiation (LTP), a form of activity-dependent synaptic plasticity that may underlie learning and memory [17], could explain the beneficial effect of crocin on SAD. However, induction of LTP in brain slices and improvement of cognition in lower doses of crocin, especially in rats [20] could explain the efficacy of crocin at a dose of 15 mg/kg in spatial memory as obtained from Y-maze task. Also, because crocin at low and high doses in each Y maze control and STZ groups test could yield different cognitive improvement potency, it guide our mid to the different possible involved mechanism(s) for crocin cognitive repair action in control and STZ-icv injected rats. With this respect, the cognitive repair mimicking action of crocin at 30 mg/kg in passive avoidance test may be related to these unknown mechanism(s). On the other hand, it has been found that STZ as a bacterial toxin in the rats could chronically decrease cerebral glucose uptake which leads to several molecular and pathological features of Alzheimer's disease. Furthermore, cerebral glucose and energy metabolism disturbance and increment of oxidative stress are the most signs in STZ-icv animals which yield to learning and memory deficits. Therefore, the neuroprotective [21, 22], antioxidant [23] and free radical scavengering properties of crocin [24] could describe the antagonized extinction recognition memory action of this component. Molecular evidences show that inflammatory processes are associated with the pathophysiology of Alzheimer's disease [25, 26].
Furthermore, it has recently been demonstrated that STZ-icv in rats could also lead to enhance inflammatory processes within the central nervous system (CNS) [27]. The inflammatory mediators could release free radicals, and the neuro-toxicity induced by free radicals is known as a leading hypothesis for cause of SAD. Also, It has been reported that an increase in free radical production and lipid peroxidation in CNS lead to impairment of recognition. However, pretreatment with crocin (especially in 30 mg/kg dose) could prevent the generation of inflammatory mediators [28] that could yield beneficial cognition. Psychomotor coordination data obtained from animal groups show a low but significant difference between STZ and crocin groups with respect to control. However, the non-significant psychomotor coordination index and also mean total number of arm entrances between STZ and crocin groups could explain that absolute cognitive learning improvement by crocins is related to central cognitive mechanism(s) not the motor coordination paradigms. In complementary experiments, we found a weight loss due to STZ-icv application which consolidated by crocin treatment. Intraventricular process on hypothalamic cells could explain the initial hypophasia and weight loss seen after icv injection of STZ [29]. However, anti-lipidemic effect of crocin [30] may be evident the more weight loss in crocins treatment rats regarding control and STZ animal groups.
In conclusion, the present study clearly demonstrated that crocin (30 mg/kg) treatment could significantly prevent the cognitive impairments following icv injection of STZ and this suggests the therapeutic potential of this component in aging and age-related neurodegenerative disorders where cognitive impairment is involved.
ACKNOWLEDGEMENTS
The authors would like to thank Mrs. Fariba Ansari for her assistance in behavioral tests and also Shahed University for the financial support of this work.
References
- 1.Veerendra Kumar M H, Gupta Y K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer's disease in rats. Clin Exp Pharmacol Physiol. 2003;30(5-6):336–342. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- 2.Standridge J B. Pharmacotherapeutic approaches to the prevention of Alzheimer's disease. Am J Geriatr Pharmacother. 2004;2(2):119–132. doi: 10.1016/s1543-5946(04)90017-7. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Gupta Y K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci . 2002;71(21):2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 4.Vinod T, Anurag K, Mahendra B, Kanwaljit C. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intra-cerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharm Biochem Behav. 2009;93(2):183–189. doi: 10.1016/j.pbb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L on the ethanol-induced impairment of passive avoidance performance in mice. Biol Pharm Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura M, Shoyama Y, Saito H, Nishiyama N. Crocin improves the ethanol-induced impairment of learning behaviors of mice in passive avoidance tasks. Proc Jpn Acad. 1995;71:319–324. [Google Scholar]
- 7.Khalili M, Roghani M, Ekhlasi M. The effect of aqueous Crocus sativus L extract on intra-cerebroventricular Streptozotocin-induced cognitive deficits in rats: a behavioral analysis. Iran J Pharmaceutical Res. 2009;8:185–191. [Google Scholar]
- 8.Tarantilis P A, Tsoupras G, Polissiou M. Determination of saffron (Crocus sativus L ) components in crude plant extract using high-performance liquid chromatography-UV/visible photodiode-array detection-mass spectrometry. J Chromatogr A. 1995;699(1-2):107–118. doi: 10.1016/0021-9673(95)00044-n. [DOI] [PubMed] [Google Scholar]
- 9.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L, crocins on recognition and spatial rats' memory. Behav Brain Res. 2007;183(2):141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Olcese J M, Cao C, Mori T, Mamcarz M B, Maxwell A, Runfeldt M J, Wang L, Zhang C, Lin X, Zhang G Arendash G W. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47(1):82–96. doi: 10.1111/j.1600-079X.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770(4):578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasiński A Grieb P. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer's disease. Acta Neurochir Suppl. 2010;106:177–181. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. San Diego, USA: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 14.Vallee M, Mayo W, Darnauder M, Corpechot C, Young J, Koehl M, LeMoal M, Baulieu E E, Robel P, Simon H. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci. 1997;94(26):14865–14870. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roghani M, Joghataie M T, Jalali M R, Baluchnejadmojarad T. Time course of changes in passive avoidance and Y-maze performance in male diabetic rats. Iran Biomed J. 2006;10(2):99–104. [Google Scholar]
- 16.Sharma M, Gupta Y K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci . 2002;71(21):2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 17.Lannert H, Hoyer S. Intra-cerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112(5):1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 18.Carr D B, Goate A, Morris J C. Current concepts in the pathogenesis of Alzheimer’s disease. Am J Med. 1997;103(3A):3–10. doi: 10.1016/s0002-9343(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 19.Gasparini L, Racchi M, Binetti G, Trabucchi M, Solerte S B, Alkon D, Etcheberrigaray R, Gibson G, Blass J, Paoletti R, Govoni S. Peripheral markers in testing pathophysiological hypothesis and diagnosing Alzheimer’s disease. FASEB J. 1998;(12):17–34. doi: 10.1096/fasebj.12.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Sugiura M, Shoyama Y, Saito H, Abe K. Crocin (crocetin di-gentiobiose ester) prevents the inhibitory effect of ethanol on long-term potentiation in the dentate gyrus in vivo. J Pharmacol Exp Ther . 1994;271(2):703–707. [PubMed] [Google Scholar]
- 21.Fukui K, Omoi N O, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci . 1998;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 22.Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food. 2006;9(2):246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 23.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocussativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;15(23):8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 24.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L extract and its bioactive constituents. Phytother Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 25.Tariot P N, Federoff H J. Current treatment for Alzheimer disease and future prospects. Alzheimer Dis Assoc Disord . 2003;17(suppl 4):105–113. doi: 10.1097/00002093-200307004-00005. [DOI] [PubMed] [Google Scholar]
- 26.Jiang F, Dusting G J. Natural phenolic compounds as cardiovascular therapeutics: potential role of their anti-inflammatory effects. Curr Cardiovasc Pharm. 2003;1:135–156. doi: 10.2174/1570161033476736. [DOI] [PubMed] [Google Scholar]
- 27.Chu W Z, Qian C Y. Expressions of Abeta1-40, Abeta1-42, tau202, tau396 and tau404 after intracerebroventricular injection of strepto-zotocin in rats. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(2):168–170. [PubMed] [Google Scholar]
- 28.Heo H J, Kim D O, Shin S C, Kim M J, Kim B G, Shin D H. Effect of antioxidant flavanone, naringenin, from Citrus junoson neuroprotection. J Agric Food Chem. 2004;52(6):1520–1525. doi: 10.1021/jf035079g. [DOI] [PubMed] [Google Scholar]
- 29.Shoham S, Bejar C, Kovalev E, Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp Neurol. 2003;184(2):1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Xu G L, Yu S Q, Gong Z N, Zhang S Q. Study of the effect of crocin on rat experimental hyperlipemia and the underlying mechanismsZhongguo. Zhong Yao Za Zhi . 2005;30(5):369–372. [PubMed] [Google Scholar]