Abstract
Objective
This study aimed to comprehensively describe inflammatory responses to trivalent influenza virus vaccine (TIV) among pregnant women and determine if responses differ compared to non-pregnancy.
Methods
Twenty-eight pregnant and 28 non-pregnant women were vaccinated. Serum cytokines were measured at baseline, one, two, and three days post-vaccination. Anti-influenza antibody titers were measured at baseline and one month post-vaccination.
Results
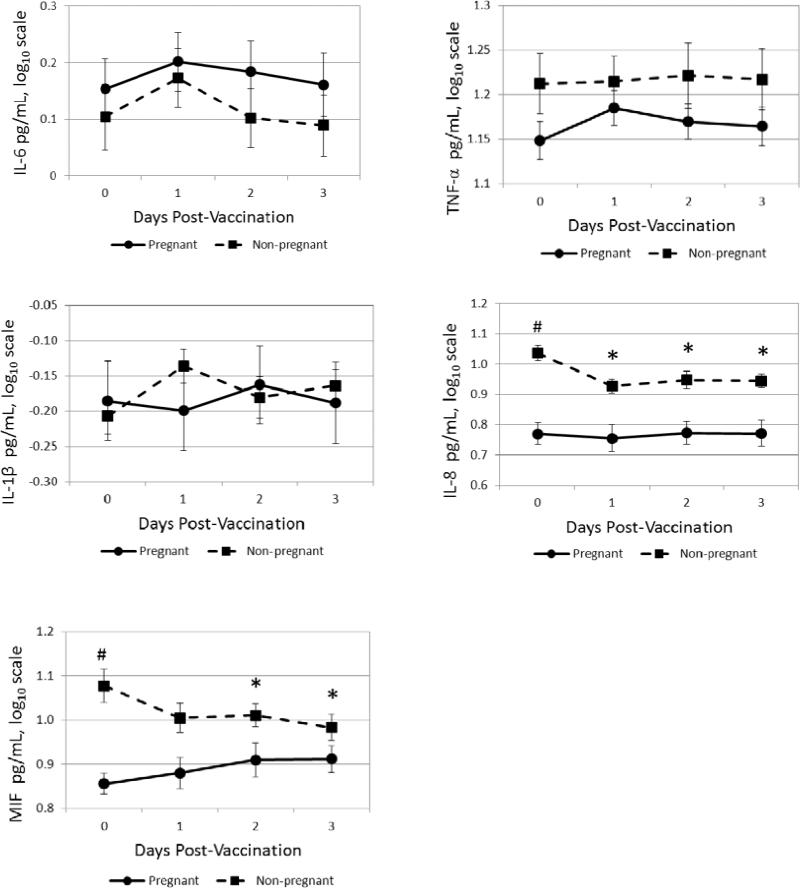
Overall, following vaccination, tumor necrosis factor (TNF)-α and interleukin(IL)-6 increased significantly, peaking at one day post-vaccination (ps<0.001). Pregnant versus non-pregnant women showed no differences in IL-6, TNF-α, or IL-1β responses. Pregnant women showed no change in IL-8 and increases in migration inhibitory factor (MIF), while non-pregnant showed decreases in both. Pregnancy did not significantly alter antibody responses.
Conclusions
Inflammatory responses to TIV are mild, transient, and generally similar in pregnant and non-pregnant women. Given the variability evidenced, vaccination may provide a useful model for studying individual differences in inflammatory response propensity.
Keywords: pregnancy, inflammatory response, vaccination, influenza, proinflammatory cytokines
1. Introduction
During pregnancy, substantial immune adaptation occurs. Pregnancy has been associated with decreased inflammatory responses and maintained/increased anti-inflammatory responses to immune challenges in women as well as in animal models.1-6 It has been postulated that this immune adaptation may prevent rejection of the fetus by the maternal immune system. Abnormalities in immune adaption to pregnancy may affect pregnancy outcomes such as risk for preeclampsia, poor fetal growth, and preterm birth.7-10
For clear ethical reasons, studies of the inflammatory response in humans during pregnancy have focused almost exclusively on in vitro stimulation models.4, 5 Because in vitro techniques involve isolation of specific cells, removal of cells from the complex in vivo environment, and exposure to higher levels of antigen than normally occurs in vivo, the clinical relevance of in vitro assessments is often unclear. By providing insight into immune function in the complex, multifaceted, naturally-occurring environment, in vivo models arguably provide data with clearer clinical relevance.
Influenza virus vaccination provides a unique opportunity to study the in vivo inflammatory response during pregnancy. Vaccination is considered safe and beneficial to pregnant women, who are at higher risk than the general population for complications, hospitalization, and death due to influenza.11-14 Routine influenza vaccination is recommended by the Centers for Disease Control (CDC) and American College of Obstetricians and Gynecologists (ACOG) for all healthy pregnant women in any trimester.15, 16
In addition to providing a model for studying propensity to inflammatory responding in general, data on inflammatory responses to TIV is of clinical value. In pregnancy, maternal exposure to influenza infection has been linked to increased risk of schizophrenia in offspring 17-19 and inflammatory responses to infection are implicated in this link. Because influenza vaccination induces an inflammatory response, potential effects on fetal development have been cited as a cause for possible concern. 20.
Previous studies have reported that influenza virus vaccine elicits a mild but statistically significant inflammatory response during pregnancy at one to two days post-vaccination.21 An important limitation of data to-date is the cross-sectional design and lack of a non-pregnant comparison group.
Given the limitations of available data, the aims of the current study were: 1) to comprehensively describe inflammatory responses to seasonal influenza vaccine among women during pregnancy with longitudinal measurement at baseline, one day, two days, and three days post-vaccination and 2) to compare inflammatory responses in women during pregnancy versus non-pregnancy. It was hypothesized that inflammatory responses in pregnant women would be mild and transient, with a peak at one to two days post-vaccination. It was also hypothesized that inflammatory responses would be attenuated in magnitude among pregnant women as compared to non-pregnant women. The current study also included assessment of antibody responses prior to and at one month post-vaccination, allowing for examination of equivalence in vaccine immunogenicity in pregnancy versus non-pregnancy.
2. Methods
2.1. Participants
This study included 28 pregnant women and 28 non-pregnant women who were assessed prior to and at one day, two days, three days, and approximately one month following seasonal trivalent influenza virus vaccination (TIV) during the 2011-2012 influenza season. Women were recruited primarily from staff and faculty at The Ohio State University Wexner Medical Center through newsletters and on-line advertisements.
Women were excluded from participation if they reported chronic health conditions with implications for immune or neuroendocrine function including HIV, lupus, arthritis, hypertension, asthma, and diabetes. Women were also excluded if they were taking medications which may alter immune or inflammatory parameters including daily antivirals (e.g., valacyclovir HCl) or statins. Pregnant women were excluded if they reported fetal anomaly or preeclampsia. All pregnant women were < 33 weeks gestation to allow for adequate time for the one month post-vaccination visit prior to delivery. Per phone call the day before the first study visit, women who reported an acute illness with cold or flu like symptoms within the past seven days were rescheduled. Women who were eligible and chose to participate completed a written informed consent. Participants received compensation for their participation. The study was approved by The OSU Biomedical Institutional Review Board.
2.2. Demographic and Psychosocial Measures
Demographic and descriptive information regarding height, current weight, pre-pregnancy weight, age, race, education level, marital status, and income was collected. The following health behaviors were assessed at the initial study visit: smoking, participation in regular physical activity (i.e., at least one hour per week of vigorous activity), and frequency of prenatal vitamin use (for pregnant women only).
2.3. Measurement of Serum Inflammatory Markers
Inflammatory markers were assessed at baseline, one day, two days, and three days post-vaccination. At each study visit, whole blood was collected into vacutainer tubes while subjects were in a seated position. On follow-up days, blood samples for the same woman were collected within a two hour window of collection of the baseline sample for that particular woman to ensure that sample timepoints were approximately 24 hours apart. Samples were immediately centrifuged, aliquoted, and placed in −80°C freezer storage until analysis. Serum levels of interleukin(IL)-6, tumor necrosis factor (TNF)-α, IL-8, and IL-1β were assayed in duplicate with ultra-sensitive multiplex kits from Meso Scale Discovery (MSD) and chemilluminescence methodology using the Immulite 1000 (Siemens Healthcare Diagnostics, Inc., 1717 Deerfield Rd., Deerfield, Il.). Serum levels of macrophage migration inhibitory factor (MIF) were assayed in duplicate using ultra-sensitive multiplex kits from R&D Systems (Minneapolis, MN) per kit instructions.
2.4. Measurement of Antibody Responses to Vaccination
Serum from baseline and one month post-vaccination was assayed using the hemagglutination inhibition (HAI) test. HAI antibody titers reported as <1:10 were valued at 1:5 for statistical purposes. Consistent with prior studies e.g., 22, seroconversion was defined as a pre-vaccination antibody titer ≤ 1:10 and a post-vaccination titer of ≥ 1:40, or among women with a pre-vaccination titer >1:10, a four-fold increase in the titer. Seroprotection was defined as an antibody titer ≥ 1:40.
2.5. Physical Measurements
For pregnant women, body mass index (BMI) was calculated (kg/m2) using height as measured by a nurse at the study visit and self-reported weight prior to pregnancy. For non-pregnant women, BMI was calculated based on current height and weight as measured by a nurse at the study visit.
2.6. Influenza Virus Vaccination
Each woman received Fluarix (GlaxoSmithKline) seasonal trivalent influenza virus vaccination. During the 2011-2012 influenza season, each 0.5mL dose contained 45 μg hemagglutinin (HA), with 15 μg HA of each of the following three virus strains: A/California/7/09 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010.
2.7. Statistical Analyses
First, pregnant and non-pregnant women were compared in terms of demographic and behavioral characteristics to assess the comparability of groups. T-tests and chi-square tests were used to evaluate group differences.
Next, inflammatory responses were analyzed using a separate linear mixed model for each outcome. Each model contained fixed effects for pregnancy status and time (baseline, 1, 2, or 3 days post-vaccination), and a time by pregnancy status interaction term. A random subject effect was included to account for correlation among measures from the same subject. Contrasts for change from baseline for each cytokine were tested at each post-vaccination time point for pregnant versus non-pregnant women by linear combinations of the mixed model parameter estimates. All analyses utilized log-transformed cytokine data to normalize the data distributions. For one subject, IL-6 and IL-1β measures were ≥ 3 SD above the mean and were considered outliers and excluded from analyses. Fifty-four women had complete data at all four study timepoints. Two subjects had one missing datapoint each, one at day 1 and one at day 3. These subjects remained in the analyses; the mixed models utilize the non-missing data to inform the missing data points during parameter estimation. Eighty-three of the IL-1β datapoints (38.6%) were below the detection limit of 0.6 pg/mL. The datapoints were set to one-half the lower detection limit, 0.3 pg/mL.
Finally, we examined antibody responses among pregnant versus non-pregnant women by chi-square analyses or Fisher's Exact Test when necessary, with the antibody response for seroconversion and seroprotection defined as described above.
3. Results
3.1. Demographic and Behavioral Characteristics
Demographic and behavioral characteristics of the study sample are presented in Table 1. In the sample overall, women were predominately White (75%). The average age was 29.1 (SD = 5.7) years. Pregnant women were predominately in the 2nd trimester (n = 16; 57%) at the time of vaccination [average weeks gestation = 28.4 (SD = 17.9)]. Twenty-seven women (48%) indicated that they had received the influenza virus vaccine in the previous year. Income was distributed similarly across ranges of less than $30,000 (36%), $30,000-$74,999 (30%), and $75,000 or more (34%). Women with bachelor's degrees or higher made up 50% of the sample. Thirty-one women (55%) were married, with another 21% unmarried but in a relationship.
Table 1.
Demographic Characteristics
| Pregnant (n=28) | Non-pregnant (n=28) | p-value | |
|---|---|---|---|
| Age, mean (SD) | 28.6 (5.5) | 29.6 (5.9) | 0.49 |
| Race | 0.53 | ||
| African-American | 8 (29%) | 6 (21%) | |
| White | 20 (71%) | 22 (79%) | |
| Ethnicity, Hispanic/Latino | 1 (4%) | 3 (11%) | 0.30 |
| Nulliparity | 11 (39.3%) | 11 (39.3%) | 0.96 |
| BMI, mean (SD) | 24.9 (5.2) | 24.1 (7.0) | 0.64 |
| Marital Status | 0.40 | ||
| Married | 18 (64%) | 13 (46%) | |
| Unmarried, but in a relationship | 5 (18%) | 7 (25%) | |
| Single | 5 (18%) | 8 (29%) | |
| Income | 0.95 | ||
| Less than $30,000 | 10 (36%) | 10 (36%) | |
| $30,000 - $74,999 | 8 (29%) | 9 (32%) | |
| $75,000 or more | 10 (36%) | 9 (32%) | |
| Education | 0.46 | ||
| High school or less | 8 (29%) | 7 (25%) | |
| Some college | 4 (14%) | 9 (32%) | |
| Bachelor's degree | 5 (18%) | 4 (14%) | |
| Some graduate school or higher | 11 (39%) | 8 (29%) | |
| Smoking | 0.15 | ||
| Current | 2 (7%) | 5 (18%) | |
| Past | 5 (19%) | 9 (32%) | |
| Never | 21 (75%) | 14 (50%) | |
| Vigorous activity, ≥ 1 hour per week | 11 (39%) | 18 (64%) | 0.06 |
| Hours of sleep night prior to vaccination, mean (SD) | 7.4 (1.7) | 6.8 (1.9) | 0.18 |
| Vaccinated Previous Year | 12 (43%) | 15 (54%) | 0.42 |
Pregnant and non-pregnant women did not differ significantly in age (t(54) = 0.70, p = 0.49), body mass index (t(54) = 0.47, p = 0.64), or race (X2(1) = 0.38, p = 0.54). In terms of prior births, 11/28 (39.3%) in each group (pregnant and non-pregnant) were nulliparous. Pregnant versus non-pregnant women did not differ significantly in rates of smoking (X2(2) = 3.83, p = 0.15), hours of sleep in the night prior to vaccination (t(54) = 1.37, p = 0.18), or rates of vaccination in the previous year (X2(1) = 0.64, p =0.42). Non-pregnant women reported marginally higher rates of vigorous activity (X2(1) = 3.50, p = 0.06), with 64% reporting one or more hour per week of vigorous activity compared to 39% of pregnant women.
3.2. Inflammatory Responses following Influenza Virus Vaccine
In terms of baseline serum inflammatory markers, pregnant and non-pregnant women did not differ in IL-6, TNF-α, or IL-1β. Baseline IL-8 and MIF were significantly higher in pregnant than non-pregnant women (ps < 0.001). Inflammatory responses to vaccination are presented in Figures 1-5. Mixed model analyses demonstrated that, in the sample overall, significant increases in IL-6 were seen at one and two days post-vaccination, with a peak at one day post-vaccination (day 1, p < 0.001; day 2, p = 0.01). Similarly, in the overall sample, an increase in TNF-α was observed at one day post-vaccination (p < 0.001). In contrast, there was a significant decrease in IL-8 at one day post-vaccination (p < 0.001). In the overall sample, there were no significant changes in either MIF or IL-1β in response to vaccination.
Fig 1-5.
Serum proinflammatory cytokine responses to trivalent influenza virus vaccine (TIV)
Pregnant and non-pregnant women did not differ significantly in IL-6, TNF- α, or IL-1β responses to vaccination. Pregnant women differed from non-pregnant in their IL-8 responses at each follow-up time point (ps < 0.005), with IL-8 decreasing from baseline in non-pregnant women and remaining unchanged in pregnant women. The MIF responses differed at two and three days post-vaccination (ps < 0.02), with responses decreasing from baseline in non-pregnant women and increasing in pregnant women.
3.3. Antibody Responses following Influenza Virus Vaccine
As described, antibody levels were measured by HAI at baseline and approximately one month post-vaccination. Data at this timepoint were missing for two women (one pregnant, one non-pregnant). The majority of women (51/54; 94%) completed this follow-up visit between 27-39 days post-vaccination, with the remaining 3 completing this visit between 43-51 days. Seroconversion and seroprotection, as defined above (Section 2.4.), were examined for each of the three strains included in the trivalent influenza virus vaccine for the 2011-2012 influenza season (A/California/7/09 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010).
Results showed that pregnant and non-pregnant women did not differ significantly in their antibody response to any strain of the vaccine (Table 2). Specifically, seroconversion was achieved against A/H1N1 among 70% of pregnant and 74% of non-pregnant (X2(1) = 0.09, p = 0.76), against A/H3N2 among 63% of pregnant and 59% of non-pregnant (X2(1) = 0.08, p = 0.78), and against influenza B among 63% of pregnant and 74% of non-pregnant (X2(1) = 0.77, p = 0.38). Seroprotection was achieved against A/H1N1 among 89% of pregnant and 85% of non-pregnant (X2(1) = 0.16, p = 0.69), against A/H3N2 among 81% of pregnant and 93% of non-pregnant (X2(1) = 1.48, p = 0.22), and against influenza B among 83% of pregnant and 100% of non-pregnant (Fisher's Exact Test, p = 0.49).
Table 2.
Antibody Responses to Vaccination among Pregnant and Non-pregnant Women
| Pregnant (n=27)* | Non-Pregnant (n=27)* | p-value (chi-square test) | |
|---|---|---|---|
| H1N1 Seroconversion | 19 (70%) | 20 (74%) | 0.76 |
| H1N1 Seroprotection | 24 (89%) | 23 (85%) | 0.69 |
| H3N2 Seroconversion | 17 (63%) | 16 (59%) | 0.78 |
| H3N2 Seroprotection | 22 (81%) | 25 (93%) | 0.22 |
| B Seroconversion | 17 (63%) | 20 (74%) | 0.38 |
| B Seroprotection | 25 (83%) | 27 (100%) | 0.15 |
One pregnant and one non-pregnant woman had missing data for the one-month follow-up, for a final sample of 54 for these analyses.
4. Discussion
In this sample of young, generally healthy women, mild and transient inflammatory responses to seasonal trivalent influenza virus vaccine (TIV) were observed in terms of IL-6 and TNF-α, with peak responses at one day post-vaccination. Inflammatory responses to vaccination were not significantly different among pregnant versus non-pregnant women for IL-6, TNF-a, or IL-1β. For IL-8, non-pregnant women showed decreases post-vaccination compared to baseline while pregnant women showed no change. For serum MIF, pregnant women showed increases post-vaccination while non-pregnant women exhibited decreases.
These data suggest that inflammatory responses to seasonal influenza virus vaccine are not strongly modified during pregnancy. These results contrast prior data showing that inflammatory responses to in vivo and in vitro immune challenges are attenuated during pregnancy in human and animal models.1-5 For example, in vitro cytokine production of whole blood exposed to lipopolysaccharide (LPS) was significantly lower among 18 women during their third trimester of pregnancy as compared to postpartum, with three-fold lower IL-12 production and 40% lower TNF-α production.4 Lack of such effect in the current study may be related to the mild response among women overall, resulting in a floor effect whereby attenuation was not observable.
In other populations, individual differences in inflammatory responses to vaccination have been used as an in vivo model to study propensity toward inflammatory responding to immune triggers in general. For example, in response to TIV, at 24 hours post-vaccination, men with carotid artery disease (CAD) showed an average of 227% increase in C-reactive protein (CRP) as compared to an increase of 40% among men without disease.23 Similarly, older adults reporting greater depressive symptoms showed elevations in IL-6 at 2 weeks after influenza vaccination while no IL-6 increase was seen at this timepoint among those reporting fewer depressive symptoms.24 Paralleling these findings, we have previously reported that pregnant women reporting greater depressive symptoms showed significantly greater inflammatory responses to TIV.25
As perinatal health conditions including gestational hypertension, preeclampsia, and preterm birth have an inflammatory component 8, 9, 26-30, a tendency toward exaggerated inflammatory responding to immune triggers may have unique implications in pregnancy. Despite mild changes observed in the group overall, meaningful variability in inflammatory responses was evidenced between individuals. Among pregnant women, for all five inflammatory markers, standard deviations of change scores from baseline to post-vaccination were substantially larger than the mean change score, indicating substantial variability. The coefficients of variation for change scores (100% × standard deviation / mean) ranged from 191% to 8760% (1.9-fold to 87.6-fold greater than the mean). The current sample size did not provide statistical power for examining differential inflammatory responding in relation to risk for adverse perinatal health outcomes. This should be a goal for future studies.
From a clinical standpoint, these results are consistent with prior data showing that inflammatory responses to TIV vaccination are mild and transient in pregnant women, supporting the safety of vaccination.21 In a study of experimental influenza virus infection in 19 healthy adults, 5-fold increases in IL-6 were evidenced with significant increases observable from two to four days after infection. In comparison, the average response among pregnant women in the current study across the five biomarkers measured was of 1.01 to 1.14-fold magnitude at peak response for the given biomarker. Moreover, elevations in IL-6 were observable at only one to two days post-vaccination and were thus of considerably shorter duration than observed in clinical infection.
Given the considerable variability in inflammatory responses to vaccination, it has been suggested that, despite mild responses on average, inflammatory responses may be considerably greater among some women, resulting in potential risk to the developing fetus.20 In this study, among pregnant women, those in the top quartile of responders showed peak increases relative to baseline of 1.3 to 3.1-fold for MIF; 1.3 to 1.9-fold for IL-6; 1.1 to 2.1-fold for IL-1β; 1.2 to 1.8-fold for TNF-α ; and 1.1 to 1.4-fold for IL-8. Thus, responses were relatively mild even among the greatest responders. However, these women were generally healthy; responses may differ in women with chronic health conditions.
These data support the notion that, despite eliciting an inflammatory response itself, vaccination should provide a protective function by reducing risk of influenza infection and related exposure to an inflammatory response of considerably greater magnitude. In support of the health benefits of vaccination, pregnant and non-pregnant women did not differ significantly in rates of achieving seroconversion or seroprotection against any of the three influenza virus strains. This is consistent with prior evidence that pregnant women vaccinated in any trimester of pregnancy show similar antibody responses to non-pregnant women.22, 31-34
In terms of generalizability, this study examined responses during a single influenza season. The composition of the trivalent influenza virus vaccine changes each season. Inflammatory responses may differ based on strains of virus or the novelty of a given strain. In addition, this sample was predominately White and insured. At 47%, rates of vaccination in the prior year were high as compared to the general population; among adults 18-49 years of age, 30.5% were vaccinated in during the 2010-2011 influenza season (the year preceding the current study) 35. However, our analyses showed no significant differences in inflammatory responses based on receipt of vaccination in the prior year. This high prior vaccination rate reflects the fact that many participants were faculty or staff at The Ohio State University Medical Center, among whom seasonal influenza virus vaccination is mandatory.
In this study, 57% of pregnant women were in the 2nd trimester. It would be informative to examine a cohort in which sufficient representation of demographically matched women in the first, second, and third trimester are assessed. However, this is challenging from a research design standpoint; for clear ethical reasons women cannot be randomized to receive vaccination in a specific trimester (i.e., delay vaccination). Women may be vaccinated in later gestation due to the timing of their pregnancy relative to the influenza season. However, later vaccination may also be related to lack of early prenatal care which, in turn, covaries with poor health behaviors, lack of access to healthcare, and demographic characteristics. In this study, the majority [19/28 (68%)] had private health insurance, suggesting ready availability to early prenatal care. If future studies examine differences in immune responses to vaccination by trimester, behavioral/demographic characteristics should be carefully considered, particularly in cohorts with greater socioeconomic diversity.
It would be of value to compare inflammatory responses to flu vaccine as an in vivo challenge to an in vitro inflammatory challenge. As described, the majority of studies in humans to-date rely on the latter. It would be informative, from a research standpoint, to understand the extent to which in vitro immune challenges correspond to vaccination as an in vivo immune challenge, thus providing support for the clinical relevance of in vitro challenge studies.
In sum, this study provides novel data on the inflammatory response in the days following influenza virus vaccine in women during pregnancy versus non-pregnancy. These data indicate that, among generally healthy women during pregnancy or non-pregnancy, the inflammatory response to TIV is mild and transient. This response was generally similar among pregnant and non-pregnant women. These data lend support for the clinical safety of vaccination during pregnancy. Given the range in magnitude of response evidenced, vaccination may provide a useful model for studying individual differences in inflammatory response propensity, a factor which may have implications for maternal health and pregnancy outcomes.
Acknowledgments
We appreciate the contributions of Clinical Research Assistants Kelly Marceau and Rebecca Long to data collection and Research Associate Hui Xu for conducting the cytokine assays. We would like to thank our study participants and the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
Role of the Funding Sources
This study was supported by NICHD (HD061644, LMC and HD067670, LMC) and in part by the NIAID contract HHSN266200700005C and the American Lebanese Syrian Associated Charities (ALSAC) to SSC. The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources, Grant UL1RR025755 and is now at the National Center for Advancing Translational Sciences, Grant 8UL1TR000090-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2004;90:95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Valles A, Poole S, Mistry Y, Williams S, Luheshi GN. Attenuated fever in rats during late pregnancy is linked to suppressed interieukin-6 production after localized inflammation with turpentine. Journal of Physiology-London. 2007;583:391–403. doi: 10.1113/jphysiol.2007.132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashdown H, Poole S, Boksa P, Luheshi GN. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2007;292:R1667–R1674. doi: 10.1152/ajpregu.00274.2006. [DOI] [PubMed] [Google Scholar]
- 4.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, Chrousos GP. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. J Clin Endocrinol Metab. 2001;86:4933–4938. doi: 10.1210/jcem.86.10.7905. [DOI] [PubMed] [Google Scholar]
- 5.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MMK, Hassan NA, Bandar A: Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol. 1999;42:273–281. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109:121–127. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- 8.Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonat M. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 10.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 11.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D: Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44–52. doi: 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 12.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201:547–552. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ. Pregnancy PHNI: Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. Jama-J Am Med Assoc. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D: Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004;21:333–339. doi: 10.1055/s-2004-831888. [DOI] [PubMed] [Google Scholar]
- 15.The American College of Obstetricians and Gynecologists: Committe Opinion Number 468: Influenza Vaccination During Pregnancy. Obstet Gynecol. 2010;116:1006–1007. doi: 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- 16.Advisory Committee on Immunization Practices (ACIP) Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. Morbidity and Mortality Weekly Report (MMWR. 2012:61. [PubMed] [Google Scholar]
- 17.Mednick SA, Machon RA, Huttunen MO, Bonett D: Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 18.Takei N, Mortensen PB, Klaening U, Murray RM, Sham PC, O'Callaghan E, Munk-Jorgensen P. Relationship between in utero exposure to influenza epidemics and risk of schizophrenia in Denmark. Biol Psychiatry. 1996;40:817–824. doi: 10.1016/0006-3223(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 19.O'Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337:1248–1250. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 20.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian LM, Iams JD, Porter K, Glaser R: Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine. 2011;29:8982–8987. doi: 10.1016/j.vaccine.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, Breiman RF, Zaman K: Influenza Immunization in Pregnancy - Antibody Responses in Mothers and Infants. New Engl J Med. 2010;362:1644–1646. doi: 10.1056/NEJMc0912599. [DOI] [PubMed] [Google Scholar]
- 23.Carty CL, Heagerty P, Nakayama K, McClung EC, Lewis J, Lum D, Boespflug E, McCloud-Gehring C, Soleimani BR, Ranchalis J, Bacus TJ, Furlong CE, Jarvik GP. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Atertio Thromb Vasc Biol. 2006;26:2738–2744. doi: 10.1161/01.ATV.0000248534.30057.b5. [DOI] [PubMed] [Google Scholar]
- 24.Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 25.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R: Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behav, Immun. 2010;24:49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286:R989–R990. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 27.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 28.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 29.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;43:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 30.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D'Anna K, Argys L, Ross RG, Brandt C, Cole S: The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behavior and Immunity. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis. 1993;168:647–656. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 32.Murray DL, Imagawa DT, Okada DM, St Geme JW., Jr. Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol. 1979;10:184–187. doi: 10.1128/jcm.10.2.184-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumaya CV, Gibbs RS. Immunization of Pregnant-Women with Influenza-a-New-Jersey-76 Virus-Vaccine - Reactogenicity and Immunogenicity in Mother and Infant. Journal of Infectious Diseases. 1979;140:141–146. doi: 10.1093/infdis/140.2.141. [DOI] [PubMed] [Google Scholar]
- 34.Jackson LA, Patel SM, Swamy GK, Frey SE, Creech CB, Munoz FM, Artal R, Keitel WA, Noah DL, Petrie CR, Wolff M, Edwards KM. Immunogenicity of an Inactivated Monovalent 2009 H1N1 Influenza Vaccine in Pregnant Women. J Infect Dis. 2011;204:854–863. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention NCfIaRDN Flu Vaccination Coverage, United States, 2011-12 Influenza Season In. 2012 [Google Scholar]