Abstract
Background
Ambient air pollution has been implicated in the development of hypertensive disorders of pregnancy (HDP). However, evidence of the association between air pollution and HDP is still limited, and the effects of gaseous air pollutants on HDP and their time windows of exposure have not been well studied.
Methods
We used the Florida birth registry data to investigate the associations between air pollutants (NO2, SO2, PM2.5, O3 and CO) and the risks of HDP in 22 041 pregnant women in Jacksonville, Florida, USA from 2004 to 2005. Further, we examined whether air pollution exposure during different time windows defined by trimesters and the entire pregnancy had different effects on HDP.
Results
The single-pollutant logistic regression model showed that exposure to four pollutants during the full pregnancy period was significantly associated with prevalence of HDP after adjusting for covariates: NO2 (OR=1.21, 95% CI 1.09 to 1.35), PM2.5 (OR=1.24, 95% CI 1.08 to 1.43), SO2 (OR=1.13, 95% CI 1.01 to 1.25) and CO (OR=1.12, 95% CI 1.03 to 1.22) per IQR increase. Similar effects were observed when first trimester exposure to NO2, SO2 and CO, and second trimester exposures to PM2.5 were examined. Consistent results were confirmed in multiple-pollutant models.
Conclusions
This study suggests that exposure to high levels of air pollution during early pregnancy and the full gestational period was associated with increased prevalence of HDP in Florida, USA.
INTRODUCTION
Women during pregnancy are susceptible to hypertensive disorders of pregnancy (HDP) including gestational hypertension, pre-eclampsia and eclampsia because changes during pregnancy lead to higher stress on the cardiovascular system.1 HDP complicates up to 10% of all pregnancies, and can have adverse health effects on the mother and the child.2 In mothers, HDP is associated with maternal pitting oedema, endothelial abnormalities, liver and renal dysfunction and increased risk of cardiovascular disease, stroke and type II diabetes later in life.2–5 With its progression to pre-eclampsia or even eclampsia, gestational hypertension dramatically increases the risks of maternal and perinatal death.6 In infants, maternal HDP is associated with small for gestational age, preterm delivery, low birthweight and increased risk of hospitalisation for a wide range of neonatal diseases.7,8 Pre-eclampsia is the underlying cause for about 25% of all medically indicated preterm deliveries in the USA, and it is also the most frequent primary reason for preterm birth without labour, which accounts for 30–35% of total preterm deliveries.9,10
Despite serious consequences, the biological mechanisms underlying HDP remain unclear. Environmental exposures such as ambient air pollution during pregnancy may play a role in the development of HDP. The association between air pollution and increased risk of hypertension in the general population has been reported by many studies.11–15 It is plausible that exposure to air pollution during pregnancy may increase the risk of HDP. However, evidence of the association between air pollution and HDP is still limited.16–19 Moreover, the effect of the time window of exposure to air pollution during pregnancy on HDP has not been well studied. In this study, we used the Florida birth registry data to investigate the association between criteria air pollutants (NO2, SO2, PM2.5, O3 and CO) and the risk of HDP in 22 041 pregnant women who delivered during 2004–2005 in Jacksonville, Florida, USA. Furthermore, we examined whether air pollution exposure during different time windows defined by trimesters and the entire pregnancy had different effects on HDP.
MATERIAL AND METHODS
Birth data
Birth record data were obtained from the Bureau of Vital Statistics & Office of Health Statistics and Assessment, Florida Department of Health. The data included all registered live births occurring in Jacksonville, Florida, USA between 1 January 2004 and 31 December 2005 (n=24 483). Six hundred and ninety-seven participants were excluded because of missing geographical information, or because of living too far away from monitors (see section Air pollution exposure assessment for detailed information). We further excluded infants with congenital abnormalities (n=159), as well as infants with a birthweight <400 g (n=39) or a gestational age <24 or >42 weeks (n=74). Women who had plural deliveries (n=798) or previous preterm births (n=305) were also excluded from the study because it is known that multifetal pregnancies and women with previous preterm births have higher rates of adverse pregnancy outcomes, and gestational hypertension associated with such outcomes may have a different underlying physiology.20,21 Additionally, women who reported having chronic hypertension (n=370) were removed from the study. Under these restrictions, a total of 22 041 births were available for analysis.
The study was conducted following a human participant’s research protocol approved by the Institutional Review Board at the University of Florida and Florida Department of Health.
Outcome ascertainment
Women identified from birth records with gestational hypertension, pre-eclampsia and/or eclampsia during their pregnancy were considered as cases with HDP.
Air pollution exposure assessment
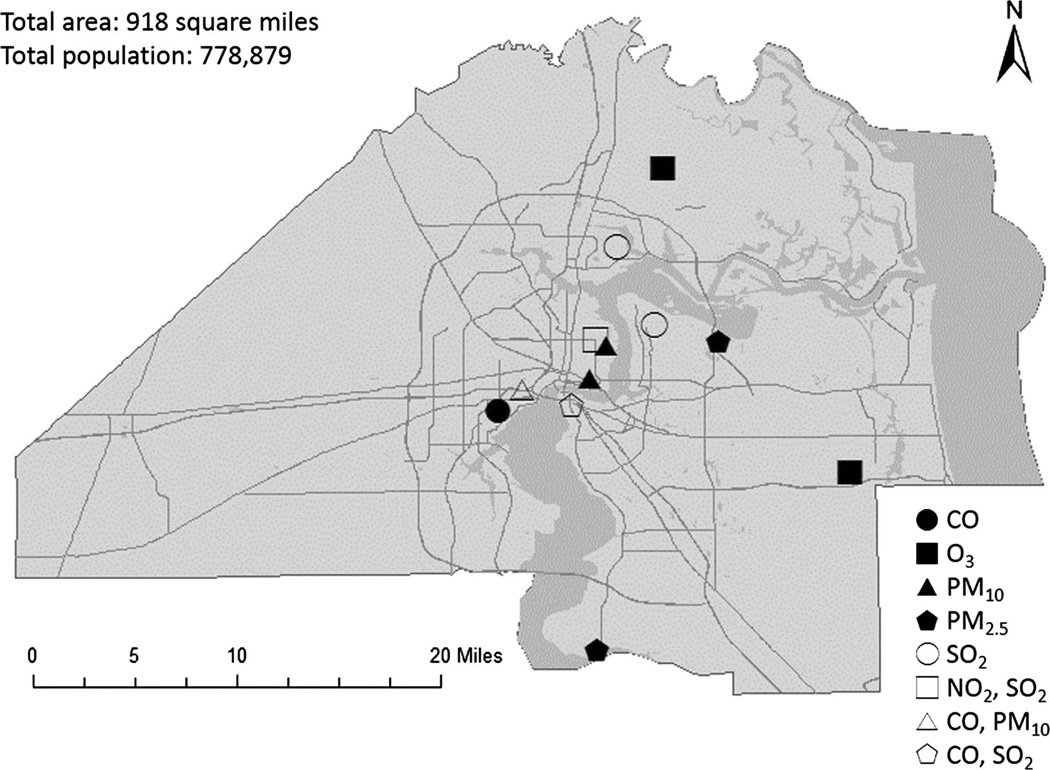
Daily measurements of NO2, SO2, PM2.5, PM10, O3 and CO were obtained from the US Environmental Protection Agency’s (EPA’s) Air Quality System (AQS) for 2003–2005 in Jacksonville, Florida, USA. During the study period, there were one active NO2 monitor, four active SO2 monitors, two active PM2.5 monitors, three active PM10 monitors, two active O3 monitors and three active CO monitors. Since PM10 was observed for only 180–182 days over the 3-year period (please refer to table 1), we did not include PM10 in this study.
Table 1.
Concentrations of air pollutants and observational days by monitor from 2003 to 2005 in Jacksonville, Florida, USA
| Monitor ID | Observational days | Daily mean | The 5th centile | The 25th centile | Median | The 75th centile | The 95th centile |
|---|---|---|---|---|---|---|---|
| NO2 (ppb) | |||||||
| 12-031-0032 | 937 | 27.95 | 12.00 | 20.00 | 26.00 | 34.00 | 48.00 |
| PM10 (µg/m3) | |||||||
| 12-031-0053 | 180 | 21.98 | 11.50 | 16.00 | 21.00 | 25.50 | 36.00 |
| 12-031-0084 | 182 | 26.17 | 13.00 | 20.00 | 25.00 | 31.00 | 41.00 |
| 12-031-0089 | 182 | 22.29 | 11.00 | 16.00 | 22.00 | 26.00 | 38.00 |
| PM2.5 (µg/m3) | |||||||
| 12-031-0098 | 1005 | 10.01 | 3.70 | 6.50 | 9.00 | 12.30 | 19.40 |
| 12-031-0099 | 1095 | 10.44 | 4.00 | 6.80 | 9.50 | 12.60 | 20.40 |
| SO2 (ppb) | |||||||
| 12-031-0032 | 1034 | 6.74 | 0.00 | 2.00 | 4.00 | 8.00 | 23.00 |
| 12-031-0080 | 986 | 4.69 | 0.00 | 1.00 | 2.00 | 6.00 | 18.00 |
| 12-031-0081 | 1002 | 8.72 | 0.00 | 1.00 | 3.00 | 8.00 | 41.00 |
| 12-031-0097 | 976 | 7.12 | 0.00 | 2.00 | 4.00 | 8.00 | 28.00 |
| CO (ppm) | |||||||
| 12-031-0080 | 990 | 0.50 | 0.50 | 0.30 | 0.40 | 0.60 | 1.10 |
| 12-031-0083 | 917 | 0.60 | 0.30 | 0.40 | 0.50 | 0.70 | 1.20 |
| 12-031-0084 | 1012 | 0.85 | 0.30 | 0.50 | 0.80 | 1.10 | 1.70 |
| O3 (ppm) | |||||||
| 12-031-0077 | 982 | 0.038 | 0.019 | 0.029 | 0.037 | 0.047 | 0.062 |
| 12-031-0100 | 1017 | 0.042 | 0.021 | 0.032 | 0.040 | 0.051 | 0.067 |
The mother’s residential address at birth was geocoded at the street level using the ArcGIS V.10.1 software (ESRI, Redlands, California, USA) with a geocoding success rate of 98%. Women whose addresses could not be geocoded were excluded from further analysis (n=524). The residential addresses were then linked to the closest active monitor for each pollutant. Birth date and gestational age were used to determine the start and end dates of the trimesters and full gestational period for each infant, allowing exposures to air pollution to be calculated for each period specific to each pregnant woman. Exposures were calculated as 24 h values averaged over the corresponding periods. We used two trimester windows of exposure: 1–13 weeks and 14–26 weeks. Similar definitions have been applied elsewhere.22,23 Women were excluded from the study if they lived more than 20 km from all AQS monitors in the study area (n=173, please refer to online supplemental material 1).
Covariates
Census data
We extracted census-tract level median household income from the 2000 Census to control for population characteristics that may confound the effect of exposure to air pollution on HDP.24 The income variable was categorised into three levels based on tertiles across all tracts in Jacksonville, Florida, USA. Tracts in the lowest tertile of socioeconomic status had a median household income under US$35 265 and tracts in the highest tertile of socioeconomic status had a median household income over US$45 087.
Maternal characteristics
Information on marital status, maternal age, race, education, smoking during pregnancy, season of birth and prenatal care was also obtained from birth records. Marital status was treated as a dichotomous variable (yes/no). Maternal age at delivery was categorised in 5-year increments for women between 20 and 39 years, with two additional categories for <20 and ≥40 years. Maternal race was categorised as non-Hispanic White, non-Hispanic Black and others. In addition, maternal education was categorised into three groups: <high school, high school or equivalent and >high school. Smoking during pregnancy was categorised into three groups based on the number of cigarettes smoked per day: non-smokers, smokers with <10 cigarettes smoked per day and smokers with ≥10 cigarettes smoked per day. Season of conception was categorised as two categories: warm (June–November) and cool (November–May). Year of conception was treated as a categorical variable (2003, 2004 or 2005). The trimester in which prenatal care began (first, second, third trimester or no care) was also obtained.
Statistical analysis
χ2 Tests and t tests were applied to compare the distributions of categorical and continuous independent variables between women with HDP and those without HDP. Logistic regression models were used to evaluate the association between exposure to different air pollutants and gestational hypertension during the three trimesters and the full gestational period. Air pollutants were analysed as continuous variables in separate models with the same covariates (maternal characteristics described above). ORs and 95% CI per IQR increase in specific pollutants during a specific pregnancy period were obtained. Two-pollutant logistic models were implemented for gestational exposures to explore potential confounding by copollutants. Furthermore, multipollutant scores were calculated for each participant during different gestational periods. Participants’ exposure to different air pollutants were categorised into tertiles. The multipollutant score was developed to study the combined effects of multiple air pollutants. It is equal to the sum of air pollutants that the individuals were exposed to. Given the limited sample size of participants with a score of 5, we combined those with a score of 4 or above together. The score was then treated as a nominal categorical variable in logistic models adjusted for the same covariates as described above. All statistical analyses were conducted using SAS V.9.3 (Cary, North Carolina, USA).
RESULTS
Air pollution exposure was estimated for 22 041 women. Of the women in the study population, 4.7% had HDP (n=1037). Table 1 shows the concentrations of air pollutants and observational days by different monitors. Figure 1 shows the locations of monitors in Jacksonville, Florida, USA during the study period. Table 2 shows the distribution of exposures to each pollutant for each trimester and the entire pregnancy period.
Figure 1.
Locations of air pollution monitors during the study period in Jacksonville, Florida, USA.
Table 2.
Exposure information concerning NO2, PM2.5, SO2, CO and O3 by gestational hypertension (GH) status
| Trimester 1 | Trimester 2 | Full gestational period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH (n=1037) | No GH (n=21 004) | Total (n=22 041) | p Value | GH (n=1037) | No GH (n=21 004) | Total (n=22 041) | p Value | GH (n=1037) | No GH (n=21 004) | Total (n=22 041) | p Value | |
| NO2 (ppb) | ||||||||||||
| Mean (SD) | 28.54 (3.68) | 27.95 (3.73) | 27.98 (3.73) | <0.01 | 27.97 (3.76) | 27.89 (3.77) | 27.90 (3.77) | 0.55 | 28.12 (1.70) | 27.83 (1.52) | 27.84 (1.53) | <0.01 |
| Median | 28.74 | 28.27 | 28.28 | 28.44 | 28.23 | 28.24 | 28.08 | 27.76 | 27.77 | |||
| IQR | 4.71 | 5.34 | 5.39 | 6.19 | 6.44 | 6.43 | 1.79 | 1.64 | 1.65 | |||
| PM2.5 (µg/m3) | ||||||||||||
| Mean (SD) | 10.18 (0.85) | 10.11 (0.92) | 10.12 (0.91) | 0.02 | 10.39 (0.75) | 10.16 (0.85) | 10.17 (0.85) | <0.01 | 10.38 (0.38) | 10.28 (0.44) | 10.29 (0.44) | <0.01 |
| Median | 10.22 | 10.21 | 10.21 | 10.39 | 10.12 | 10.13 | 10.40 | 10.33 | 10.33 | |||
| IQR | 1.20 | 1.18 | 1.18 | 1.15 | 1.26 | 1.25 | 0.54 | 0.67 | 0.67 | |||
| SO2 (ppb) | ||||||||||||
| Mean (SD) | 6.33 (2.67) | 5.96 (2.79) | 5.98 (2.79) | <0.01 | 5.99 (2.73) | 6.08 (2.72) | 6.07 (2.72) | 0.29 | 6.15 (1.78) | 6.08 (1.72) | 6.08 (1.72) | 0.17 |
| Median | 6.31 | 5.84 | 5.85 | 5.86 | 5.89 | 5.89 | 5.91 | 5.74 | 5.75 | |||
| IQR | 3.69 | 3.72 | 3.73 | 3.77 | 3.70 | 3.70 | 2.70 | 2.54 | 2.55 | |||
| CO (ppm) | ||||||||||||
| Mean (SD) | 0.65 (0.23) | 0.63 (0.21) | 0.63 (0.21) | <0.01 | 0.60 (0.22) | 0.61 (0.22) | 0.61 (0.22) | 0.13 | 0.60 (0.17) | 0.60 (0.17) | 0.60 (0.17) | 0.68 |
| Median | 0.59 | 0.56 | 0.57 | 0.52 | 0.55 | 0.55 | 0.55 | 0.57 | 0.57 | |||
| IQR | 0.29 | 0.24 | 0.24 | 0.25 | 0.25 | 0.25 | 0.16 | 0.15 | 0.15 | |||
| O3 (ppm) | ||||||||||||
| Mean (SD) | 0.041 (0.006) | 0.040 (0.006) | 0.040 (0.006) | 0.40 | 0.041 (0.006) | 0.041 (0.005) | 0.041 (0.005) | 0.13 | 0.041 (0.003) | 0.040 (0.003) | 0.040 (0.003) | <0.01 |
| Median | 0.041 | 0.040 | 0.040 | 0.042 | 0.041 | 0.041 | 0.041 | 0.041 | 0.041 | |||
| IQR | 0.011 | 0.009 | 0.010 | 0.008 | 0.009 | 0.009 | 0.003 | 0.003 | 0.003 | |||
Table 3 presents the correlation between criteria air pollutants in different gestational periods. Only a moderate negative correlation between CO and O3 during all the gestational periods examined was observed in this study (Pearson’s correlation coefficients range from −0.58 to −0.62). Weak correlations were observed among other criteria air pollutants during different gestational periods.
Table 3.
Correlation between criteria air pollutants in different gestational periods
| NO2 | PM2.5 | SO2 | CO | O3 | |
|---|---|---|---|---|---|
| Trimester 1 | |||||
| NO2 | 1.00 | 0.02* | 0.15* | 0.01 | 0.35* |
| PM2.5 | – | 1.00 | 0.20* | 0.03* | 0.02* |
| SO2 | – | – | 1.00 | 0.29* | −0.36* |
| CO | – | – | – | 1.00 | −0.61* |
| Trimester 2 | |||||
| NO2 | 1.00 | −0.18* | 0.08* | 0.09* | 0.29* |
| PM2.5 | – | 1.00 | 0.13* | 0.04* | −0.14* |
| SO2 | – | – | 1.00 | 0.25* | −0.39* |
| CO | – | – | – | 1.00 | −0.62* |
| Full gestational period | |||||
| NO2 | 1.00 | −0.04* | 0.06* | 0.08* | 0.22* |
| PM2.5 | – | 1.00 | 0.10* | −0.15* | 0.36* |
| SO2 | – | – | 1.00 | 0.18* | −0.28* |
| CO | – | – | – | 1.00 | −0.62* |
p<0.05.
Table 4 shows the maternal characteristics of women by HDP status. A higher proportion of normotensive women were of a race/ethnicity other than non-Hispanic White or non-Hispanic Black (14.2% vs 9.4%), had conceived in 2003 (36.2% vs 29.5%), had begun prenatal care later and had a high socioeconomic status (33.9% vs 30.9%).
Table 4.
Maternal characteristics of women by hypertensive disorders of pregnancy (HDP) status among women who gave birth from 2004 to 2005 in Jacksonville, Florida, USA
| HDP (n=1037) |
No HDP (n=21 004) |
||
|---|---|---|---|
| n (%) | n (%) | p Value | |
| Maternal age (years) | |||
| <20 | 117 (11.3) | 2549 (12.1) | 0.50 |
| 20–24 | 309 (29.8) | 6322 (30.1) | |
| 25–29 | 266 (25.7) | 5588 (26.6) | |
| 30–34 | 212 (20.4) | 4257 (20.3) | |
| 35–39 | 108 (10.4) | 1852 (8.8) | |
| ≥40 | 25 (2.4) | 435 (2.1) | |
| Race | |||
| Non-Hispanic White | 536 (51.7) | 10 787 (51.4) | <0.01 |
| Non-Hispanic Black | 404 (39.0) | 7233 (34.4) | |
| Others | 97 (9.4) | 2984 (14.2) | |
| Maternal education | |||
| <High school | 155 (15.2) | 3676 (17.7) | 0.06 |
| High school or equivalent | 341 (33.4) | 7085 (34.1) | |
| >High school | 526 (51.5) | 10 043 (48.3) | |
| Marital status | |||
| Married | 611 (59.0) | 11 986 (57.1) | 0.24 |
| Not married | 426 (41.1) | 9017 (42.9) | |
| Smoked during pregnancy | |||
| No | 954 (93.4) | 18 919 (91.6) | 0.11 |
| Yes, and <10 cigarettes/day | 28 (2.7) | 781 (3.8) | |
| Yes, and ≥10 cigarettes/day | 39 (3.8) | 947 (4.6) | |
| Season of conception | |||
| Warm | 509 (49.1) | 10 032 (47.8) | 0.41 |
| Cool | 528 (50.9) | 10 972 (52.2) | |
| Year of conception | |||
| 2003 | 306 (29.5) | 7593 (36.2) | <0.01 |
| 2004 | 563 (54.3) | 10 499 (50.0) | |
| 2005 | 168 (16.2) | 2912 (13.9) | |
| Prenatal care began | |||
| No care | 8 (1.0) | 230 (1.6) | 0.04 |
| First trimester | 602 (77.2) | 10 792 (73.8) | |
| Second trimester | 149 (19.1) | 2963 (20.3) | |
| Third trimester | 21 (2.7) | 641 (4.4) | |
| Tract median household income | |||
| Low | 340 (32.8) | 6899 (32.9) | 0.07 |
| Moderate | 377 (36.4) | 6996 (33.3) | |
| High | 320 (30.9) | 7109 (33.9) |
Table 5 provides the crude and adjusted ORs of the single-pollutant logistic regression between HDP and gestational exposure to air pollutants during different time windows in the full gestational period. The increase in risk of HDP per IQR increase in pollutant during the full pregnancy was 21% for NO2, 24% for PM2.5, 13% for SO2 and 12% for CO after adjusting for covariates. In addition, women with higher (per IQR increase) exposure to NO2, SO2 and CO during the first trimester were 14%, 14% and 19% more likely to be associated with HDP, respectively. Furthermore, women with higher exposure to PM2.5 during the second trimester were also associated with higher prevalence of HDP (OR=1.28, per IQR increase). All p values were <0.05.
Table 5.
ORs for risk of hypertensive disorders of pregnancy for an IQR increase in air pollution (NO2, PM10, PM2.5, SO2, CO and O3) exposure by air pollutants and pregnancy period of exposure among women who gave birth from 2004 to 2005 in Jacksonville, Florida, USA
| Unadjusted OR (95% CI) | Adjusted OR† (95% CI) | |
|---|---|---|
| NO2 | ||
| Trimester 1 | 1.25 (1.15 to 1.37)* | 1.14 (1.01 to 1.29)* |
| Trimester 2 | 1.03 (0.93 to 1.15) | 0.98 (0.83 to 1.15) |
| Pregnancy | 1.21 (1.14 to 1.29)* | 1.21 (1.09 to 1.35)* |
| PM2.5 | ||
| Trimester 1 | 1.09 (1.01 to 1.19)* | 1.10 (0.99 to 1.23) |
| Trimester 2 | 1.51 (1.38 to 1.67)* | 1.28 (1.13 to 1.46)* |
| Pregnancy | 1.40 (1.27 to 1.55)* | 1.24 (1.08 to 1.43)* |
| SO2 | ||
| Trimester 1 | 1.19 (1.10 to 1.29)* | 1.14 (1.03 to 1.26)* |
| Trimester 2 | 0.96 (0.88 to 1.04) | 1.00 (0.89 to 1.11) |
| Pregnancy | 1.07 (0.97 to 1.17) | 1.13 (1.01 to 1.25)* |
| CO | ||
| Trimester 1 | 1.12 (1.04 to 1.19)* | 1.19 (1.07 to 1.31)* |
| Trimester 2 | 0.95 (0.88 to 1.02) | 1.06 (0.95 to 1.18) |
| Pregnancy | 0.99 (0.93 to 1.05) | 1.12 (1.03 to 1.22)* |
| O3 | ||
| Trimester 1 | 1.05 (0.95 to 1.16) | 1.00 (0.84 to 1.19) |
| Trimester 2 | 1.08 (0.98 to 1.19) | 0.94 (0.82 to 1.07) |
| Pregnancy | 1.21 (1.12 to 1.31)* | 0.98 (0.87 to 1.11) |
p<0.05.
Adjusted for maternal age, race, education, marital status, smoking during pregnancy, season of conception, year of conception, prenatal care began and tract median household income.
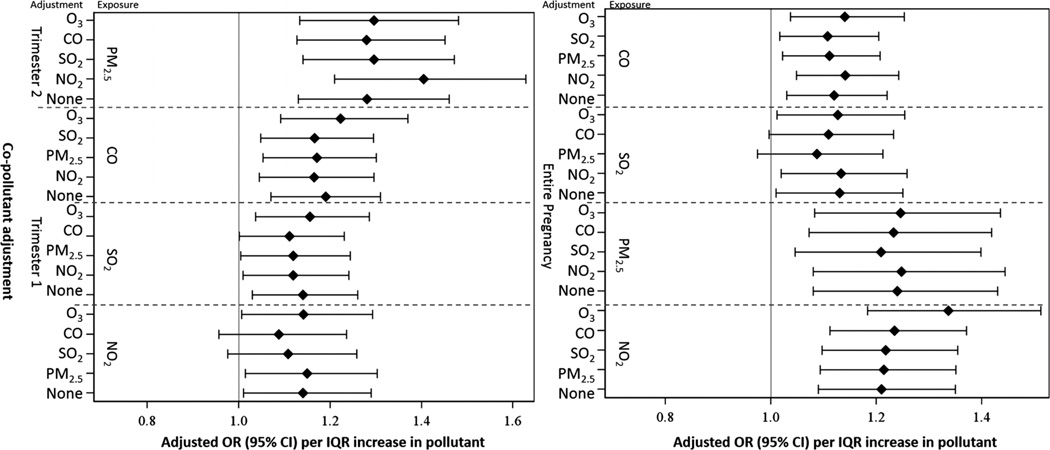
Two-pollutant logistic models were applied for gestational exposure to pollutants exhibiting significant associations in table 5. Since no pairs of pollutants were highly correlated (|r|>0.7) in this study, we analysed all pairs of pollutants (please refer to online supplemental material 2 for pollutants exhibiting insignificant associations in table 5). Figure 2 compares results with and without copollutant adjustment. Except for SO2 during the full pregnancy and NO2 during the first trimester, results for all other pollutants considered in the single-pollutant regression analysis are robust to copollutant adjustment and remain statistically significant. On the other hand, after adjusting for CO/SO2, the higher level of NO2 during the first trimester is not significantly, but still marginally, associated with HDP. Similar results were observed for SO2 during the full gestational period after adjusting for CO and NO2.
Figure 2.
Adjusted OR for risk of hypertensive disorders of pregnancy per IQR increase in gestational exposure to pollutant, for single and two-pollutant models among women who gave birth from 2004 to 2005 in Jacksonville, Florida, USA. The point reflects the central estimate; the horizontal line represents the 95% CI.
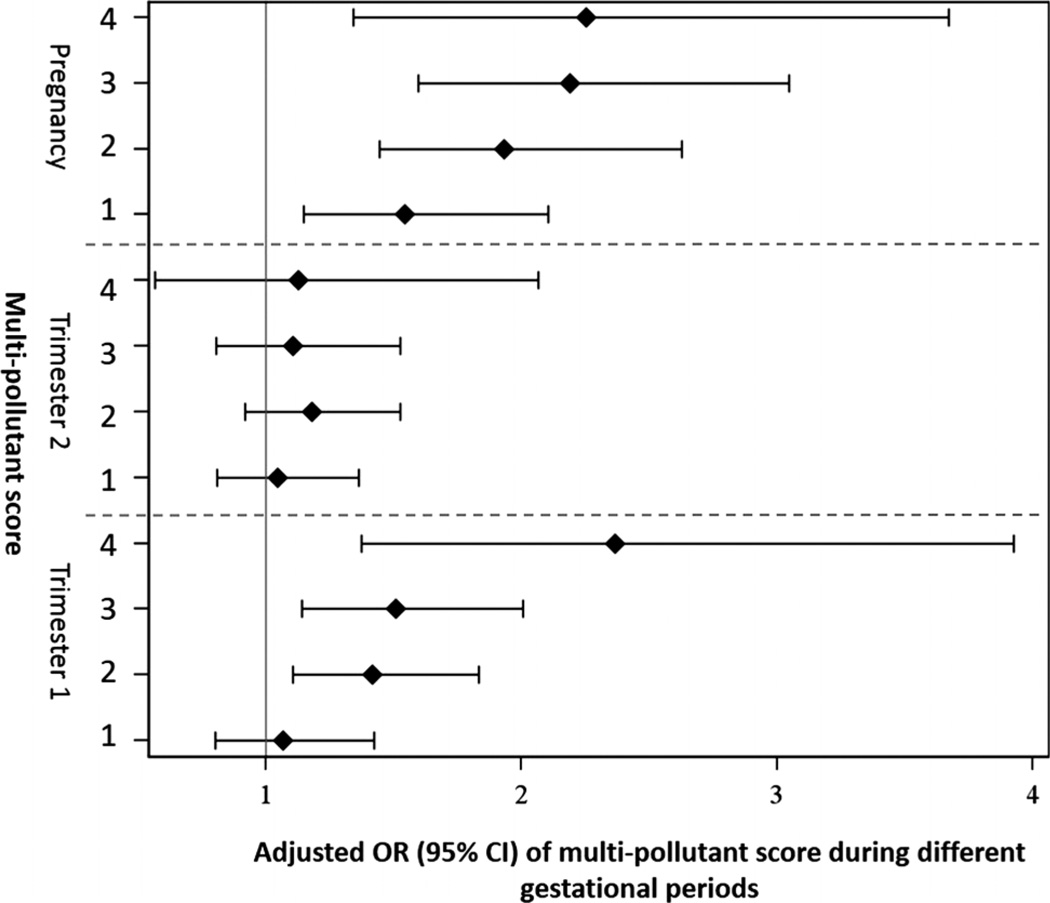
Figure 3 shows the adjusted ORs for risk of HDP for multi-pollutant score for participants during different gestational periods. The risks of HDP increase with the increase in the number of highly exposed pollutants for women during the first trimester or the entire pregnancy period. Such a trend was not observed during the second trimester.
Figure 3.
Adjusted ORs for risk of hypertensive disorders of pregnancy for multipollutant score during different gestational periods among women who gave birth from 2004 to 2005 in Jacksonville, Florida, USA. The point reflects the central estimate; the horizontal line represents the 95% CI.
DISCUSSION
This study examined the association of HDP with NO2, PM2.5, PM10, SO2, CO and O3 exposure during both the full gestational period and different trimesters during pregnancy. When elevated air pollution exposures were assessed across the entire period of pregnancy, the increase in HDP was statistically significant for each IQR increase in NO2, PM2.5, SO2 and CO. Similar results were observed when the first trimester exposures to NO2, SO2 and CO, and the second trimester exposures to PM2.5 were examined. The observed associations were independent of the confounding effects of maternal age, race/ethnicity, education, marital status, smoking during pregnancy, season of birth, time prenatal care began, socioeconomic status and copollutants. These results suggest that an elevated risk of HDP was associated with increased exposure earlier in pregnancy. In addition, the risk associated with increased air pollution exposure throughout the full gestational period may indicate a dose–response relationship, or only reflect the early pregnancy effects of air pollution. The results of our study add to the emerging evidence pointing to the deleterious effects of air pollution on pregnant women.
We observed that air pollution exposure at an early stage of pregnancy is associated with elevated odds of HDP. This finding may be explained by the fact that air pollution exposure at the early stage of pregnancy may change the normal pattern of blood pressure during pregnancy. In normal pregnancy, blood pressure starts to fall during the first trimester, reaching the lowest point in midpregnancy, and then gradually returns to the prepregnancy level.25,26 However, women who develop HDP have a different pattern. Their blood pressure is stable during the first half of pregnancy and then continuously increases until delivery.26 Therefore, it is plausible that exposure to air pollution may contribute to the different patterns of blood pressure change in the early stage of pregnancy between women with and without HDP. The associations we observed between air pollution exposure during the entire pregnancy period and HDP are consistent with previous studies.16–19 In addition, the observed association of SO2/CO and HDP may be due to different causes. It is possible that the adverse health effects of SO2 and CO may induce HDP; many studies have suggested that these pollutants may induce hypertension in different populations.27,28 On the other hand, it is also possible that SO2 and CO served as an indirect indicator of other factors, given the relatively low concentration of SO2 and CO in this study.
Although the causal mechanisms between exposure to air pollution and HDP remain unclear, it is known that air pollution can speed the development and progression of atherosclerosis, which may potentially contribute to hypertension.29–31 HDP and vascular atherosclerosis may share common pathways in relation to air pollutants.32,33 In addition, air pollution has been linked with endothelial dysfunction, which is a precursor associated with HDP.20,34,35 The abnormal placentation and failed vascular remodelling due to endothelial dysfunction may trigger pre-eclampsia.36
There are several strengths to this study. First, we used different models to examine the association between air pollution and HDP among pregnant women. The results from different models such as single pollutant models and multiple-pollutant models were consistent, which suggests that gaseous pollutants such as SO2 and CO may have effects independent of particulate air pollutants on HDP. In addition, we further developed the multipollutant score to investigate the combined effects of exposure to multiple air pollutants and our findings indicate that women simultaneously exposed to high levels of several air pollutants had a greater risk of developing HDP than those exposed to one high level of air pollutant or none. These findings reinforce the fact that air pollution consists of a mixture of pollutants and that the health effects of air pollution need to consider the combined effects of all measured pollutants together. However, this approach has some limitations. It did not consider between-pollutant correlations and that different pollutants may have different effects on the outcome since we used the same weight for each air pollutant. Thus, a potential bias may exist when evaluating the combined effects of different air pollutants.
This study had several limitations. First, information on HDP was obtained from vital statistics records. It is possible that HDP may be underdiagnosed on the birth certificate since it could be asymptomatic, especially among women who do not have access to prenatal care. Second, the date of HDP diagnosis is not available in this study. However, since HDP is defined as the development of new arterial hypertension in a pregnant woman after 20 weeks’ gestation, the date of an HDP diagnosis should be later than 20 weeks after conception. As we observed elevated odds of HDP associated with air pollution early in pregnancy, these results may not be biased by the unknown diagnostic date of HDP. Third, stillbirth, birth defects and preterm birth might lie on the causal pathway. Although we excluded participants with stillbirth and birth defects from this analysis, some preterm births between 24 and 37 weeks were included in this analysis and may influence our estimates. But, given that the number of preterm births was relatively small, their inclusion would not have a significant effect on our estimates. Fourth, in this study, individual air pollution exposure was estimated using the US EPA monitored data nearest to their location of residence. No direct individual measurement was performed to measure the actual exposure of pregnant women. This method of exposure assessment may suffer from misclassification because the difference between the monitored data near their address and individual air pollution exposure may be due to the fact that the individual daily mobility and behaviour patterns could substantially influence exposure to air pollution over time and in space. Moreover, information on residential mobility during pregnancy was not available. It is possible that some women in the study lived elsewhere and were exposed to different air pollution levels during a part of their pregnancy. However, the biases are likely to be non-differential, which would make the estimation more conservative and trending towards null. Furthermore, the number of air monitors available in the study area is very small. It further limits the ability of capturing spatial heterogeneity in exposure, which is especially important for assessing exposure to some air pollutants such as nitrogen dioxide. Thus, compared with other studies with substantial spatial heterogeneity in exposure,16,19 the small number of air monitors available in the study area is a limitation. Fifth, the results obtained may be influenced by the fixed cohort bias, which occurs in retrospective cohorts which include all births occurring within a fixed start and end date.37 This selection bias is more likely to happen when shorter pregnancies are missed at the start of the study, and longer pregnancies are missed at the end. However, since this study had the day and month of the start date just before the day and month of the end date, the potential to the bias was reduced. Finally, although we were able to control for some important covariates, other factors such as environmental tobacco smoke and individual-level socioeconomic status are unavailable on the birth certificate. Thus, we were unable to control for the confounding of these factors.
CONCLUSION
We observed a positive association between exposure to NO2, PM2.5, SO2 and CO during the entire pregnancy period, exposure to NO2, SO2 and CO during the first trimester of pregnancy, and exposure to PM2.5 during the second trimester of pregnancy and increased prevalence of HDP among women giving birth in Jacksonville, Florida, USA between 2004 and 2005. This finding indicates that there is a need for better air pollution control in order to reduce the disease burden of HDP among pregnant women.
Supplementary Material
What is already known on this subject.
-
►
The association between air pollution and increased risk of hypertension in the general population has been reported by many studies.
-
►
However, evidence of the association between air pollution and hypertensive disorders of pregnancy (HDP) is still limited. Moreover, the effect of the time window of exposure to air pollution during pregnancy on HDP has not been well studied.
What this study adds.
-
►
Our findings suggest that exposure to NO2, PM2.5, SO2 and CO during the first trimester of pregnancy is positively associated with an increased risk of hypertensive disorders of pregnancy (HDP) among women giving birth in Jacksonville, Florida, USA between 2004 and 2005. Similar findings were also observed during the entire pregnancy period.
-
►
Our findings also suggest potentially combined effects of different air pollutants on HDP.
-
►
These findings indicate that there is a need for a better air pollution control in order to reduce the disease burden of HDP among pregnant women.
Acknowledgments
Funding The project described was supported by Grant Number K01ES019177 from the National Institute of Environmental Health Sciences.
Footnotes
Contributors XX conceived the study, participated in its design and coordination and helped to draft the manuscript. HH participated in the design of the study, performed the statistical analysis and drafted the manuscript. SH was involved in the design of the study, interpretation of results and drafting of the manuscript. JR helped interpret the results and draft the manuscript. All authors read and approved the final version of the manuscript.
Competing interests None.
Patient consent Obtained.
Ethics approval Institutional Review Board at the University of Florida and Florida Department of Health.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Yoder SR, Thornburg LL, Bisognano JD. Hypertension in pregnancy and women of childbearing age. Am J Med. 2009;122:890–895. doi: 10.1016/j.amjmed.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Semin Perinatol. 2009;33:158–165. doi: 10.1053/j.semperi.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang IK, Tsai IJ, Chen PC, et al. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: a retrospective cohort study. Am J Med. 2012;125:251–257. doi: 10.1016/j.amjmed.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Wallis AB, Saftlas AF, Hsia J, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 7.Allen VM, Joseph K, Murphy KE, et al. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CS, Nohr EA, Bech BH, et al. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201:e1–e10. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 9.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen M, Hoffmann B, Hvidberg M, et al. Long-term exposure to traffic-related air pollution associated with blood pressure and self-reported hypertension in a Danish cohort. Environ Health Perspect. 2012;120:418–424. doi: 10.1289/ehp.1103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basile JN, Bloch MJ. Exposure to air pollution increases the incidence of hypertension and diabetes in black women living in Los Angeles. J Clin Hypertens. 2012;14:819–820. doi: 10.1111/jch.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Tong S, Zhang Y, et al. The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China. Sci Total Environ. 2010;408:4446–4450. doi: 10.1016/j.scitotenv.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Tong S, Li S, et al. Gaseous air pollution and emergency hospital visits for hypertension in Beijing, China: a time-stratified case-crossover study. Environ Health. 2010;9:57. doi: 10.1186/1476-069X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira G, Haggar F, Shand AW, et al. Association between pre-eclampsia and locally derived traffic-related air pollution: a retrospective cohort study. J Epidemiol Community Health. 2013;67:147–152. doi: 10.1136/jech-2011-200805. [DOI] [PubMed] [Google Scholar]
- 17.Vinikoor-Imler LC, Gray SC, Edwards SE, et al. The effects of exposure to particulate matter and neighbourhood deprivation on gestational hypertension. Paediatr Perinat Epidemiol. 2012;26:91–100. doi: 10.1111/j.1365-3016.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 18.van den Hooven EH, de Kluizenaar Y, Pierik FH, et al. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: the generation R study. Hypertension. 2011;57:406–412. doi: 10.1161/HYPERTENSIONAHA.110.164087. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Ren C, Delfino RJ, et al. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 21.Xiong X, Fraser WD, Demianczuk NN. History of abortion, preterm and term birth, and risk of gestational hypertension: a population-based study. J Reprod Med. 2004;49:899–907. [PubMed] [Google Scholar]
- 22.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker JD, Woodruff TJ, Basu R, et al. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- 24.Census 2000 Summary File 3—FL. Washington, DC: US Census Bureau; 2002. [Google Scholar]
- 25.Ayala DE, Hermida RC, Mojon A, et al. Blood pressure variability during gestation in healthy and complicated pregnancies. Hypertension. 1997;30:611–618. doi: 10.1161/01.hyp.30.3.611. [DOI] [PubMed] [Google Scholar]
- 26.Hermida RC, Ayala DE, Iglesias M. Predictable blood pressure variability in healthy and complicated pregnancies. Hypertension. 2001;38:736–741. doi: 10.1161/01.hyp.38.3.736. [DOI] [PubMed] [Google Scholar]
- 27.Dong GH, Qian ZM, Xaverius PK, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61:578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 28.Vigeh M, Yunesian M, Shariat M, et al. Environmental carbon monoxide related to pregnancy hypertension. Women Health. 2011;51:724–738. doi: 10.1080/03630242.2011.633599. [DOI] [PubMed] [Google Scholar]
- 29.Allen RW, Adar SD, Avol E, et al. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120:824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Curr Opin Pulm Med. 2012;18:155–160. doi: 10.1097/MCP.0b013e32834f210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill EA, Curl CL, Adar SD, et al. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Prog Cardiovasc Dis. 2011;53:353–360. doi: 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 34.Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook RD, Rajagopalan S. Chronic air pollution exposure and endothelial dysfunction: what you can’t see—can harm you. J Am Coll Cardiol. 2012;60:2167–2169. doi: 10.1016/j.jacc.2012.08.974. [DOI] [PubMed] [Google Scholar]
- 36.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. doi: 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.