Abstract
Breast cancers are not responsive to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI), although 30% of breast cancers overexpress EGFR. The mechanism of intrinsic resistance to EGFR TKIs in breast cancer is the focus of current studies. Here, we observed that EGFR remains tyrosine phosphorylated in breast cancer cells that proliferate in the presence of EGFR TKIs. In one such cell line, SUM229, inhibiting c-Src kinase activity with either a dominant-negative c-Src or a c-Src TKI decreased EGFR phosphorylation on Tyr845, Tyr992, and Tyr1086 in the presence of EGFR TKIs. Conversely, overexpressing wild-type (wt) c-Src in the EGFR TKI–sensitive breast cancer cell line SUM149 increased EGFR kinase–independent EGFR tyrosine phosphorylation. In addition, in the presence of EGFR TKIs, inhibiting c-Src kinase activity decreased cell growth in SUM229 cells, and over-expressing wt-c-Src increased cell growth in SUM149 cells. We identified the receptor tyrosine kinase Met to be responsible for activating c-Src in SUM229 cells. Inhibiting Met kinase activity with a small molecule inhibitor decreased c-Src phosphorylation and kinase activation. In addition, inhibiting Met kinase activity in SUM229 cells decreased EGFR tyrosine phosphorylation and growth in the presence of EGFR TKIs. Stimulating Met kinase activity in SUM149 cells with hepatocyte growth factor increased EGFR tyrosine phosphorylation and cell growth in the presence of EGFR TKIs. These data suggest a Met/c-Src–mediated signaling pathway as a mediator of EGFR tyrosine phosphorylation and cell growth in the presence of EGFR TKIs.
Introduction
The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor important to normal and pathogenic cellular processes. Classic EGFR activation involves an EGFR ligand that binds the extracellular domain of the receptor and induces dimerization between two EGFR family molecules (reviewed in ref. 1). Dimerization leads to a conformational change in the intracellular domains of EGFR and allows for activation of the kinase and autophosphorylation of the COOH-terminal tyrosines (reviewed in ref. 1). Autophosphorylation permits the docking of signaling proteins, which stimulate cellular processes such as cell growth and survival. Breast cancer is one pathologic process where EGFR has been found to be overexpressed. Specifically, >60 studies have been performed using various methods of determining EGFR expression with the range of overexpression from 20% to 70% in breast cancer tissue compared with normal epithelial tissue (2–5). The majority of these studies conclude that EGFR overexpression correlates with poor prognosis in breast cancer (3–5). However, the use of small molecule inhibitors and inhibitory antibodies has not improved survival rates or decreased disease burden in breast cancer patients (6, 7).
As with other targeted therapies, determining biomarkers for sensitivity or resistance to EGFR tyrosine kinase inhibitors (TKI) is an important area of research. Mutations within the kinase domain of EGFR in a subset of lung cancers have been shown to sensitize tumors to EGFR TKIs (8, 9). In addition, a polymorphic variation in intron 1 of EGFR is predictive for response to EGFR TKI treatment in a study of pediatric solid tumors (10). Although relative protein levels do not seem to be predictors of EGFR TKI response, several studies suggest gene copy number as a predictor of response to EGFR TKIs (11–14). Although amplification and mutation of EGFR frequently occur in cancers of other origins, breast cancers have a <10% rate of amplification and mutation of EGFR (15, 16).
Similar to EGFR, c-Src overexpression occurs in breast cancers (17, 18). However, c-Src overexpression does not correlate with poor prognosis (17, 18). An analysis of human breast tumors found that 20% of breast cancers overexpress both EGFR and c-Src, suggesting a cooperativity between the kinase activities of EGFR and c-Src in breast cancer (17). In support of the ability of EGFR and c-Src to functionally interact, studies overexpressing both EGFR and c-Src in cell culture models showed synergistic increases in DNA synthesis, transformation, and tumor growth in nude mice (19, 20). The mechanism for this synergy is currently unknown. However, when EGFR and c-Src are co-overexpressed, two tyrosines in the intracellular domain of EGFR are phosphorylated: Tyr845 and Y1101 (21). Mutating Tyr845 on the EGFR to an unphosphorylatable phenylalanine (Y845F), and subsequent expression in fibroblasts and human breast cancer cells, leads to abrogation of EGF-induced DNA synthesis (21, 22). Tyr845 on the EGFR is a conserved tyrosine found within the kinase domains in the majority of tyrosine kinases; however, unlike other tyrosine kinases, Tyr845 is not required for kinase activity of EGFR (21). These data suggest phosphorylation of Tyr845 may be important to EGFR/c-Src biological synergy.
Here, we describe a pathway independent of EGFR kinase activity where EGFR is tyrosine phosphorylated and growth is maintained. We previously identified seven EGFR-expressing breast cancer cell lines that grow in the presence of EGFR TKIs. Of these seven, five cell lines maintained EGFR tyrosine phosphorylation in the presence of gefitinib. In this report, we describe how maintenance of EGFR tyrosine phosphorylation in one such cell line, SUM229, is mediated by the kinase activity of c-Src. The kinase activity of c-Src also contributed to DNA synthesis and proliferation in the presence of EGFR TKI. In addition, we identified Met as a highly phosphorylated receptor tyrosine kinase regulating c-Src activity in the presence of gefitinib. Interestingly, treatment with a Met TKI in the presence of gefitinib also decreased EGFR EGFR tyrosine phosphorylation and growth. Taken together, these data suggest proliferation of EGFR-expressing breast cancer cells in the presence of gefitinib was mediated, at least in part, by a Met/c-Src signaling pathway via phosphorylation of EGFR.
Materials and Methods
Reagents
Gefitinib was provided by AstraZeneca and PP2 and SU11274 were purchased from EMD Biosciences. Hepatocyte growth factor (HGF) was purchased from Sigma. All other reagents were purchased from Sigma or VWR unless indicated.
Cell lines and growth conditions
SUM149 and SUM229 cell lines were a gift from Dr. Stephen Ethier (Wayne State University, Detroit, MI). The cells were cultured in 5% IH medium (Ham’s F-12 medium supplemented with 5% fetal bovine serum, 1 µg/mL hydrocortisone, 5 µg/mL insulin, 2.5 µg/mL amphotericin B, and 25 µg/mL gentamicin) in 10% CO2.
Transfections
SUM 149 and SUM 229 cells were transfected using a 3:1 ratio of FuGENE6 (Roche) to DNA (pcDNA3-wt-c-Src or pcDNA3-K-c-Src; gift from S. Parsons, University of Virginia, Charlottesville, VA). For growth assays, 6 µL FuGENE6 and 2 µg DNA were added to the cells, and for immunoblotting, 18 µL Fugene6 and 6 µg DNA were added. The transfection mixture was added to cells growing in normal growth medium. The cells were then incubated with the mixture for 48 h.
Immunoblotting
Cells were plated at one million cells per 100-mm dish and grown for 48 h. Cells were then lysed in CHAPs lysis buffer [10 mmol/L CHAPs, 50 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, and 2 mmol/L EDTA with 10 µmol/L sodium orthovanadate and 1× protease inhibitor cocktail (EMD Biosciences)]. For immunoblotting, 10 to 50 µg of protein lysate were separated by SDS-PAGE and transferred to Immobilon P (Millipore). Membranes were blocked in either 5% nonfat dry milk or 5% bovine serum albumin for 1 h at 25°C. The following primary antibodies were used in the experiments: anti-EGFR, anti–pY845-EGFR, anti–pY992-EGFR, anti–pY1045-EGFR, anti–pY1068-EGFR, anti–pY1148-EGFR, anti–pY1173-EGFR, anti-Met, and anti–pY1234/1235-Met (Cell Signaling), anti-phosphotyrosine, anti–pY1086-EGFR, and anti–pY416-Src [Py-Plus-horseradish peroxidase (HRP); Invitrogen], and anti-Src (2–17; gift from S. Parsons). All antibodies were incubated overnight at 4°C, except for the HRP-linked phosphotyrosine antibody (1 h at 25°C). Membranes were washed with TBS + 0.1% Tween 20 thrice for 10 min, followed by incubation with corresponding secondary antibody and another series of three washes. Incubation with enhanced chemiluminescence (GE Healthcare) was followed by exposure to film. Experiments were repeated at least thrice and quantitated using densitometry (AlphaEaseFC; AlphaInnotech).
Immunoprecipitations
Cells were lysed as described above, and 500 µg of cell lysate was incubated with 5 µL of EGFR antibody (mab108; gift from M. Weber, University of Virginia, Charlottesville, VA) and rocked for 1 h at 4°C. Forty microliters of protein A beads (Millipore) were added for 30 min at 4°C rocking. The beads were washed thrice with CHAPs lysis buffer, resuspended in 2× sample buffer, boiled, and loaded onto a 7.5% SDS-PAGE. Proteins were transferred to Immobolin-P and immunoblotted using the indicated antibodies described above.
In vitro kinase assays
For EGFR kinase assays, cells were washed in PBS and lysed in solubilization buffer [50 mmol/L HEPES (pH 7.5), 10% glycerol, 0.5% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L phenylmethylsulfonyl fluoride, 50 µg/mL aprotinin, and 400 nmol/L sodium orthovanadate]. Lysates were cleared by centrifugation, quantitated, and 0.5 mg of protein was immunoprecipitated using EGFR antibodies (mab108; gift from M. Weber). Antibody-bound proteins were collected using protein A beads (Millipore) and washed thrice in HTG buffer [20 mmol/L HEPES (pH 7.5), 0.1% Triton X-100, and 10% glycerol]. For the kinase reaction, 40 µL HTG buffer, 4 µL MnCl2 (of 100-mmol/L stock), and 10 µCi 32P-γATP (GE Healthcare) were incubated for 10 min at 30°C. The beads were pelleted and the supernatant removed and discarded. Sample buffer was added to the pellets; the samples were boiled, and proteins were separated using 7.5% SDS-PAGE. The gels were dried and exposed to film. Experiments were repeated at least thrice with densitometry used for quantitation.
Cell growth assays
The indicated breast cancer cells were plated in triplicate in 6-well plates at 35,000 cells per well (day 0). The next day, the cells were treated with indicated inhibitors every day for 7 d at the indicated dosage. The number of cells was determined using a Beckman Coulter Counter on days 1, 4, and 8. Experiments were repeated at least twice, and the average and SE was graphed using a log10 scale for cell numbers.
BrdUrd incorporation assays
After transfection with the c-Src constructs for 24 h, 0.5 µmol/L gefitinib was added for 20 h. One hundred micromoles per liter of BrdUrd was added for 4 h before fixation of the cells with 4% paraformaldehyde for 20 min at 25°C. Cells were permeabilized using 0.01% Triton X-100 for 3 min at 4°C and washed with PBS thrice. DNA was then exposed by incubation of the cells with 2 mol/L HCl for 1 h at 37°C and neutralized with two borate buffer washes. Cells were then blocked in 20% goat serum for 1 h at 25°C and incubated with alexa-fluor 594 anti-BrdUrd–conjugated antibody (Invitrogen; 1:50) for 1 h at 37°C. Excess antibody was washed away, coverslips were mounted, and BrdUrd-incorporated nuclei were counted as a ratio of the total number of cells. One hundred cells per coverslip were counted with experiments performed in duplicate.
Phospho-proteomic array
The Human Phospho-RTK Array kit was purchased from R&D Systems. The membranes contain spotted antibodies corresponding to 42 distinct receptor tyrosine kinases with nine species and antibody controls. SUM149 and SUM229 cells were seeded at one million cells on 100-mm plates and allowed to proliferate for 48 h. Cells were then treated with 0.5 µmol/L gefitinib for 30 min and lysed in the recommended NP40 lysis buffer [1% NP40, 20 mmol/L Tris-HCl (pH 8.0), 137 mmol/L NaCl, 10% glycerol, 2 mmol/L EDTA, 1 mmol/L sodium orthovanadate, and 1× protease inhibitor cocktail]. The arrays were blocked in the provided blocking buffer and incubated with 500 µg of lysate overnight at 4°C. The arrays were washed and incubated with a phosphotyrosine detection antibody, treated with enhanced chemiluminescence (Amersham), and exposed to film.
Statistical analysis
To perform the statistical analyses in this report, the Student’s t test was used using the statistical software in GraphPad Prism. P values of <0.05 were considered statistically significant.
Results
Growth and EGFR tyrosine phosphorylation in the presence of EGFR TKIs
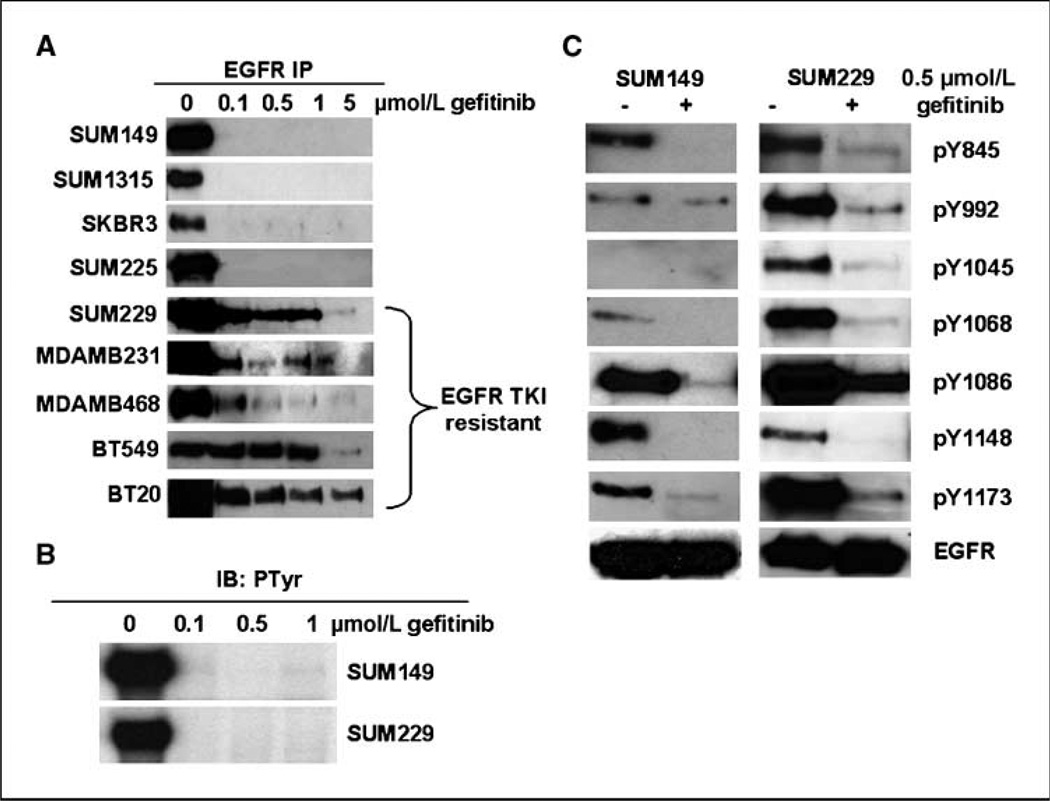
Clinical studies have not shown an improvement in survival of breast cancers treated with the EGFR TKI, gefitinib. To determine a mechanism for EGFR TKI intrinsic resistance, we identified seven breast cancer cell lines to be intrinsically resistant to EGFR TKIs. In analyzing the responsiveness of the seven cell lines to EGFR TKIs, we measured phosphorylation of EGFR as a surrogate marker for EGFR kinase activity. Interestingly, five of the seven EGFR TKI–resistant cell lines showed EGFR tyrosine phosphorylation in the presence of gefitinib (Fig. 1A). In addition, four EGFR TKI–sensitive cell lines showed complete inhibition of EGFR tyrosine phosphorylation in the presence of gefitinib (Fig. 1A). To ensure gefitinib was inhibiting EGFR kinase activity, SUM149 and SUM229 cells were treated with increasing concentrations of gefitinib for 30 min, and in vitro kinase activity of the EGFR was measured (Fig. 1B). We found that 0.1 µmol/L gefitinib for 30 min was sufficient to inhibit the EGFR kinase activity in both SUM149 and SUM229 cells (Fig. 1B). These results show a lack of correlation between EGFR tyrosine phosphorylation and the kinase activity of EGFR in breast cancer cells.
Figure 1.
EGFR tyrosine phosphorylation in the presence of EGFR TKIs. A, a panel of breast cancer cell lines was plated at 1 million cells per 100-mm plate for 48 h. Increasing doses of gefitinib were added for 30 min before lysing of the cells. Lysates were immunoprecipitated (IP) with EGFR antibodies and immunoblotted (IB) with phosphotyrosine antibodies. B, SUM149 and SUM229 cells were cultured and treated as in A; however, the immunoprecipitates were subjected to an in vitro kinase activity assay. EGFR autophosphorylation was measured by autoradiography after SDS-PAGE separation. C, EGFR immunoprecipitates from SUM149 and SUM229 cells were immunoblotted with antibodies to the specific tyrosine phosphorylation sites on EGFR (Y845, Y992, Y1045, Y1068, Y1086, Y1148, and Y1173) and EGFR itself.
To determine the specific sites of phosphorylation maintained in the presence of gefitinib, SUM149 and SUM229 cells were treated with 0.5 µmol/L gefitinib for 30 min. Lysates were immunoprecipitated with EGFR antibodies and immunoblotted using the indicated phospho-specific antibodies for tyrosines on EGFR. Except for Tyr1148, all sites of tyrosine phosphorylation were still detectable after gefitinib treatment in SUM229 cells, whereas only Tyr992, Tyr1086, and Tyr1173 were detectable in SUM149 cells (Fig. 1C). These data show that in SUM229 cells, multiple sites of EGFR tyrosine phosphorylation are maintained in the presence of gefitinib.
C-Src kinase activity mediates EGFR tyrosine phosphorylation in the presence of EGFR TKIs
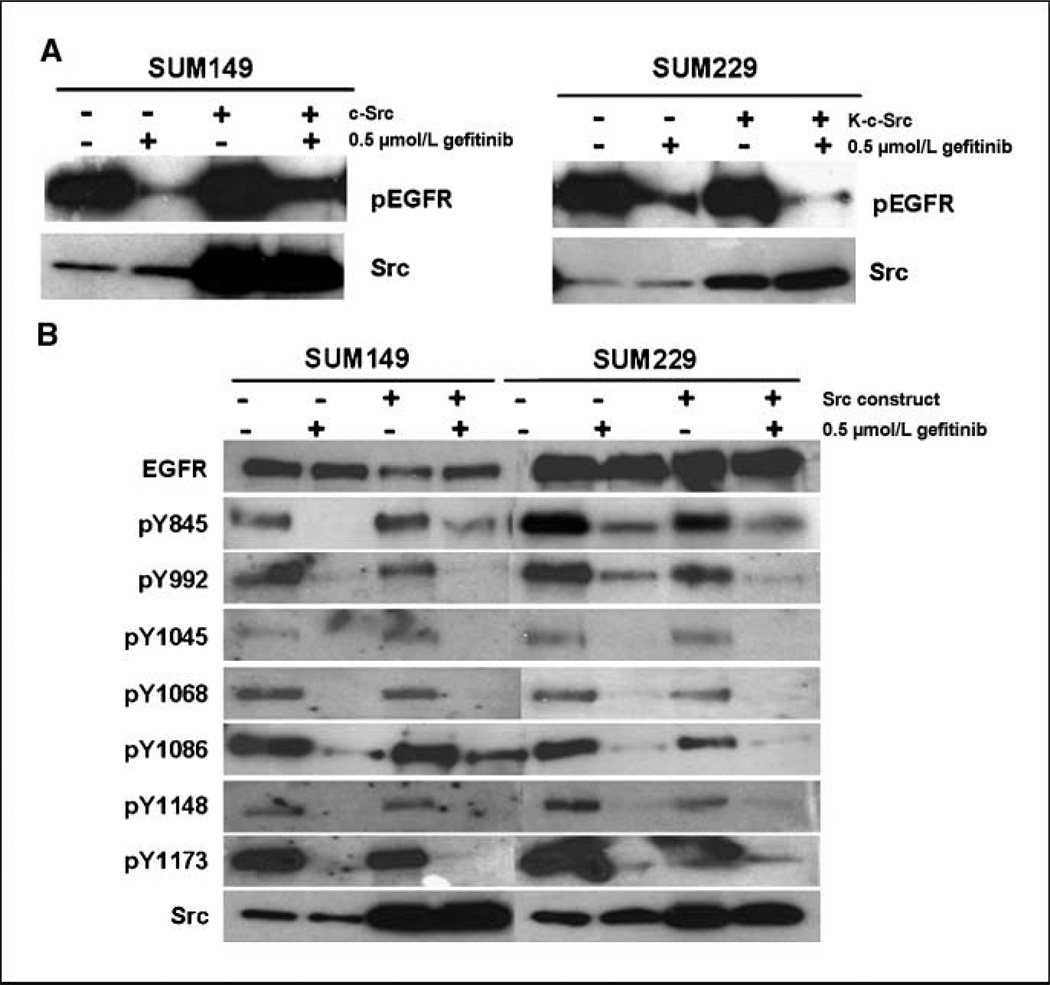
One site of phosphorylation maintained in SUM229 cells, but not in SUM149 cells treated with gefitinib, was the c-Src phosphorylation site, Tyr845. To determine the influence of c-Src kinase activity on EGFR tyrosine phosphorylation after gefitinib treatment, we transfected SUM149 cells with wt-c-Src and SUM229 cells with a dominant-negative kinase-dead c-Src (K-c-Src). The K-c-Src construct contains mutations in the kinase domain that prevent ATP binding. After 48 h of incubation with the constructs, the cells were treated with 0.5 µmol/L gefitinib for 30 min and EGFR phosphorylation was measured using immunoprecipitation and immunoblotting. In SUM149 cells, transfection with wt-c-Src increased EGFR tyrosine phosphorylation in the presence of gefitinib (Fig. 2A). Conversely, transfection of K-c-Src into SUM229 cells decreased EGFR tyrosine phosphorylation in the presence of gefitinib (Fig. 2A). To determine the sites of phosphorylation modified with wt-c-Src or K-c-Src expression, whole cell lysates were immunoblotted with phosphospecific antibodies corresponding to EGFR tyrosines and with antibodies against EGFR and c-Src (Fig. 2B). EGFR phosphorylation of Tyr845 and Tyr1086 were increased in SUM149 cells with expression of wt-c-Src in the presence of gefitinib (Fig. 2B). In SUM229 cells, EGFR tyrosine phosphorylation on Tyr845, Tyr992, and Tyr1086 was decreased with the expression of K-c-Src and treatment with gefitinib (Fig. 2B). The phosphorylation of Tyr1045, Tyr1068, Tyr1148, and Tyr1173 on EGFR was unaffected by transfection with either wt-c-Src or K-c-Src (Fig. 2B). Taken together, these data implicate c-Src in the phosphorylation of EGFR in the presence of gefitinib.
Figure 2.
c-Src kinase activity mediating EGFR tyrosine phosphorylation. A, SUM149 breast cancer cells were transiently transfected with pcDNA-c-Src, and SUM229 breast cancer cells were transiently transfected with K-c-Src. Forty-eight hours later, cells were treated with 0.5 µmol/L gefitinib for 30 min, lysed and immunoprecipitated with EGFR antibodies, and immunoblotted with phosphotyrosine antibodies (top). In addition, whole cell lysates were analyzed for c-Src expression as a determination of transfection of the c-Src constructs (bottom). B, SUM149 and SUM229 were transfected with either c-Src or K-c-Src and treated with gefitinib as described above. Whole cell lysates were collected and immunoblotted using site-specific phosphorylation antibodies on EGFR as well as EGFR and c-Src.
C-Src kinase activity mediates growth in the presence of EGFR TKIs
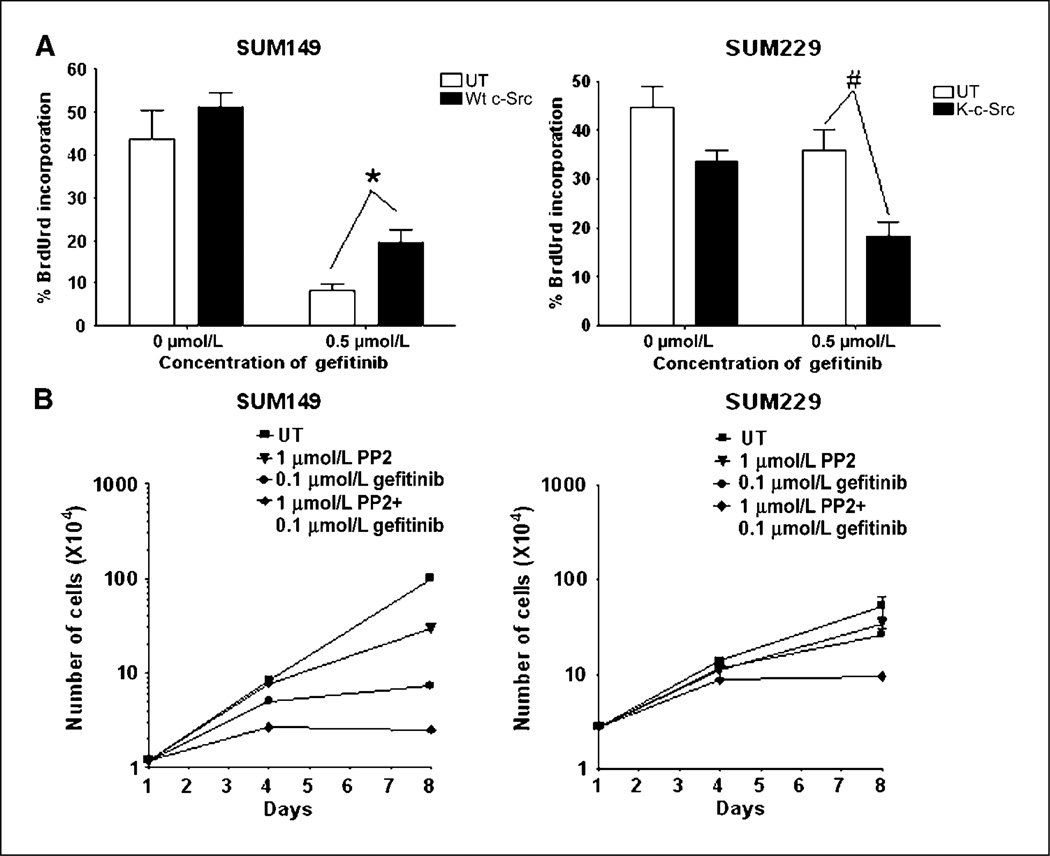
Breast cancer cells that maintain EGFR tyrosine phosphorylation in the presence of EGFR TKIs also continue to proliferate in the presence of EGFR TKIs. Therefore, to determine if c-Src kinase activity regulates proliferation in the presence of EGFR TKIs, we examined BrdUrd incorporation after transfection of c-Src constructs and cell counting with EGFR and c-Src small molecule inhibitors. In SUM149 breast cancer cells, treatment with 0.5 µmol/L gefitinib decreased BrdUrd incorporation from 44% to 8% (Fig. 3A, open bars). SUM149 cell transfected with wt-c-Src showed no increase in BrdUrd incorporation under normal growth conditions (Fig. 3A, filled bars). However, in the gefitinib-treated cells, the percent cells incorporating BrdUrd increased from 8% to 19%, which was a statistical increase in BrdUrd incorporation (P = 0.009; Fig. 3A). In SUM229 breast cancer cells, treatment with 0.5 µmol/L gefitinib did not significantly change the BrdUrd incorporation compared with untreated cells (44% versus 38%; Fig. 3A, open bars). However, transfection with K-c-Src decreased BrdUrd incorporation in untreated SUM229 cells, from 44% to 34%, with a P value approaching significance at 0.05 (Fig. 3A). Importantly, in gefitinib-treated cells, BrdUrd incorporation significantly decreased from 38% to 18% with transfection of K-c-Src (P = 0.011; Fig. 3A). Taken together, these data suggest the kinase activity of c-Src modulates the response of breast cancer cells to gefitinib.
Figure 3.
c-Src kinase activity mediating proliferation in the presence of EGFR TKIs. A, SUM149 and SUM229 were transfected with either c-Src or K-c-Src. Twenty-four hours later, cells were treated with 0.5 µmol/L gefitinib. Twenty hours later, BrdUrd was added for 4 h. Cells were fixed, nuclei were extracted via HCl treatment, and anti-BrdUrd antibodies tagged with AlexaFluor563 were used to detect BrdUrd incorporation. White bars, untransfected cells; black bars, transfected cells with the indicated c-Src construct. The experiment was repeated at least thrice in duplicate, and 100 cells were counted for each coverslip. The percent BrdUrd incorporation was determined for each experiment and averaged; columns, mean; bars, SE. *, P = 0.0086; #, P = 0.011. B, SUM149 and SUM229 breast cancer cells were plated at 35,000 cells per well of the 6-well plate on day 0. Cells were treated with 0.1 µmol/L gefitinib, 1 µmol/L PP2, or the combination of the two every day for 8 d. Cell counts were performed at day 4 and day 8. Points, mean from two independent experiments performed in triplicate; bars, SE. UT, untreated.
To determine if the reduction in DNA synthesis observed in the BrdUrd experiments reflected a decrease in proliferation, SUM149 and SUM229 cells were treated with the combination of gefitinib and the c-Src inhibitor PP2. Inhibiting EGFR kinase activity with 0.1 µmol/L gefitinib in SUM149 cells completely inhibited proliferation, whereas in SUM229 cells, proliferation continued to occur with gefitinib treatment (Fig. 3B, circles). Treatment of SUM149 and SUM229 cells with 1 µmol/L PP2 decreased c-Src activity as measured by the phosphorylation of Src substrates (data not shown) and slightly decreased proliferation (Fig. 3B, triangles). The combination of 0.1 µmol/L gefitinib and 1 µmol/L PP2 further decreased proliferation in SUM149 and inhibited proliferation in SUM229 cells (Fig. 3B, diamonds). These data are consistent with a role for the kinase activity of c-Src in the proliferation of SUM229 cells in the presence of EGFR TKIs.
Met is highly phosphorylated in the presence of EGFR TKIs
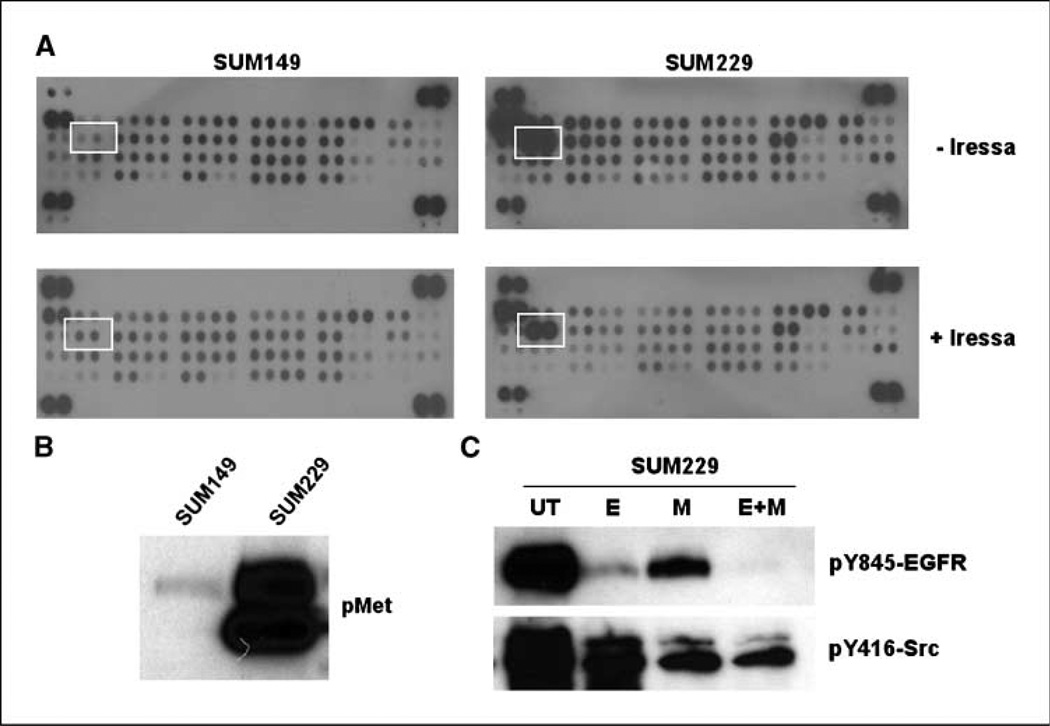
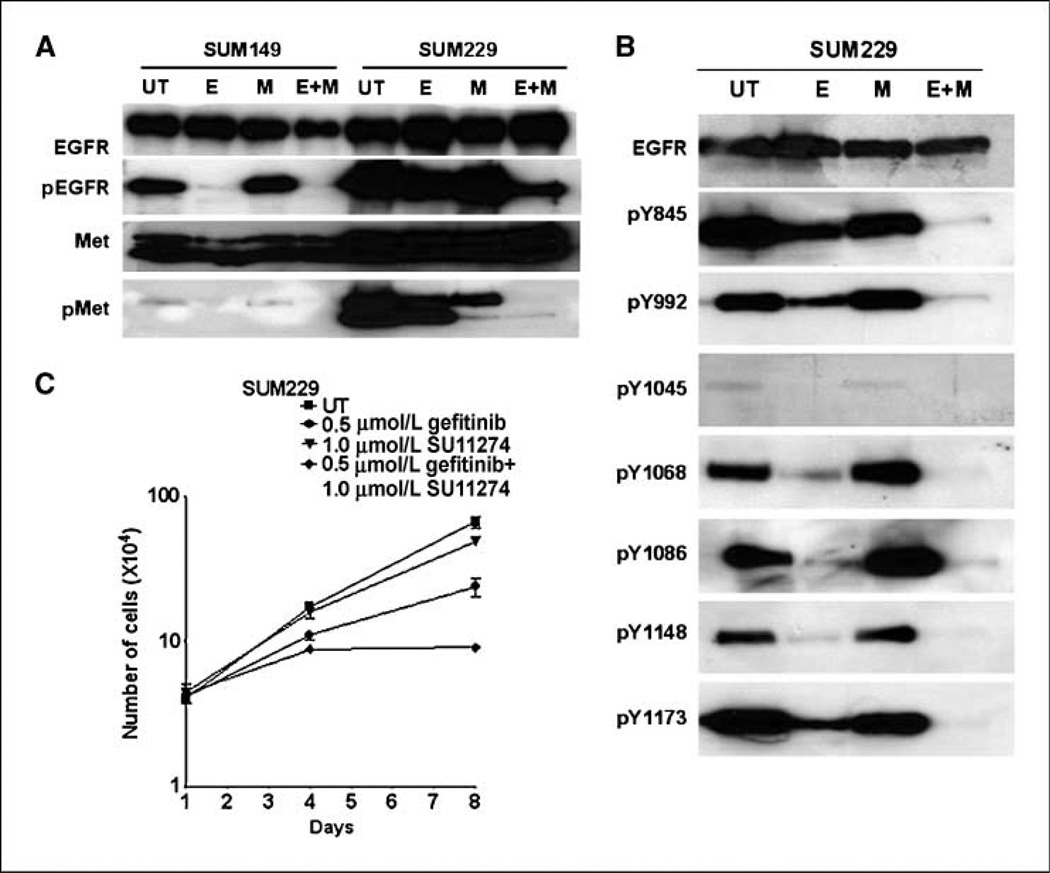
The regulation of c-Src activity occurs through multiple mechanisms, including the phosphorylation of Tyr527 (inactivating) and Tyr416 (activating; ref. 23). Receptor tyrosine kinases, including EGFR family members, activate c-Src. In our model, we inhibited EGFR kinase activity; therefore, in SUM229 cells, activation of c-Src may require another kinase. To identify additional receptor tyrosine kinases activated in SUM229 cells, we used a phospho-proteomic array spotted with antibodies for 42 receptor tyrosine kinases. SUM149 or SUM229 cells were treated with 0.5 µmol/L gefitinib for 30 minutes and lysates were collected and incubated with arrays. We found the pattern of tyrosine-phosphorylated receptors in SUM149 cells compared with SUM229 to be distinct (Fig. 4A). SUM229 cells had high levels of Met phosphorylation both in the untreated and the gefitinib-treated conditions, whereas SUM149 cells had undetectable levels of Met tyrosine phosphorylation (Fig. 4A). Increased Met tyrosine phosphorylation was confirmed using immunoprecipitation and immunoblotting, where the phosphorylation of Met was 5-fold higher in SUM229 cells compared with the Met phosphorylation levels in SUM149 cells (Fig. 4B). These data show that Met was highly tyrosine phosphorylated in the presence of EGFR TKIs in SUM229 cells.
Figure 4.
Met is constitutively tyrosine phosphorylated in SUM229 cells and regulates c-Src phosphorylation and activity. A, using a phospho-proteomic array (R&D Systems), lysates from gefitinib-treated SUM149 and SUM229 breast cancer cells were exposed to membranes spotted with antibodies representing 42 different receptor tyrosine kinases. The unbound lysate was washed away and phospho-tyrosine specific antibodies linked to HRP were added to detect receptors that are tyrosine phosphorylated. White boxes, differences seen in the tyrosine phosphorylation of Met in the cells that proliferate in the presence of EGFR TKIs compared with those that do not. B, lysates from SUM149 or SUM229 cells were prepared and separated by SDS-PAGE followed by immunoblotting using phospho-specific Met antibodies. C, SUM229 cells were either untreated or treated with 0.5 µmol/L gefitinib (E), 1.0 µmol/L SU11274 (M), or the combination (E+M) for 30 min. Lysates were prepared and separated by SDS-PAGE. Membranes were immunoblotted with pY845-EGFR or pY416 antibodies.
Met kinase activity stimulates c-Src phosphorylation and activation
To determine if Met is the kinase that phosphorylates and activates c-Src in the presence of EGFR TKIs in SUM229 cells, we treated cells with 0.5 µmol/L gefitinib, 1.0 µmol/L SU11274 (a Met inhibitor), or the combination of gefitinib and SU11274 for 30 minutes. Lysates were isolated, separated by SDS-PAGE, and immunoblotted using pY845-EGFR or pY416-Src antibodies. As mentioned above, Tyr845 on EGFR is a substrate for c-Src kinase activity. SUM229 cells treated with gefitinib (E) showed a decrease but not a complete inhibition of Tyr845 phosphorylation (Fig. 4C). The Met inhibitor (M) also showed a decrease in EGFR Tyr845 phosphorylation, and the combination of EGFR and Met inhibitors (E+M) completely abrogated Tyr845 phosphorylation (Fig. 4C, top). The inhibition of Met decreased the phosphorylation of Tyr416, an autophosphorylation site often used to represent kinase activity of c-Src (ref. 23; Fig. 4C, M). Taken together, these data suggest that Met kinase activity mediated c-Src phosphorylation and activation in SUM229 cells.
Met kinase activity mediates EGFR tyrosine phosphorylation in the presence of EGFR TKIs
We then tested whether inhibiting Met activation would result in a similar decrease in EGFR tyrosine phosphorylation in the presence of EGFR TKIs. SUM149 and SUM229 cells were treated with 0.5 µmol/L gefitinib (E), 1.0 µmol/L SU11274 (M), or the combination (E+M) for 30 minutes. In SUM149 cells, as previously shown, gefitinib completely inhibited EGFR tyrosine phosphorylation. Conversely, in SUM229 cells, EGFR remained phosphorylated in the presence of gefitinib (pEGFR; Fig. 5A). The Met inhibitor alone had no effect on EGFR tyrosine phosphorylation in SUM149 or SUM229 cells. However, the combination of gefitinib and SU11274 (E+M) reduced EGFR tyrosine phosphorylation in SUM229 cells (pEGFR; Fig. 5A). Therefore, similar to our observation with inhibition of c-Src kinase activity, abrogating Met kinase activity in the presence of EGFR TKIs decreased EGFR tyrosine phosphorylation in SUM229 cells.
Figure 5.
Met kinase activity mediates EGFR kinase–independent EGFR tyrosine phosphorylation. SUM149 and SUM229 cells were left untreated or treated with 0.5 µmol/L gefitinib, 1 µmol/L SU11274, or the combination for 30 min. Lysates were prepared and (A) immunoprecipitated with antibodies for EGFR or Met, and immunoblotted with phosphotyrosine, EGFR, or Met antibodies. B, whole cell lysates from the treated cells were immunoblotted with EGFR or EGFR phospho-specific antibodies. C, SUM149 and SUM229 breast cancer cells were plated at 35,000 cells per well of the 6-well plate on day 0. Cells were left untreated or treated with 0.1 µmol/L gefitinib, 1 µmol/L SU11274, or the combination every day for 8 d. Cell counts were performed at day 4 and 8. Points, mean from two independent experiments performed in triplicate; bars, SE.
We used phospho-specific antibodies to determine the specific sites of phosphorylation modified with the combination of Met and EGFR TKIs in SUM229 cells. Phosphorylation on EGFR Tyr845, Tyr992, and Tyr1173 were reduced when treated with gefitinib and SU11274 (E+M), whereas SU11274 alone (M) had no effect on EGFR site–specific phosphorylation (Fig. 5B). These data suggest that Met mediates EGFR tyrosine phosphorylation in the presence of EGFR TKIs on Tyr845, Tyr992, and Tyr1173.
Met kinase activity mediates proliferation in the presence of EGFR TKIs
Inhibiting c-Src kinase activity in SUM229 cells in combination with EGFR TKIs caused a decrease in cell growth. Therefore, to determine if Met mediation of c-Src kinase activity also signals to regulate cell growth, 8-day growth assays were performed as described above. SUM229 cells were treated with 0.5 µmol/L gefitinib every day for 8 days, and we observed a decrease in cell growth but not complete inhibition (Fig. 5C, circles). Treatment with 1 µmol/L SU11274 (triangles) had no effect on the growth of SUM229 cells; however, cotreatment with gefitinib and SU11274 inhibited proliferation (Fig. 5C, diamonds). These data imply, similar to c-Src, that inhibiting Met synergistically decreased proliferation in the presence of EGFR TKIs.
Stimulating Met activation with HGF increases EGFR tyrosine phosphorylation in the presence of EGFR TKIs
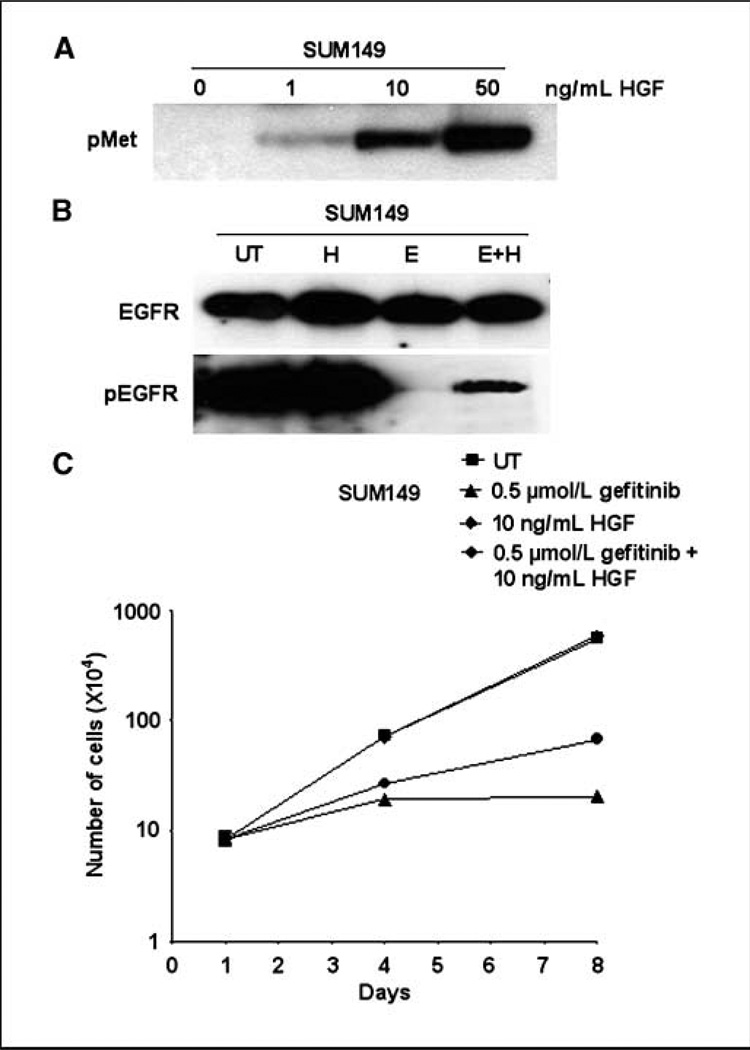
We have shown that inhibiting Met decreased EGFR tyrosine phosphorylation and growth in SUM229 cells in the presence of EGFR TKIs. To determine if the high level of Met tyrosine phosphorylation observed in SUM229 cells was sufficient to induce EGFR tyrosine phosphorylation and growth in the presence of EGFR TKIs, SUM149 cells were stimulated with the Met growth factor HGF. Increasing concentrations of HGF enhanced the phosphorylation of Met in SUM149 cell line (Fig. 6A). These data show that Met was capable of being tyrosine phosphorylated in SUM149 cell line. Next, to determine if HGF treatment stimulated EGFR tyrosine phosphorylation in the presence of EGFR TKIs, SUM149 cells were treated with 50 ng/mL HGF, 0.5 µmol/L gefitinib, or the combination for 30 minutes. Treatment with HGF did not alter EGFR tyrosine phosphorylation in SUM149 cells, yet in the presence of gefitinib, HGF stimulated a small amount of EGFR phosphorylation (pEGFR; Fig. 6B).
Figure 6.
HGF stimulates Met phosphorylation and EGFR tyrosine phosphorylation and cell growth in the presence of EGFR TKIs. A, SUM149 cells were stimulated with increasing concentrations of HGF for 10 min. Lysates were collected and immunoblotted using a phospho-Met specific antibody. B, SUM149 cells were left untreated or were stimulated with HGF for 30 min (H), treated with 0.5 µmol/L gefitinib for 30 min, or the combination (H+E) for 30 min. Lysates were prepared and immunoprecipitated using EGFR antibodies followed by immunoblotting with EGFR or phosphotyrosine antibodies. C, SUM149 cells were plated at 35,000 cells per well of the 6-well plate on day 0. HGF at 10 ng/mL was added to the culture medium. Cells were left untreated or were treated with 0.5 µmol/L gefitinib every day for 8 d. Cell counts were performed at day 4 and 8. Points, mean from two independent experiments performed in triplicate; bars, SE.
Stimulating Met activation with HGF mediates proliferation in the presence of EGFR TKIs
To determine if the stimulation of Met phosphorylation was capable of inducing proliferation in the presence of EGFR TKIs, SUM149 cells were cultured with 10 ng/mL HGF in the presence or absence of 0.5 µmol/L gefitinib. Culturing SUM149 cells with 10 ng/mL HGF (diamonds) maintained proliferation at similar levels as normal growth medium (Fig. 6C, diamonds). As previously described, treating cells with 0.5 µmol/L gefitinib completely inhibited cell proliferation (Fig. 6C, triangles). SUM149 cells cultured in HGF increased cell proliferation in the presence of gefitinib (Fig. 6C, circles). These data suggest that the stimulation of Met tyrosine phosphorylation via HGF treatment induced proliferation in the presence of EGFR TKIs.
Discussion
In this report, we have shown that in SUM229 breast cancer cells, EGFR was tyrosine phosphorylated and cells proliferated in the presence of gefitinib via a Met/c-Src–mediated signaling pathway. Specifically, we have shown preservation of EGFR phosphorylation to be mediated by the kinase activity of c-Src, on EGFR Tyr845, Tyr992, and Tyr1086. Inhibiting c-Src kinase activity reduced proliferation in the presence of gefitinib. Interestingly, proliferation and EGFR tyrosine phosphorylation in the presence of gefitinib occurred when c-Src was overexpressed SUM149 cells. We found the kinase activity of c-Src was in part regulated by constitutive tyrosine phosphorylation of Met in SUM229 cells. Abrogating Met and EGFR kinase activity inhibited phosphorylation on EGFR Tyr845, Tyr992, and Tyr1173. We also found Met kinase activity to be important for proliferation in the presence of gefitinib. Stimulating SUM149 cells with HGF to induce Met tyrosine phosphorylation increased EGFR tyrosine phosphorylation and growth in the presence of EGFR TKIs. Taken together, these data suggest that EGFR signaling and growth in the presence of gefitinib is at least, in part, mediated by a Met/c-Src pathway.
Our observation that EGFR tyrosine phosphorylation can occur in the absence of intrinsic EGFR tyrosine kinase activity has been made by others using different model systems. Specifically, mass spectrometry analysis of EGFR after treatment with the small molecule inhibitor AG1478 shows that Tyr992 and Tyr1068 remain phosphorylated even in the presence of inhibitor (24). Others confirmed these observations using immunoblotting techniques with additional EGFR inhibitors (25, 26). Our data suggest that EGFR may still be able to stimulate growth and survival in the presence of EGFR TKIs. In addition, these data suggest measuring tyrosine phosphorylation, particularly phosphorylation at specific tyrosine sites, is not a good predictor of EGFR kinase activity. Specifically, we found that EGFR remains tyrosine phosphorylated when kinase activity was abrogated with a small molecule inhibitor. In addition, we showed the phosphorylation level of EGFR at specific tyrosines varies not only between cell lines but also in responsiveness to EGFR TKIs. Therefore, these data suggest careful selection of tyrosine sites analyzed for phosphorylation and in vitro kinase assays to be used measure kinase activity of EGFR in breast cancer cells.
Receptor tyrosine kinases have the ability to directly phosphorylate and/or associate with EGFR. Specifically, Tyr845, Tyr992, Tyr1068, and Tyr1101 on EGFR are phosphorylated by c-Src and HER2 (27–29). Phospho-proteomic technology and phosphosite–specific antibodies have improved the ability to detect small changes in specific sites of tyrosine phosphorylation (26, 30, 31). The data presented here confirm the kinase activity of c-Src is at least partly responsible for EGFR phosphorylation on Tyr845, Tyr992, and Tyr1086 in the presence of EGFR TKIs. In addition, in the SUM229 cells, both K-c-Src and a c-Src small molecule inhibitor reduced DNA synthesis and proliferation in the presence of EGFR TKIs. Taken together, these data suggest that phosphorylation at Tyr845, Tyr992, and Tyr1086 on the EGFR transduce signals to cell proliferation in the presence of EGFR TKIs. Therefore, treating EGFR-expressing breast cancers with a c-Src inhibitor in combination with EGFR inhibitor may decrease EGFR tyrosine phosphorylation and subsequent signaling pathways and inhibit cell growth.
Multiple lines of evidence support a physical and functional interaction between Met and c-Src. A member of the Src kinase family, Fyn, was first shown to associate with Met 15 years ago (32). Because then, other c-Src family members, including c-Src, have been found to associate with Met (33). The association between Met and c-Src has functional significance, as c-Src kinase activity is required for HGF-mediated motility and transformation in mammary carcinoma cells and papillary renal carcinoma cells (34, 35). In this report, we show Met kinase activity regulated c-Src phosphorylation and activation. However, c-Src kinase activity was not completely abrogated by Met inhibition in SUM229 cells, suggesting an alternate mechanism for c-Src activation and/or modification of dephosphorylation and turnover mechanisms of c-Src. In that regard, others showed an incomplete inhibition of c-Src phosphorylation of Tyr416 with c-Src inhibitor treatment and implicated the inactivation of a tyrosine phosphatase (36). This phenomenon will need to be further evaluated in SUM229 cells.
Transphosphorylation of EGFR by other receptor tyrosine kinases has been well-documented (37). Examples of receptor tyrosine kinases transphosphorylating the EGFR include HER2, HER4, Met, and insulin-like growth factor I–IR (38–40). It has been hypothesized that transphosphorylation occurs from direct interaction of the other receptor tyrosine kinases with EGFR. Recently, Met tyrosine phosphorylation has been associated with acquired EGFR TKI resistance in lung cancer cell lines containing EGFR tyrosine kinase domain mutations (38). In the lung cancer model, Met amplification is responsible for the high levels of Met tyrosine phosphorylation (38). Met has also been shown to be physically associated with EGFR, and this association mediates transphosphorylation of EGFR (41). In addition, Met is a tyrosine kinase implicated in breast cancer progression (42–46). Specifically, HGF/Met signaling pathways have been shown to increase tumor vasculature volume, promote local and distant invasion of tumor cells, and increase tumor growth and survival in mouse models (42, 47, 48). In human breast tumor samples, an average of 30% of samples express high levels of Met and 66% of samples express high levels of HGF in the primary tumor and/or the associated lymph node metastasis (44). Here, our data show that high levels of Met tyrosine phosphorylation mediated c-Src tyrosine phosphorylation and activation, resulting in the regulation of EGFR tyrosine phosphorylation and growth in the presence of EGFR TKIs. In combination with the c-Src data, these results suggest that the mediation of EGFR tyrosine phosphorylation by Met may occur through c-Src and not via a direct interaction with EGFR. However, inhibiting Met kinase activity decreased phosphorylation on Tyr1068 and Tyr1173, whereas inhibiting c-Src kinase activity did not. These data imply that Met is either directly phosphorylating Tyr1068 and Tyr1173 on EGFR or is activating yet another tyrosine kinase. Interestingly, although there are differences in the sites of tyrosine phosphorylation regulated by Met and c-Src, the inhibition of either kinase had similar effects on cell growth, suggesting a lack of redundancy in the pathways activated through specific sites of phosphorylation on EGFR.
Stimulation of Met with HGF in SUM149 cells increased EGFR tyrosine phosphorylation and proliferation in the presence of EGFR TKIs. However, the phospho-specific sites of EGFR tyrosine phosphorylation were not altered in the presence of HGF (data not shown). Therefore, complimentary to the wt-c-Src overexpression in SUM149 cells, HGF increased EGFR tyrosine phosphorylation and growth in the presence of EGFR TKIs, but neither c-Src overexpression nor HGF stimulation seem to be sufficient to replicate EGFR kinase independence of SUM229 cells. These data suggest the requirement of other genetic and signaling alterations in SUM229 cells to mediate EGFR tyrosine phosphorylation and proliferation in the presence of EGFR TKIs.
In this study, we have implicated a Met/c-Src signaling pathway to mediate proliferation in the presence of EGFR TKIs in breast cancer cells. These data suggest inhibiting multiple tyrosine kinases, including Met and c-Src in breast cancer cells, may be an effective mechanism of inhibiting cell growth.
Acknowledgments
Grant support: Karmanos Cancer Institute Strategic Research Initiative Fund.
We thank Dr. Sarah J. Parsons for the c-Src constructs and the 2–17 antibody; Dr. Michael Weber for the mab108 antibody; Dr. Ramsi Haddad, Dr. Raymond Mattingly, and Scott Boerner for their careful reading of the manuscript; and the Ethier laboratory group for their helpful discussions and comments.
References
- 1.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 2.Sainsbury JR, Farndon JR, Harris AL, Sherbet GV. Epidermal growth factor receptors on human breast cancers. Br J Surg. 1985;72:186–188. doi: 10.1002/bjs.1800720309. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero JM, Ramaioli A, Largillier R, et al. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12:841–846. doi: 10.1023/a:1011183421477. [DOI] [PubMed] [Google Scholar]
- 4.Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71:67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny MV, Darzynkiewicz Z. Why Iressa failed: toward novel use of kinase inhibitors (outlook) Cancer Biol Ther. 2003;2:137–140. doi: 10.4161/cbt.2.2.286. [DOI] [PubMed] [Google Scholar]
- 7.Twombly R. Failing survival advantage in crucial trial, future of Iressa is in jeopardy. J Natl Cancer Inst. 2005;97:249–250. doi: 10.1093/jnci/97.4.249. [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 9.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 10.Jimeno A, Daw NC, Amador ML, et al. Analysis of biologic surrogate markers from a Children’s Oncology Group Phase I trial of gefitinib in pediatric patients with solid tumors. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20753. [DOI] [PubMed] [Google Scholar]
- 11.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 12.Dziadziuszko R, Holm B, Skov BG, et al. Epidermal growth factor receptor gene copy number and protein level are not associated with outcome of non-small-cell lung cancer patients treated with chemotherapy. Ann Oncol. 2007;18:447–452. doi: 10.1093/annonc/mdl407. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23:6838–6845. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava R, Gerald WL, Li AR, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–1033. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 16.Reis-Filho JS, Milanezi F, Carvalho S, et al. Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Res. 2005;7:R1028–R1035. doi: 10.1186/bcr1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Verbeek BS, Vroom TM, Adriaansen-Slot SS, et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol. 1996;180:383–388. doi: 10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimri M, Naramura M, Duan L, et al. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 2007;67:4164–4172. doi: 10.1158/0008-5472.CAN-06-2580. [DOI] [PubMed] [Google Scholar]
- 21.Ware MF, Tice DA, Parsons SJ, Lauffenburger DA. Overexpression of cellular Src in fibroblasts enhances endocytic internalization of epidermal growth factor receptor. J Biol Chem. 1997;272:30185–30190. doi: 10.1074/jbc.272.48.30185. [DOI] [PubMed] [Google Scholar]
- 22.Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005 doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- 23.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Kozlosky CJ, Ericsson LH, Daniel TO, Cerretti DP, Johnson RS. Studies of ligand-induced site-specific phosphorylation of epidermal growth factor receptor. J Am Soc Mass Spectrom. 2003;14:1022–1031. doi: 10.1016/S1044-0305(03)00206-X. [DOI] [PubMed] [Google Scholar]
- 25.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cδ/c-Src pathways in glioblastoma cells. J Biol Chem. 2005;280:7729–7738. doi: 10.1074/jbc.M409056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thelemann A, Petti F, Griffin G, et al. Phosphotyrosine signaling networks in epidermal growth factor receptor overexpressing squamous carcinoma cells. Mol Cell Proteomics. 2005;4:356–376. doi: 10.1074/mcp.M400118-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Lombardo CR, Consler TG, Kassel DB. In vitro phosphorylation of the epidermal growth factor receptor autophosphorylation domain by c-src: identification of phosphorylation sites and c-src SH2 domain binding sites. Biochemistry. 1995;34:16456–16466. doi: 10.1021/bi00050a029. [DOI] [PubMed] [Google Scholar]
- 28.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 α. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 29.Spivak-Kroizman T, Rotin D, Pinchasi D, Ullrich A, Schlessinger J, Lax I. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J Biol Chem. 1992;267:8056–8063. [PubMed] [Google Scholar]
- 30.Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004;22:1139–1145. doi: 10.1038/nbt1005. [DOI] [PubMed] [Google Scholar]
- 31.Wolf-Yadlin A, Kumar N, Zhang Y, et al. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol Syst Biol. 2006;2:54. doi: 10.1038/msb4100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bardelli A, Maina F, Gout I, et al. Autophosphorylation promotes complex formation of recombinant hepatocyte growth factor receptor with cytoplasmic effectors containing SH2 domains. Oncogene. 1992;7:1973–1978. [PubMed] [Google Scholar]
- 33.Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 34.Rahimi N, Hung W, Tremblay E, Saulnier R, Elliott B. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem. 1998;273:33714–33721. doi: 10.1074/jbc.273.50.33714. [DOI] [PubMed] [Google Scholar]
- 35.Nakaigawa N, Weirich G, Schmidt L, Zbar B. Tumorigenesis mediated by MET mutant M1268T is inhibited by dominant-negative Src. Oncogene. 2000;19:2996–3002. doi: 10.1038/sj.onc.1203628. [DOI] [PubMed] [Google Scholar]
- 36.Fu YN, Yeh CL, Cheng HH, et al. EGFR mutants found in non-small cell lung cancer show different levels of sensitivity to suppression of Src: implications in targeting therapy. Oncogene. 2008;27(7):957–965. doi: 10.1038/sj.onc.1210684. Epub 2007 Jul 23. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 39.Riedemann J, Sohail M, Macaulay VM. Dual silencing of the EGF and type 1 IGF receptors suggests dominance of IGF signaling in human breast cancer cells. Biochem Biophys Res Commun. 2007;355:700–706. doi: 10.1016/j.bbrc.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 40.Riedemann J, Takiguchi M, Sohail M, Macaulay VM. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun. 2007;355:707–714. doi: 10.1016/j.bbrc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 42.Beviglia L, Matsumoto K, Lin CS, Ziober BL, Kramer RH. Expression of the c-Met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer. 1997;74:301–309. doi: 10.1002/(sici)1097-0215(19970620)74:3<301::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 43.Camp RL, Rimm EB, Rimm DL. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer. 1999;86:2259–2265. doi: 10.1002/(sici)1097-0142(19991201)86:11<2259::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Lengyel E, Prechtel D, Resau JH, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 45.Tolgay Ocal I, Dolled-Filhart M, D’Aquila TG, Camp RL, Rimm DL. Tissue microarray-based studies of patients with lymph node negative breast carcinoma show that met expression is associated with worse outcome but is not correlated with epidermal growth factor family receptors. Cancer. 2003;97:1841–1848. doi: 10.1002/cncr.11335. [DOI] [PubMed] [Google Scholar]
- 46.Tsarfaty I, Alvord WG, Resau JH, et al. Alteration of Met protooncogene product expression and prognosis in breast carcinomas. Anal Quant Cytol Histol. 1999;21:397–408. [PubMed] [Google Scholar]
- 47.Nagy J, Curry GW, Hillan KJ, et al. Hepatocyte growth factor/scatter factor expression and c-met in primary breast cancer. Surg Oncol. 1996;5:15–21. doi: 10.1016/s0960-7404(96)80017-x. [DOI] [PubMed] [Google Scholar]
- 48.Shinomiya N, Gao CF, Xie Q, et al. RNA interference reveals that ligand-independent met activity is required for tumor cell signaling and survival. Cancer Res. 2004;64:7962–7970. doi: 10.1158/0008-5472.CAN-04-1043. [DOI] [PubMed] [Google Scholar]