Abstract
Background and Objectives:
Amiodarone (AM), one of the most commonly prescribed antiarrhythmics, is frequently associated with thyroid dysfunction. Green tea extract (GTE) supplementation would attenuate oxidative stress and activate progenitor cells. However, the potential toxicity of GTE on various organs when administered at high doses has not been completely investigated. The present study aimed at investigating the possible relation between endogenous stem cells and GTE in overconsumption and AM induced thyroid damage in albino rat.
Methods and Results:
Twenty four male albino rats were divided into control group, GTE group (rats given 50 mg/kg), Overconsumption group (rats given 1,000 mg/kg GTE), AM group (rats given 30 mg/kg) and combined AM, GTE therapy group. AM and GTE were administered orally 5 days/week for 8 weeks. Serological tests were performed. Thyroid sections were exposed to histological, immunohistochemical and morphometric studies. In overconsumption group, multiple distorted follicles with cellular debris in the lumen and multiple follicles devoid of colloid were found. In AM group, multiple follicles exhibiting crescent of colloid and few follicles devoid of colloid were detected. In combined therapy group, multiple follicles were filled with colloid. Significant decrease in area of colloid and significant increase in the area% of collagen were recorded in overconsumption and AM groups. Area% of CD 105 +ve cells denoted significant increase in combined therapy group. Serological tests were confirmative.
Conclusions:
Endogenous SCs activation was proved in AM and GTE combined therapy group with regression of AM induced morphological, morphometric and serological changes. However, overconsumption of GTE recruited endogenous SCs suppression.
Keywords: Mesenchymal stem cells, Amiodarone, Thyroid, Green tea extract
Introduction
Amiodarone (AM) remains one of the most commonly prescribed antiarrhythmics effectively treating atrial and ventricular fibrillation (1). AM is frequently associated with thyroid dysfunction. Identifying predictors for AM-associated thyroid dysfunction may aid clinicians in daily practice (2).
It was hypothesized that green tea extract (GTE) supplementation would elevate antioxidant potential and attenuate oxidative stress (3). Green tea is associated with beneficial health effects mainly because of its body fat-reducing and hypocholesterolemic activities. It improves cardiovascular risk indicators such as body weight, visceral fat content and atherogenic index of plasma (4).
The potential toxicity of green tea on various organs when administered at high doses via concentrated extracts has not been completely investigated (5). Green tea was proved to be associated with various beneficial health effects, but several cases of toxic side effects have been reported (6).
Recent studies suggest that (-)-epigallocatechin-3-gallate (EGCG), which is the main polyphenolic constituent of green tea, may be used for the prevention and treatment of various neurodegenerative diseases. EGCG was proved to activate neural progenitor cells (7).
The present study aimed at investigating the possible relation between endogenous stem cells and green tea extract in overconsumption and amiodarone induced thyroid damage in albino rat.
Materials and Methods
Drugs
Amiodarone (Cordarone): Used as tablets 200 mg (Sanofi Corporation), crushed, the required dose was weighed using a digital scale and dissolved in tween 80.
Green tea extract: Used as tablets 1,000 mg (Technomad Group), crushed, the required dose was weighed using a digital scale and dissolved in distilled water.
Animals
Twenty four adult male albino rats weighing 150∼200 g were divided into 5 groups. Each group was kept in a separate cage under good hygienic conditions, fed ad libitum and allowed for free water supply in Histology Department Animal House, Faculty of Medicine, Cairo University. The rats were treated in accordance with guidelines approved by the Animal Use Committee of Cairo University. The rats were divided into the following groups.
Control group: 4 rats, one for each experimental group. Two rats were given 0.5 ml distilled water (solvent of GTE) and two given 0.5 ml tween 80 (solvent of AM). The latter being water insoluble at room temperature. The solvents were given daily orally five days/week for 8 weeks.
GTE group: 5 rats, each given 50 mg/kg (8) daily orally five days/week for 8 weeks. The required dose for each rat was dissolved in 0.5 ml distilled water.
Overconsumption: 5 rats, each given 1,000 mg/kg of GTE five days/week (9) for 8 weeks. The required dose for each rat was dissolved in 2.5 ml distilled water given as 0.5 ml/hour orally.
AM group: 5 rats, each given 30 mg/kg (10), daily orally five days/week for 8 weeks.
AM and GTE group: 5 rats, each given AM 30 mg/kg (10) and GTE 50 mg/kg (8) daily orally five days/week for 8 weeks.
In groups 2, 3, 4 and 5 a stock solution was prepared weekly and kept at 4℃. The required dose for each rat was introduced into the mouth using a syringe with a metal tube instead of the needle.
Before sacrifice blood samples were collected using capillary tubes introduced into the eye of rats to perform thyroid function tests (T3, TSH) in the Clinical Pathology Department, Faculty of Medicine, Cairo University. Rats were sacrificed by lethal dose of ether. Thyroid gland was exposed by frontal incision immediately below the head. Thyroid specimens were placed in 10% formol saline, paraffin blocks prepared and 5 microns thick sections were subjected to the following:
Histological study
Immunohistochemical study
CD105 immunostaining, MSC marker (13) was added as 0.1 ml ready-to-use primary antibody (CD105) goat polyclonal antibody (catalogue number 559286, BD Biosciences, San Jose, California, USA), and incubated at room temperature in a moist chamber for 60 min. Tonsil was used as a positive control specimen. Reaction localization is the cell membrane. However, one of the thyroid sections was used as a negative control by passing the step of applying the primary antibody.
Morphometric study
Using Leica Qwin 500 (Leica LTD, Cambridge, UK) image analysis system, assessment of the mean area (μ2) of colloid was performed using interactive measurements menu in 10 high power fields (HPF). In addition, area% of collagen fibers and area% of CD105 +ve cells was done in 10 HPF using binary mode menu.
Statistical analysis (14)
Quantitative data were summarized as means and standard deviations and compared using one-way analysis-of variance (ANOVA). p-values<0.05 were considered statistically significant. Calculations were made on SPSS software.
Results
Hematoxylin and eosin (H&E) stained sections
Sections in the thyroid gland of control rats revealed a connective tissue capsule covering lobules demonstrating follicles of variable sizes. The follicles contained colloid with peripheral vacuoles and were separated by interfollicular tissue (Fig. 1). In GTE group, the morphological features of thyroid sections were comparable to the control group (Fig. 2). In overconsumption group, some distorted follicles with cellular debris in the lumen were observed and multiple follicles devoid of colloid were detected (Fig. 3). In AM group, multiple follicles exhibiting crescent of colloid were noticed and few follicles devoid of colloid were seen (Fig. 4). In combined AM and GTE group, multiple follicles filled with colloid and some follicles with crescent of colloid were demonstrated (Fig. 5).
Fig. 1. Section in the thyroid gland of a rat in group 1 (Control Group) showing capsule (arrow) covering lobules demonstrating follicles (F) of variable sizes containing colloid and interfollicular tissue (I) (H&E, ×200).
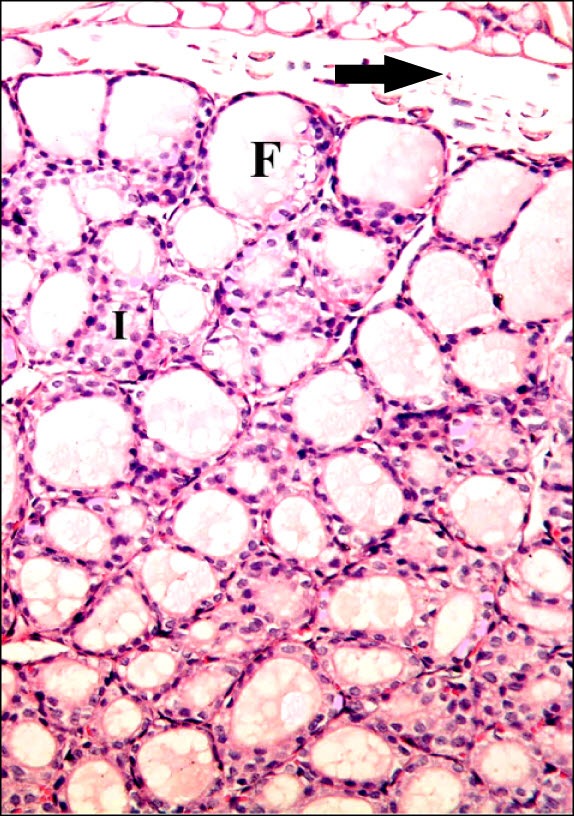
Fig. 2. Section in the thyroid gland of a rat in group 2 (GTE Group) showing follicles (F) of variable sizes containing colloid inside the lobules and interfollicular tissue (I) (H&E, ×200).

Fig. 3. Section in the thyroid gland of a rat in group 3 (Overconsumption Group) showing some distorted follicles with cellular debris in the lumen (arrows) and multiple follicles devoid of colloid (*) (H&E, ×200).
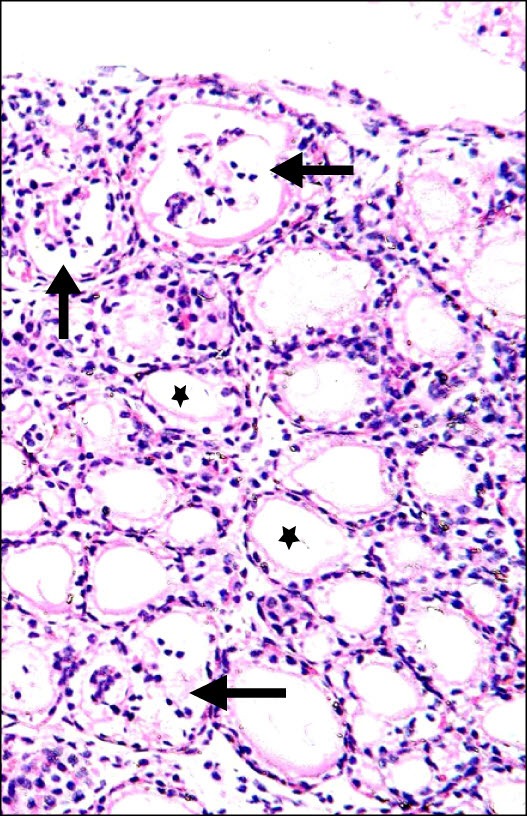
Fig. 4. Section in the thyroid gland of a rat in group 4 (AM Group) showing multiple follicles with a crescent of colloid (*) and few follicles devoid of colloid (arrows) (H&E, ×200).
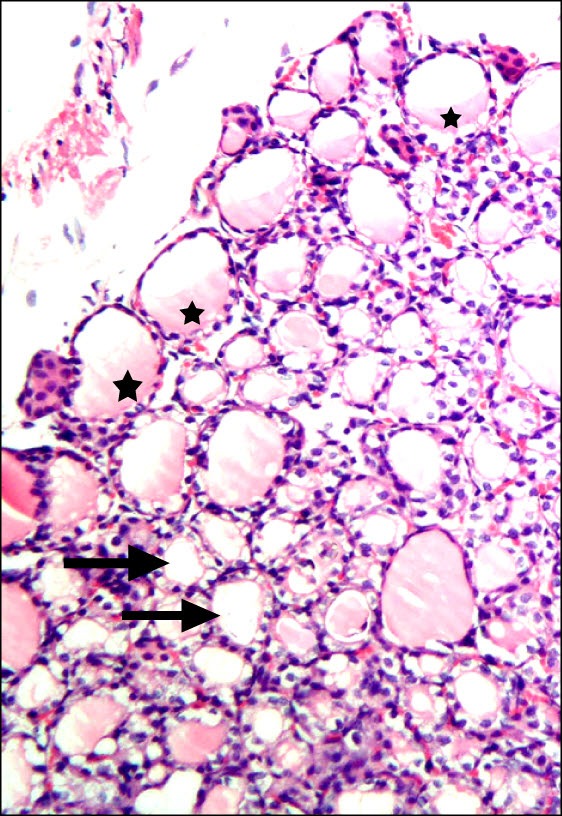
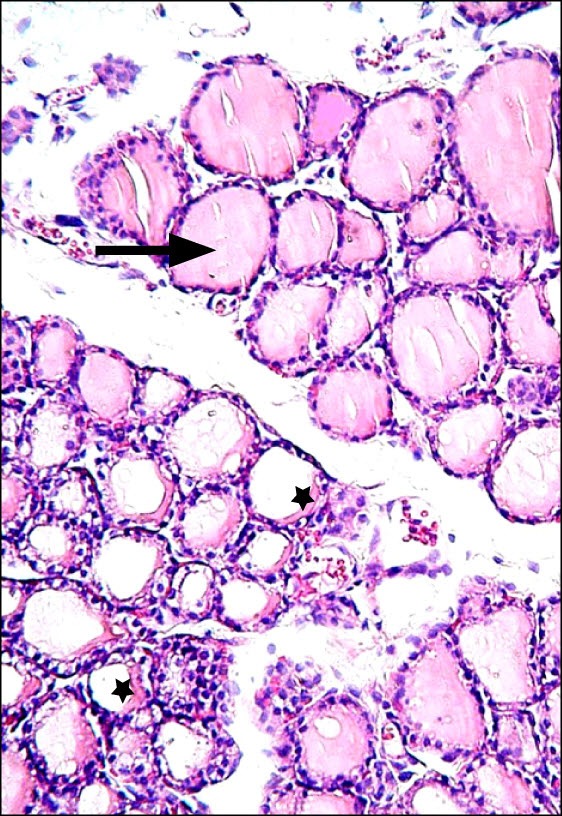
Fig. 5. Section in the thyroid gland of a rat in group 5 showing multiple follicles filled with colloid (arrow) and some follicles with crescent of colloid (*) (H&E, ×200).
Masson's trichrome stained sections
Sections in the thyroid gland of control rats and rats in GTE group showed fine collagen fibers between the lobules and between the follicles (Figs. 6,7). In overconsumption and AM groups, dense interlobular collagen fibers and dense interfollicular fibers were seen. Congested blood vessels were detected in overconsumption group. The latter change was more obvious in AM group (Figs. 8, 9). In combined therapy group fine interlobular collagen fibers and fine interfollicular fibers were noted (Fig. 10).
Fig. 6. Section in the thyroid gland of a control rat showing fine collagen fibers (arrows) between the lobules and between the follicles (arrowhead) (Masson's trichrome, ×200).
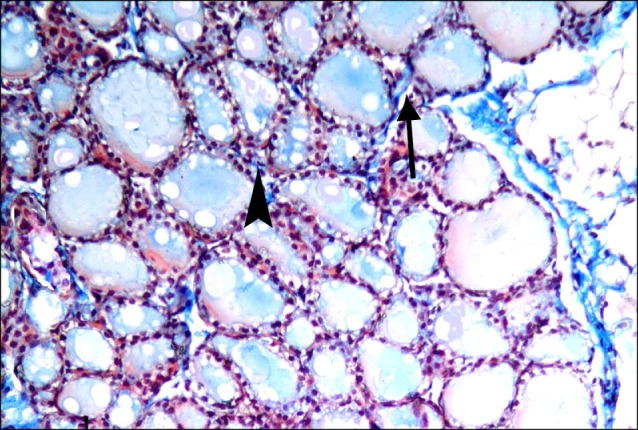
Fig. 7. Section in the thyroid gland of a rat in group 2 showing fine collagen fibers (arrows) between the lobules and between the follicles (arrowhead) (Masson's trichrome, ×200).
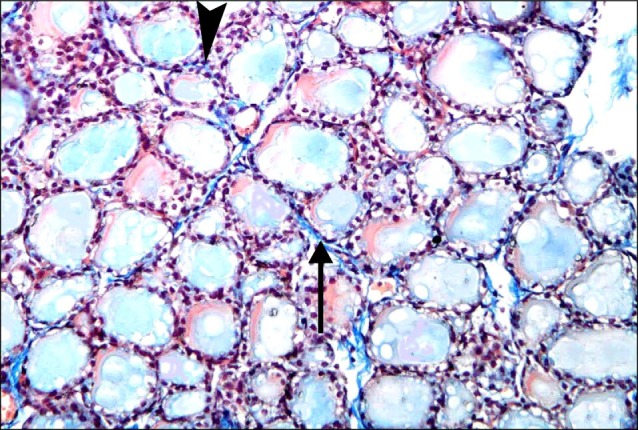
Fig. 8. Section in the thyroid gland of a rat in group 3 showing dense interlobular collagen fibers (arrows) and dense interfollicular fibers (arrowheads). Note congested blood vessels (C) (Masson's trichrome, ×200).
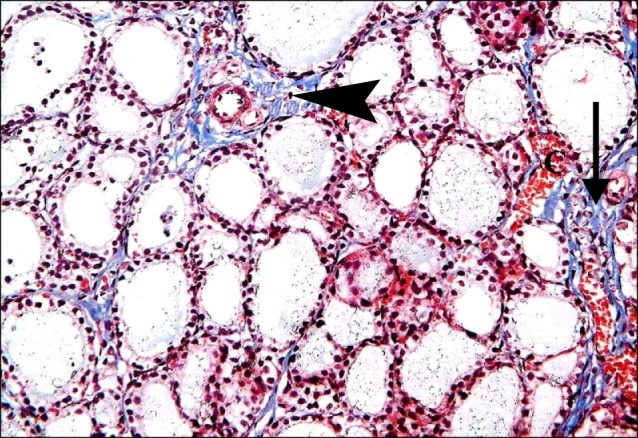
Fig. 9. Section in the thyroid gland of a rat in group 4 showing dense interlobular collagen fibers (arrow) and dense interfollicular fibers (arrowhead). Note obviously congested blood vessel (C) (Masson's trichrome, ×200).
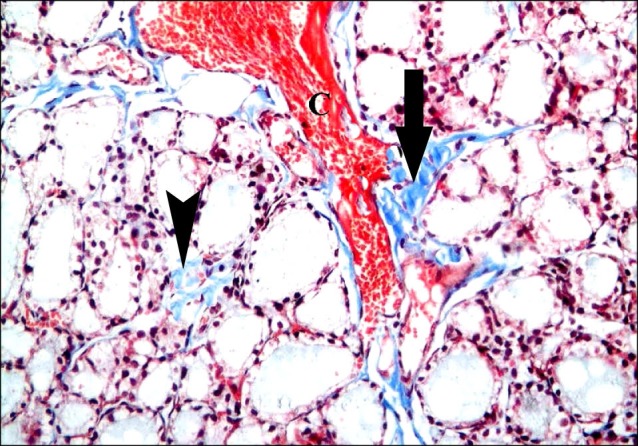
Fig. 10. Section in the thyroid gland of a rat in group 5 showing fine interlobular (arrow) and fine interfollicular (arrowhead) collagen fibers compared to figure 9 (Masson's trichrome, ×200).

CD105 immunostained sections
Sections in the thyroid gland of control rats and rats in GTE group showed -ve immunoreaction among the follicles and interfolliular tissue (Figs. 11,12). In overconsumption group, few spindle +ve cells were found among the epithelial lining of the follicles (Fig. 13). In AM group, some spindle +ve cells were obvious among the epithelial lining of the follicles (Fig. 14). In combined therapy group, multiple spindle +ve cells were detected among the epithelial lining of the follicles and multiple spindle +ve cells were observed in the interfollicular tissue (Fig. 15).
Fig. 11. Section in the thyroid gland of a control rat showing -ve immunoreaction (CD 105 immunostaining, x400).
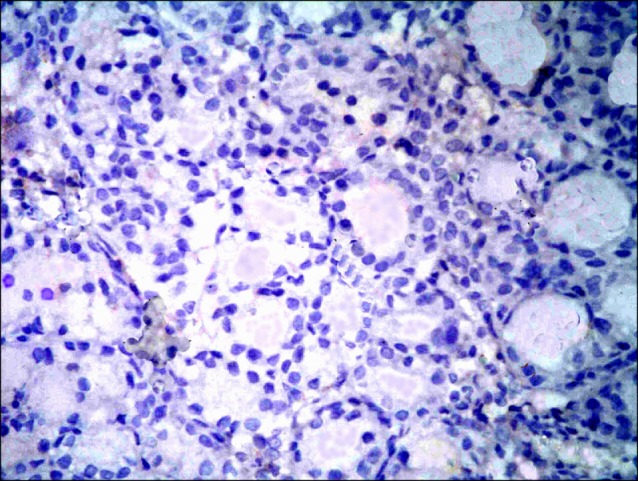
Fig. 12. Section in the thyroid gland of a rat in group 2 showing -ve immunoreaction (CD 105 immunostaining, ×400).
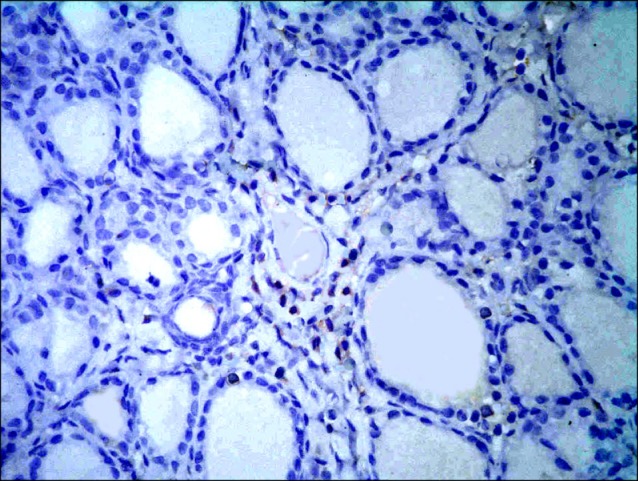
Fig. 13. Section in the thyroid gland of a rat in group 3 showing few spindle +ve cells (arrows) among the epithelial lining of the follicles (CD 105 immunostaining, ×400).
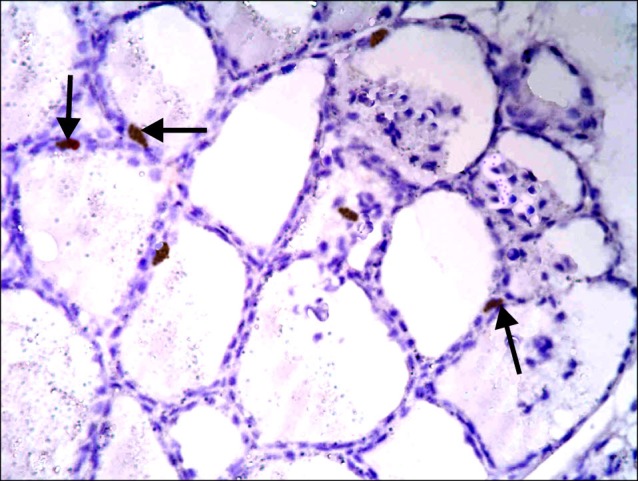
Fig. 14. Section in the thyroid gland of a rat in group 4 showing some spindle +ve cells (arrows) among the epithelial lining of the follicles (CD105 immunostaining ×400).
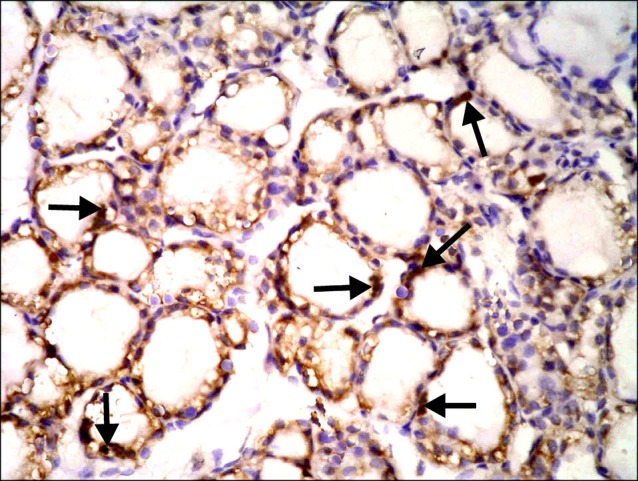
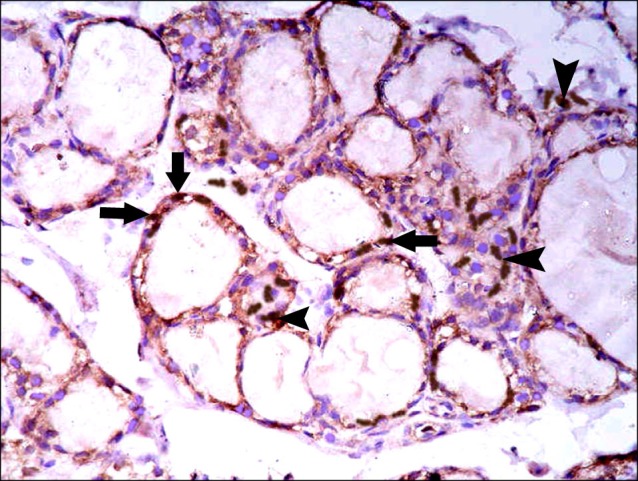
Fig. 15. Section in the thyroid gland of a rat in group 5 showing multiple spindle +ve cells (arrows) among the epithelial lining of the follicles and multiple spindle +ve cells (arrowheads) in the interfollicular tissue (CD105 immunostaining ×400).
Morphometric results
The mean area of colloid indicated a significant (p<0.05) decrease, while the mean area% of collagen fibers denoted a significant (p<0.05) increase in overconsumption and AM groups. On the other hand, a significant (p<0.05) increase in the mean area% of CD105 +ve cells was reported in combined therapy group (Table 1).
Table 1.
Mean area (μ2) of colloid, mean area% of collagen fibers and mean area% of CD105 +ve cells in control and experimental groups
| Groups | Mean area of colloid | Mean area% of collagen fibers | Mean area% of CD105 +ve cells |
|---|---|---|---|
|
| |||
| Control group | 509.14±68.83 | 12.98±1.99 | - |
| GTE group | 530.53±59.19 | 12.15±2.34 | - |
| Overconsumption group | 311.01±18.38a | 21.98±4.39a | 3.5±0.59 |
| AM group | 352.76±17.99a | 25.43±4.18aa | 4.4±0.89 |
| AM and GTE group | 441.21±52.38 | 13.43±2.97 | 18.2±3.84a |
aSignificant (p <0.05).
Serological results
Statistical analysis by ANOVA test revealed a significant (p<0.05) decrease of the mean value of TSH in overconsumption and AM groups.
Discussion
The current study demonstrated ameliorating effect of GTE supplemenation on AM induced thyroid damage, while overconsumption of GTE proved obvious changes in an experimental model of albino rat. Associated variations in stem cells existence were noted. This was evidenced by histological, immunohistochemical, morphometric and serological methods.
In overconsumption group, some distorted follicles with cellular debris in the lumen were observed and multiple follicles devoid of colloid were detected. The latter finding was confirmed by a significant decrease in mean area of colloid. The effect of GTE in a dose of 1,000 mg/kg was studied on the liver. Necrotic hepatocytes were found in the centrilobular regions (9). It was stated that several plant antioxidants including GT catechins act in low dose as antioxidants but high doses are cytotoxic (15). It was proved by histological examination of the thyroid gland the existence of follicles with depleted colloid in case of dietary administration of GTE catechins at high doses, this may be due to antithyroid effects of catechins (16).
In AM group, multiple follicles exhibiting crescent of colloid were noticed and few follicles devoid of colloid were seen. This finding was also confirmed by a significant decrease in mean area of colloid. It was reported that AM-associated thyrotoxicosis was higher compared to hypothyroidism (17). On the other hand, Narayana et al (18) stated that AM can lead to both hypothyroidism and less commonly hyperthyroidism.
Table 2.
Mean values of T3 (pg/ml) and TSH (μI/ml) in control and experimental groups
| Groups | T3 | TSH |
|---|---|---|
|
| ||
| Control group | 4.2±0.55 | 1.32±0.32 |
| GTE group | 4.0±0.50 | 1.58±0.31 |
| Overconsumption group | 5.0±0.51 | 0.05±0.002a |
| AM group | 5.2±0.53 | 0.09±0.005a |
| AM and GTE group | 4.8±0.93 | 1.05±0.021 |
aSignificant (p<0.05).
In combined AM and GTE group, multiple follicles filled with colloid and some follicles exhibiting crescent of colloid were demonstrated. It was found that GTE produce best antioxidant activity among all individual extracts (19). Administration of GT was proved to exert protective effect on intestinal mucosa in ischemia reperfusion (20).
Changes in the collagen content of the gland recorded dense interlobular and dense interfollicular fibers in overconsumption and AM groups, confirmed by a significant increase in the mean area% of collagen fibers. Concomitantly, it was postulated that fibrosis is stimulated by fibroblasts/ myofibroblasts, the effector cells in collagen deposition/ fibrosis (21).
In contrast, in combined therapy group fine interlobular collagen fibers and fine interfollicular fibers were noted. Similarly, it was reported that GTE can suppress activation of hepatic stellate cells, consequently collagen production and liver fibrosis (22).
Congested blood vessels were detected in overconsumption group. The latter change was more obvious in AM group. It was stated that nitric oxide is involved in the vasodilatation caused by AM and its metabolite. Therapeutic concentrations enhanced endothelial nitric oxide synthase in human endothelial cells (23). Jochmann et al, (24) explained that vasodilatation developing on GTE administration is endothelial induced.
As regards CD105 immunoreaction, in overconsumption group, few spindle +ve cells were found among the epithelial lining of the follicles. In AM group, some spindle +ve cells were obvious among the epithelial lining of the follicles. On the other hand, combined therapy group recruited multiple spindle +ve cells among the epithelial lining of the follicles and multiple spindle +ve cells were observed in the interfollicular tissue. These results suggested increase in the existence of MSCs in relation to GTE therapy. On the other hand, overconsumption of GTE was associated with decrease in the existence of MSCs. Morphometric assessment of the area% of CD105 +ve cells was confirmative. In agreement, increase in the number of neural stem cells around the damaged area after rat traumatic brain injury was reported (25). Recently, it was proved that GTE catechins affect the activators of colony-stimulating factors in mesenchymal stromal cells (26).
It could be concluded that endogenous SCs activation was proved in AM and GTE combined therapy group with regression of AM induced histological, immunohistochemical, morphometric and serological changes. However, overconsumption of GTE recruited endogenous SCs suppression.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Morris D, Cameron N. Did you know? Why amiodarone requires diligent monitoring? Can J Cardiovasc Nurs. 2011;21:3–6. [PubMed] [Google Scholar]
- 2.Ahmed S, Van Gelder IC, Wiesfeld AC, Van Veldhuisen DJ, Links TP. Determinants and outcome of amiodarone-associated thyroid dysfunction. Clin Endocrinol (Oxf) 2011;75:388–394. doi: 10.1111/j.1365-2265.2011.04087.x. [DOI] [PubMed] [Google Scholar]
- 3.Jówko E, Sacharuk J, Balasińska B, Ostaszewski P, Charmas M, Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res. 2011;31:813–821. doi: 10.1016/j.nutres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Bajerska J, Wozniewicz M, Jeszka J, Drzymala-Czyz S, Walkowiak J. Green tea aqueous extract reduces visceral fat and decreases protein availability in rats fed with a high-fat diet. Nutr Res. 2011;31:157–164. doi: 10.1016/j.nutres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Hsu YW, Tsai CF, Chen WK, Huang CF, Yen CC. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food Chem Toxicol. 2011;49:2624–2630. doi: 10.1016/j.fct.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Rohde J, Jacobsen C, Kromann-Andersen H. Toxic hepatitis triggered by green tea. Ugeskr Laeger. 2011;173:205–206. [PubMed] [Google Scholar]
- 7.Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res. 2012;56:1292–1303. doi: 10.1002/mnfr.201200035. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br J Nutr. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- 9.Chan PC, Ramot Y, Malarkey DE, Blackshear P, Kissling GE, Travlos G, Nyska A. Fourteen-week toxicity study of green tea extract in rats and mice. Toxicol Pathol. 2010;38:1070–1084. doi: 10.1177/0192623310382437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolettis TM, Agelaki MG, Baltogiannis GG, Vlahos AP, Mourouzis I, Fotopoulos A, Pantos C. Comparative effects of acute vs. chronic oral amiodarone treatment during acute myocardial infarction in rats. Europace. 2007;9:1099–1104. doi: 10.1093/europace/eum196. [DOI] [PubMed] [Google Scholar]
- 11.Kiernan JA. Histological and histochemical methods: Theory and Practice. 3rd ed. New York & New Delhi Arnold Publisher; London: 2001. pp. 111–162. [Google Scholar]
- 12.Bancroft JD, Gamble M. Connective tissue stains. In: Theory and Practice of Histological Techniques. 6th ed. Elsevier Health Sciences, Churchill Livingstone; Edinburgh, London, Oxford, New York, Philadelphia, St Louis, Sydney and Toronto: 2008. p. 150. [Google Scholar]
- 13.Fan ZX, Lu Y, Deng L, Li XQ, Zhi W, Li-Ling J, Yang ZM, Xie HQ. Placenta- versus bone-marrow-derived mesenchymal cells for the repair of segmental bone defects in a rabbit model. FEBS J. 2012;279:2455–2465. doi: 10.1111/j.1742-4658.2012.08625.x. [DOI] [PubMed] [Google Scholar]
- 14.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 15.Speciale A, Chirafisi J, Saija A, Cimino F. Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med. 2011;11:770–789. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto Y, Mikuriya H, Tayama K, Takahashi H, Nagasawa A, Yano N, Yuzawa K, Ogata A, Aoki N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch Toxicol. 2001;75:591–596. doi: 10.1007/s00204-001-0286-6. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Van Gelder IC, Wiesfeld AC, Van Veldhuisen DJ, Links TP. Determinants and outcome of amiodarone-associated thyroid dysfunction. Clin Endocrinol (Oxf) 2011;75:388–394. doi: 10.1111/j.1365-2265.2011.04087.x. [DOI] [PubMed] [Google Scholar]
- 18.Narayana SK, Woods DR, Boos CJ. Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab. 2011;2:115–126. doi: 10.1177/2042018811398516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain DP, Pancholi SS, Patel R. Synergistic antioxidant activity of green tea with some herbs. J Adv Pharm Technol Res. 2011;2:177–183. doi: 10.4103/2231-4040.85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdeen SM, Mathew TC, Dashti HM, Asfar S. Protective effects of green tea on intestinal ischemia-reperfusion injury. Nutrition. 2011;27:598–603. doi: 10.1016/j.nut.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Yang TH, Cho HY. Antifibrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J Gastroenterol. 2009;15:5200–5205. doi: 10.3748/wjg.15.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishida S, Nakajima T, Ma J, Jo T, Imuta H, Oonuma H, Iida H, Taka H, Morita T, Nagai R. Amiodarone and N-desethylamiodarone enhance endothelial nitric oxide production in human endothelial cells. Int Heart J. 2006;47:85–93. doi: 10.1536/ihj.47.85. [DOI] [PubMed] [Google Scholar]
- 24.Jochmann N, Lorenz M, Krosigk Av, Martus P, Böhm V, Baumann G, Stangl K, Stangl V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr. 2008;99:863–868. doi: 10.1017/S0007114507838992. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Imano M, Nishida S, Tsubaki M, Mizuguchi N, Hashimoto S, Ito A, Satou T. (-)-Epigallocatechin-3-gallate increases the number of neural stem cells around the damaged area after rat traumatic brain injury. J Neural Transm. 2012;119:877–890. doi: 10.1007/s00702-011-0764-9. [DOI] [PubMed] [Google Scholar]
- 26.Zgheib A, Lamy S, Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony- stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem. 2013;288:13378–13386. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]


