Abstract
Background and Objectives:
The myocyte death that follows intestinal ischemia reperfusion (I/R) injury is a major factor contributing to high mortality and morbidity in ischemic heart disease. The purpose of stem cell (SC) therapy for myocardial infarction is to improve clinical outcomes. The present study aimed at investigating the possible therapeutic effect of intravenous human cord blood mesenchymal stem cells (HCBMSCs) on intestinal ischemia reperfusion induced cardiac muscle injury in albino rat.
Methods and Results:
Thirty male albino rats were divided equally into control (Sham-operated) group, I/R group where rats were exposed to superior mesenteric artery ligation for 1 hour followed by 1 hour reperfusion. In SC therapy group, the rats were injected with HCBMSCs into the tail vein. The rats were sacrificed four weeks following therapy. Cardiac muscle sections were exposed to histological, histochemical, immunohistochemical and morphometric studies. In I/R group, multiple fibers exhibited deeply acidophilic sarcoplasm with lost striations and multiple fibroblasts appeared among the muscle fibers. In SC therapy group, few fibers appeared with deeply acidophilic sarcoplasm and lost striations. Mean area of muscle fibers with deeply acidophilic sarcoplasm and mean area% of fibroblasts were significantly decreased compared to I/R group. Prussion blue and CD105 positive cells were found in SC therapy group among the muscle fibers, inside and near blood vessels.
Conclusions:
Intestinal I/R induced cardiac muscle degenerative changes. These changes were ameliorated following HCBMSC therapy. A reciprocal relation was recorded between the extent of regeneration and the existence of undifferentiated mesenchymal stem cells.
Keywords: Mesenchymal stem cells, Ischemia reperfusion, Cord blood, Cardiac injury
Introduction
Intestinal IR injury is an important problem in many situations, such as abdominal aortic aneurysm surgery, cardiopulmonary bypass, strangulated hernias, neonatal necrotizing enterocolitis, intestinal transplantation, and hemorrhagic or septic shock (1). Histopathological scores (HPS) were evaluated in biopsies of the right kidney, heart and colon following intestinal ischemia-reperfusion injury (IIR) (2). The myocyte death that follows acute myocardial ischemia and subsequent reperfusion (I/R) injury is a major factor contributing to high mortality and morbidity in ischemic heart disease. Echocardiography and hemodynamic measurements were used to assess post-I/R cardiac function, which correlated with infarct sizes (3)
The purpose of stem cell therapy for myocardial infarction is to improve clinical outcomes. Autologous bonemarrow-derived stem cells were administered in patients with acute myocardial infarction. Ischemia-related outcomes recorded reduced heart failure consequences more than tenfold following administration (4). Intramyocardial delivery of bone marrow stem-cells resulted in improvement of quality of life during follow-up of coronary artery disease. One year after the procedure, myocardial thickening was observed in cardiac magnetic resonance imaging (5).
The mesenchymal stem cells (MCSs) derived from umbilical cord tissue have low immunogenicity and contain few immune cells. Previously, studies demonstrated that human umbilical cord MSC therapy in Rhesus Monkey could be induced to differentiate into various types of cells (6).
The present study aimed at investigating the possible therapeutic effect of intravenous human cord blood mesenchymal stem cells on intestinal ischemia reperfusion induced cardiac muscle injury in albino rat.
Materials and Methods
Thirty male albino rats weighing 150∼200 g were used and divided into 3 groups placed in separate cages. The animals were kept under good hygienic conditions, fed ad libitum and allowed for free water supply. The experiment was performed in the Animal House of Kasr Al Ainy, Faculty of Medicine, Cairo University. The rats were treated in accordance with guidelines approved by the Animal Use Committee of Cairo University. The rats were divided into the following groups:
Control group: 10 Sham-operated rats each underwent laparotomy without performing superior mesenteric artery occlusion (2). To induce anesthesia, ketamine hydrochloride (Ketalar; Parke Davis, Barcelona, Spain) at 35 mg/kg was injected into the gluteus maximus muscle of the animal. Using aseptic techniques, a 3 cm longitudinal skin incision was performed. The fascia and skin were sutured (7). Betadine was applied to the wound site and rinsing with normal saline was performed at the site of injury on a daily basis. Aseptic dressing was applied on the wound daily.
Ischemia reperfusion group (I/R group): 10 rats each underwent laparotomy followed by exposure and ligation of superior mesenteric artery using a silk ligature for 1 hour followed by reperfusion (2). Induction of anesthesia, aseptic techniques, suturing (7) and care of the wound were performed in the same way as in Sham operated group.
Stem cell (SC) therapy group: 10 rats each underwent laparotomy followed by exposure and ligation of superior mesenteric artery using a silk ligature for 1 hour followed by reperfusion (2). Induction of anesthesia, aseptic techniques, suturing (7) and care of the wound were performed in the same way as in sham operated group. They were injected with 0.5 ml of cultured and labeled human mesenchymal stem cells (HMSCs) suspended in phosphate buffer saline (PBS) in the tail vein (8). Stem cells were isolated from cord blood (9). Cord blood collection was performed at the Gynaecology Department, Faculty of Medicine, Cairo University. Stem cell isolation, culture, labeling and phenotyping were performed at Hematology Unit, New Kasr El Aini Teaching Hospital.
Cord blood collection (10)
The storage and transport temperature was 15∼22℃, transport time was 8∼24 hours, sample volume was 65∼ 250 ml. No sample had signs of coagulation or hemolysis.
Mononuclear cell fraction isolation (10)
The mononuclear cell fraction (MNCF) was isolated by carefully loading 30 ml of whole blood onto 10 ml of Ficoll density media (Healthcare Bio-Sciences) in 50 ml polypropylene tubes. Centrifuge for 30 minutes at room temperature at 450×g and the interphase collected after aspirating and discarding the supernatant. The interphase was washed with 20 ml PBS and centrifuged at 150×g for 5 minutes at room temperature. The supernatant was aspirated and the cells were washed with PBS a second time. The cells were re-suspended in the isolation media to prevent adherence of monocytic cells. The isolation media was low-glucose DMEM (Dulbecco's modified Eagles medium) (Cambrex Bio Science, Minnesota, USA), penicillin (100 IU/ml) (Invitrogen), streptomycin (0.1 mg/ml) and ultraglutamine (2 mM) (Cambrex Bio-Science). Incubation was at 38.5℃ in humidified atmosphere containing 5% CO2.
Culture (10)
The isolation media were replaced after overnight incubation (12∼18 hours) in order to remove non-adherent cells. The media were replaced every 3 days until MSC colonies were noted. The cultures were inspected daily for formation of adherent spindle-shaped fibroblastoid cell colonies. Sub-culturing was done by chemical detachment using 0.04% trypsin. Later, when cell numbers allowed expansion was done in 25 cm2 or 75 cm2 tissue culture flasks.
Labeling (11)
Mesenchymal stem cells were labeled by incubation with ferumoxides injectable solution (25 microgramFe/ml, Feridex, Berlex Laboratories) in culture medium for 24 hours with 375 nanogram/ml poly L lysine added 1 hour before cell incubation. Labeling was histologically assessed using Prussian blue. Feridex labeled MSCs were washed in PBS, trypsinized, washed and resuspended in 0.01 Mol/L PBS at concentration of 1×1,000,000 cells/ml.
Cell viability analysis
Cell viability was done using trypan blue dye exclusion test. This method is based on the principle that viable cells do not take up certain dyes, whereas dead cells do.
Flow cytometry (12)
Flow cytometric analyses were performed on a Fluorescence Activated Cell Sorter (FACS) flow cytometer (Coulter Epics Elite, Miami, FL, USA). HMSC were trypsinized and washed twice with PBS. A total number of 1×100,000 HMSC were used for each run. To evaluate the HMSC marker profile, cells were incubated in 100 μ l of PBS with 3 μ l of CD105-FITC for 20 min at room temperature. Antibody concentration was 0.1 mg ml-1. Cells were washed twice with PBS and finally diluted in 200 μ l of PBS. The expression of surface marker was assessed by the mean fluorescence. CD105 (mesenchymal stem cell marker), CD133 (early hematopoietic & endothelial progenitor stem cell marker) and CD45 (panleucocytic marker) were also used. The percentage of cells positive for CD105 was determined by subtracting the percentage of cells stained non-specifically with isotype control antibodies.
The rats were sacrificed using lethal dose of ether 4 weeks following therapy. A midline incision was performed followed by thoracotomy. Cardiac muscle specimens were obtained, fixed in 10% formol saline for 48 hours, paraffin blocks were prepared and 5μ m thick sections were subjected to the following studies.
Histological study
Hematoxylin and eosin (H&E) stain (13).
Histochemical study
Prussian blue (Pb) stain (14) for demonstration of iron oxide labeled therapeutic stem cells.
Immunohistochemical study
CD105 immunostaining (15) the marker for HMSCs. 0.1 ml prediluted primary antibody CD105 rabbit polyclonal Ab (ab27422) and incubate at room temperature in moist chamber for 60 minutes. Tonsil used as positive control specimens. Cellular localization is the cell membrane. On the other hand, one of the cardiac muscle sections was used as a negative control by passing the step of applying the primary antibody.
Morphometric study
Using Leica Qwin 500 (Leica LTD, Cambridge, UK) image analysis, assessment of the mean area (μ2) of cardiac muscle fibers exhibiting strong acidophilic sarcoplasm using interactive measurements menu was done in 10 high power fields (HPF). The mean area% of fibroblasts was estimated in 10 HPF using binary mode.
Statistical analysis (16)
Quantitative data were summarized as means and standard deviations and compared using one-way analysis-of variance (ANOVA). p-values<0.05 were considered statistically significant. Calculations were made on SPSS software.
Results
Hematoxylin and eosin (H&E) stained sections
Sections in the cardiac muscle of control rats showed transverse and longitudinal fibers with multiple capillaries in between (Fig. 1). Close observation revealed acidophilic sarcoplasm with pale nuclei. Irregular striations were seen in the longitudinal fibers (Fig. 2).
Fig. 1. Section in the cardiac muscle of a control rat showing transverse (T) and longitudinal (L) fibers. Note capillaries (c) inbetween (H&E, ×200).
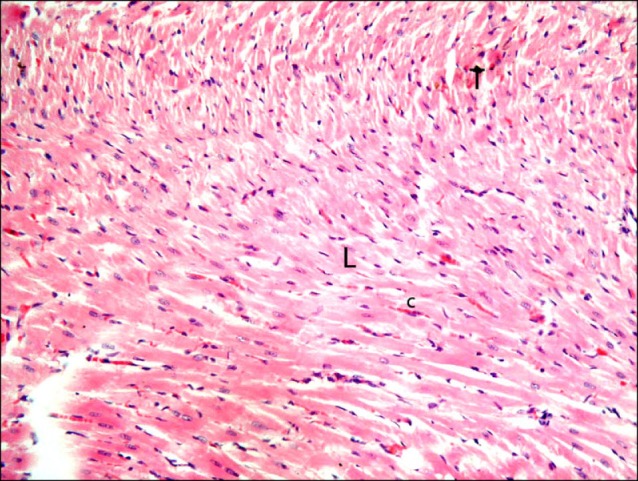
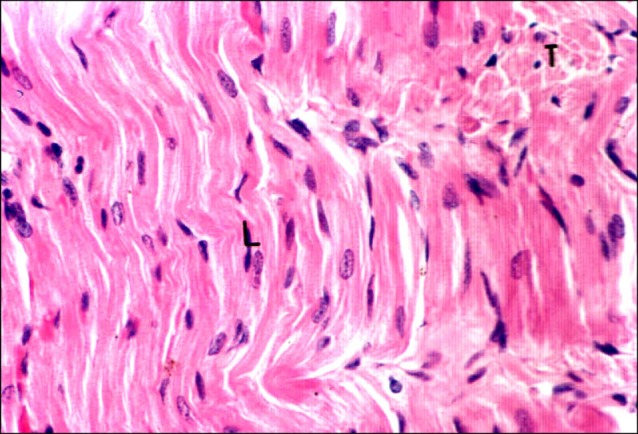
Fig. 2. Section in the cardiac muscle of a control rat showing longitudinal (L) fibers exhibiting acidophilic sarcoplasm with irregular striations and pale nuclei. Note transverse fibers (T) (H&E, ×400).
On the other hand, sections in the cardiac muscle of a rat in I/R group demonstrated multiple obviously congested capillaries between the fibers (Fig. 3). Dense mononuclear infiltration was detected in some fields among the muscle fibers (Fig. 4). Close observation revealed multiple fibers exhibiting deeply acidophilic sarcoplasm. Fibroblasts and fibrocytes were commonly found among these fibers. Normal fibers were less commonly noticed compared to the control group (Fig. 5). Closer observation demonstrated that the deeply acidophilic sarcoplasm appeared with lost striations (Fig. 6) and confirmed fibroblastic and fibrocytic infiltration (Fig. 7).
Fig. 3. Section in the cardiac muscle of a rat in I/R group showing multiple obviously congested capillaries (cc) between the fibers (H&E, ×200).
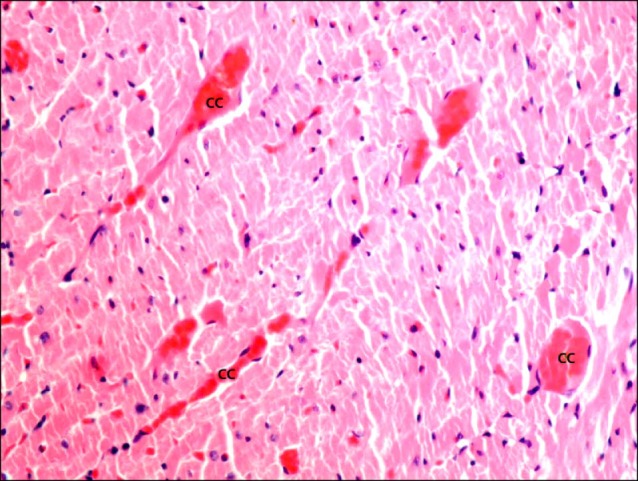
Fig. 4. Section in the cardiac muscle of a rat in I/R group showing dense mononuclear infiltration (I) among the muscle fibers (H&E, ×200).
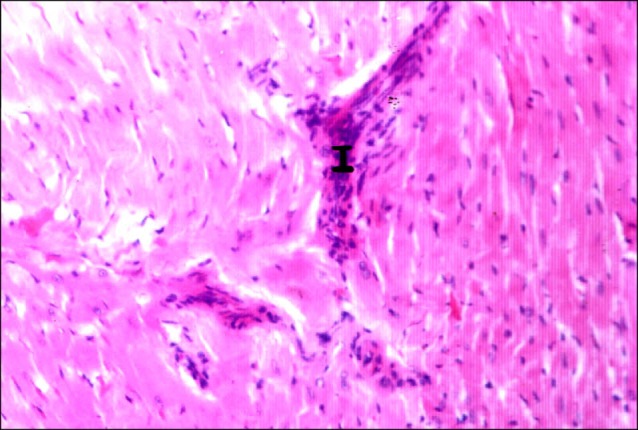
Fig. 5. Section in the cardiac muscle of a rat in I/R group showing multiple fibers exhibiting deeply acidophilic sarcoplasm (D) and surrounded by few normal fibers (N). Note multiple fibroblasts and fibrocytes (F) among the fibers (H&E ×400).
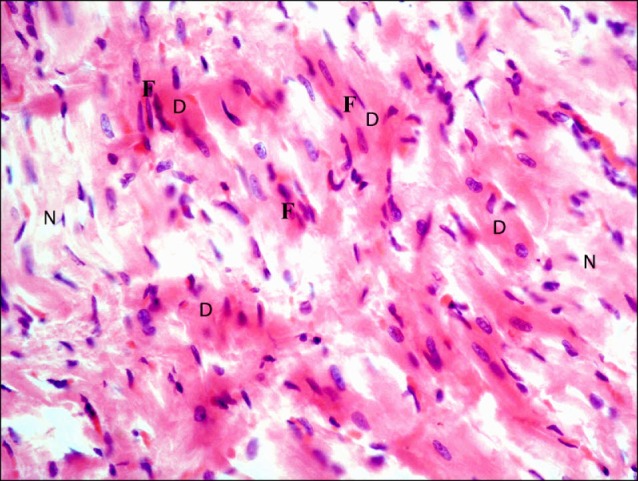
Fig. 6. Section in the cardiac muscle of a rat in I/R group showing a muscle fiber with deeply acidophilic sarcoplasm (D) with lost striations. Note adjacent normal (N) fibers and an obviously congested capillary (cc) (H&E, ×1,000).
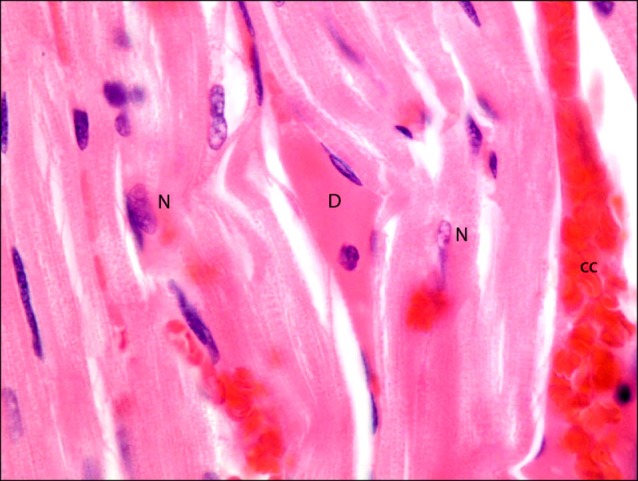
Fig. 7. Section in the cardiac muscle of a rat in I/R group showing multiple fibroblasts (F) among the muscle fibers (H&E, ×1,000).
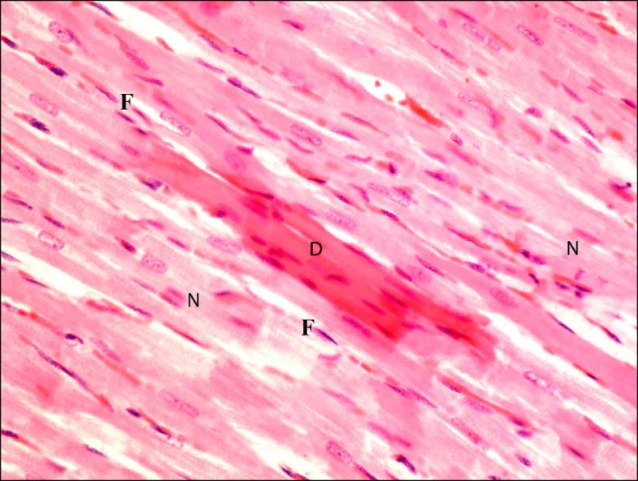
Sections in the cardiac muscle of rats in SC therapy group showed some obviously congested capillaries between the fibers (Fig. 8). Occasional fibers exhibiting deeply acidophilic sarcoplasm and surrounded by multiple normal fibers were noticed (Fig. 9). Close observation recruited lost striations in the deeply acidophilic sarcoplasm. Occasional fibroblasts and fibrocytes were seen infiltrating the muscle fibers (Fig. 10).
Fig. 8. Section in the cardiac muscle of a rat in SC therapy group showing some obviously congested capillaries (cc) between the fibers (H&E, ×200).
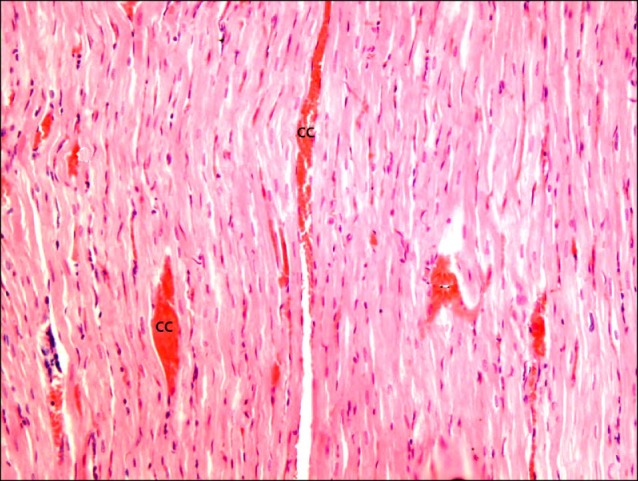
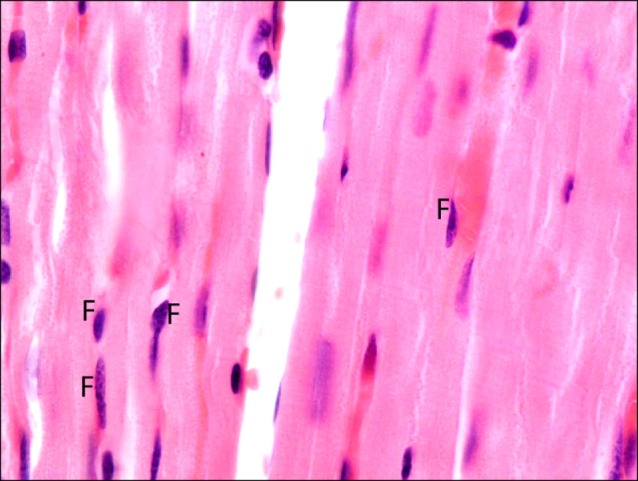
Fig. 9. Section in the cardiac muscle of a rat in SC therapy group showing a fiber exhibiting deeply acidophilic sarcoplasm (D) and surrounded by multiple normal fibers (N) (H&E, ×200).
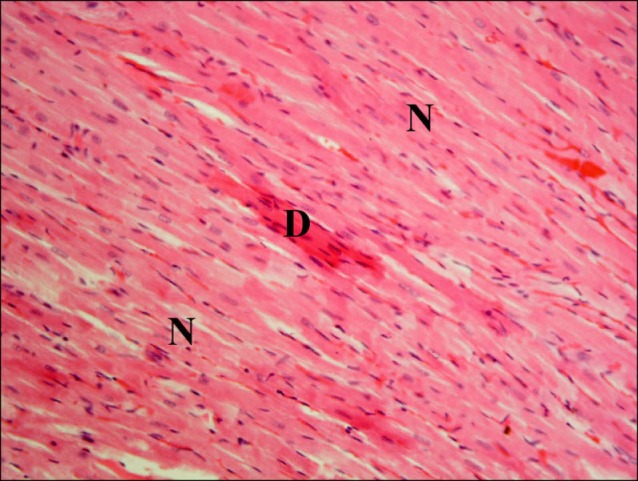
Fig. 10. Higher magnification of the previous figure showing lost striations in the deeply acidophilic sarcoplasm (D) of a muscle fiber surrounded by multiple normal fibers (N). Note few fibroblasts and fibrocytes (F) among the fibers (H&E, ×400).
Prussian blue stained sections
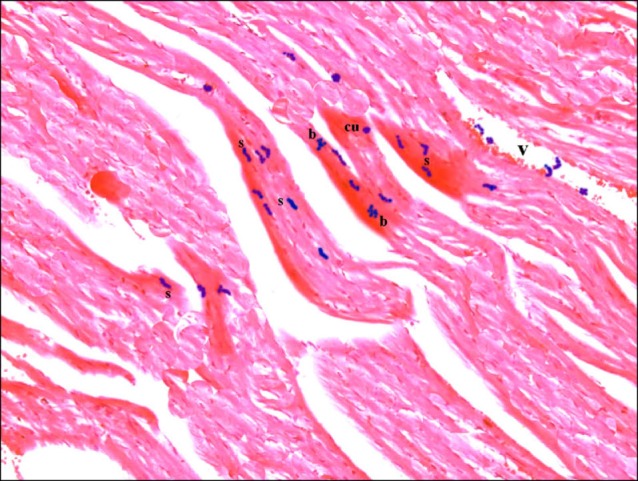
Sections in the cardiac muscle of control rats showed negative reaction (Fig. 11). In SC therapy group, multiple spindle, few cuboidal and branched PB+ve cells were evident inside and near blood vessels (Fig. 12).
Fig. 11. Section in the cardiac muscle of a control rat showing negative reaction (Prussian blue, ×400).
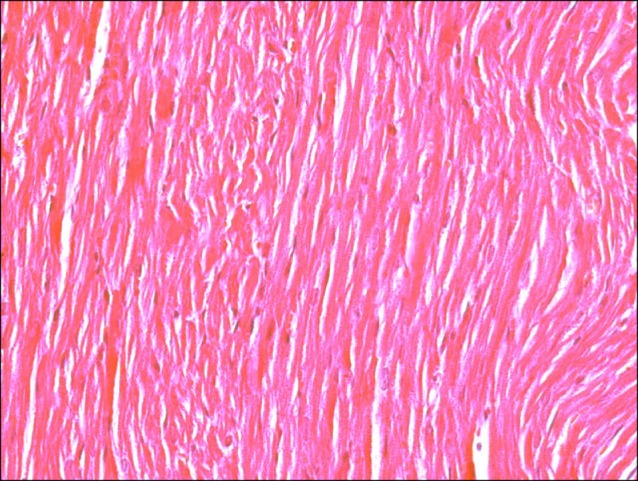
Fig. 12. Section in the cardiac muscle of a rat in SC therapy group showing multiple spindle (s), few cuboidal (cu) and branched (b) PB+ve cells inside and near a blood vessel (v) (Prussian blue, ×400).
CD105 immunostained sections
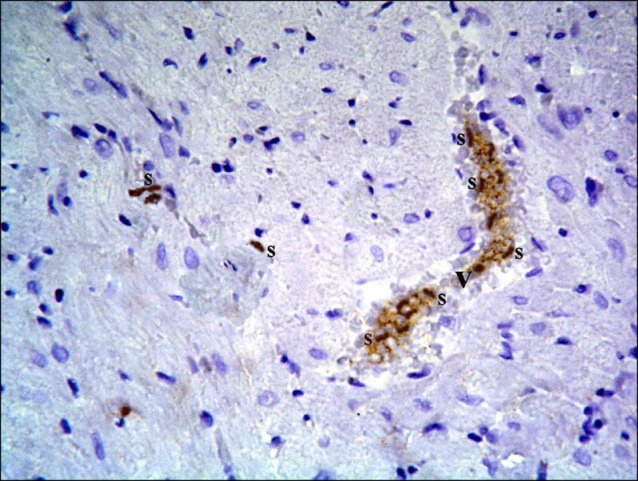
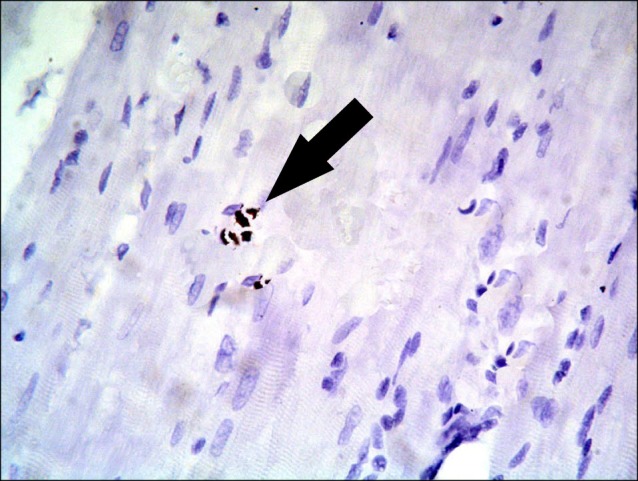
Sections in the cardiac muscle of control rats revealed negative immunostaining (Fig. 13). In SC therapy group, multiple spindle and cuboidal CD105 +ve cells were found among the muscle fibers (Fig. 14), inside and near blood vessels (Fig. 15). Few spindle CD105 +ve cells were detected among more or less normal muscle fibers (16).
Fig. 13. Section in the cardiac muscle of a control rat showing negative immunostaining (CD105 immunostaining, ×400).
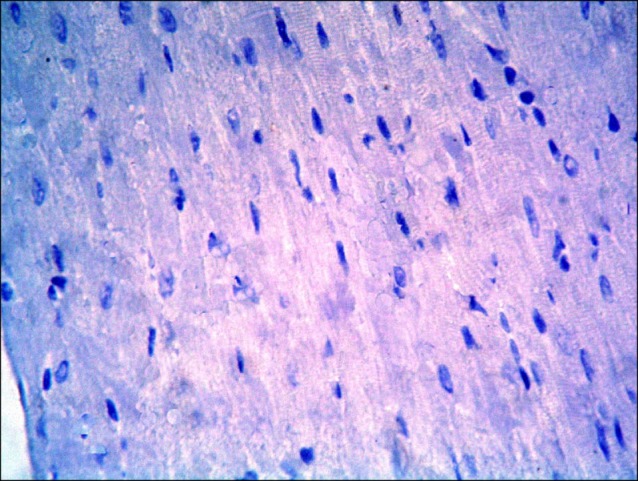
Fig. 14. Section in the cardiac muscle of a rat in SC therapy group showing multiple spindle (s) and cuboidal (cu) CD105 +ve cells among the muscle fibers (CD105 immunostaining, ×400).
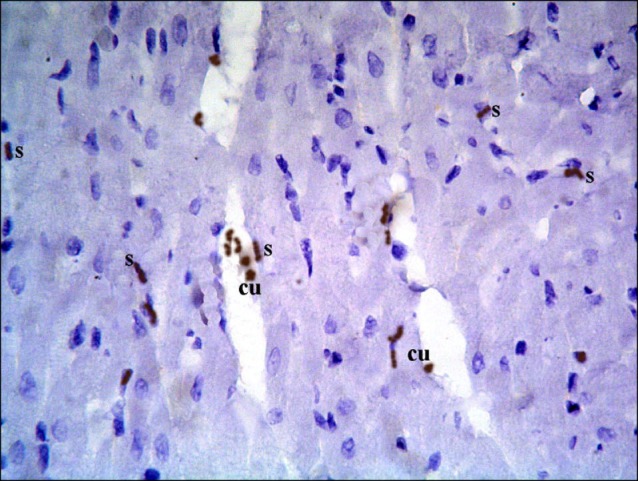
Fig. 15. Section in the cardiac muscle of a rat in SC therapy group showing multiple spindle (s) CD105 +ve cells inside and near a blood vessel (v) (CD105 immunostaining, ×400).
Morphometric results
The mean area (μ2) of cardiac muscle fibers with deeply acidophilic sarcoplasm and the mean area% of fibroblasts recorded a significant (p<0.05) decrease in SC therapy group compared to I/R group (Table 1).
Table 1.
Mean area of muscle fibers with deeply acidophilic sarcoplasm and mean area% of fibroblasts
| Groups | Mean area of muscle fibers with deeply acidophilic sarcoplasm | Mean area% of fibroblasts |
|---|---|---|
|
| ||
| Control (Sham-operated) group | - | - |
| I/R group | 3225.81±198.07 | 16.2±2.84 |
| SC therapy group | 1329.88±308.03a | 4.4±0.89a |
aSignificant (p<0.05).
Discussion
The current study demonstrated ameliorating effect of HCBMSC therapy on intestinal IR induced cardiac muscle injury in an experimental model of albino rat. This was evidenced by histological, histochemical, immunohistochemical and morphometric methods.
In the present study, sections in the cardiac muscle of a rat in I/R group demonstrated multiple obviously congested capillaries. Dense mononuclear infiltration was also detected among the muscle fibers. It was proved that elevated inflammatory response levels were observed in the cortex after cerebral ischemia-reperfusion (17). It was recorded that increased nitric oxide production was related to congestion of reperfused myocardium (18).
Multiple fibers exhibited deeply acidophilic sarcoplasm with lost striations, indicating degeneration. The previous findings detected in I/R group may be related to release of endogenous endothelin during acute myocardial infarction mediated by ischemia/reperfusion (I/R) injury. Consequent increased intracellular calcium concentration and apoptosis were noted (19). In addition, I/R was found to reduce the myocardial viability and cardiac function which was related to inhibition of mitochondrial ATP (20).
Fibroblasts and fibrocytes were commonly found among the degenerated fibers in I/R group. This finding can correlate to morphological and functional changes from acute myocardial infarction to chronic scar transformation (21).
Sections in the cardiac muscle of rats in SC therapy group showed some obviously congested capillaries between the fibers. Occasional fibers exhibited deeply acidophilic sarcoplasm, this was proved by a significant decrease in the mean area of degenerated fibers compared to I/R group. Occasional fibroblasts and fibrocytes were seen infiltrating the muscle fibers, this was also confirmed by a significant decrease in the mean area% of fibroblasts compared to I/R group. In agreement, intramyocardial MSCs injection was proved to reduce inflammation, fibrosis or apoptosis in myocardial ischemia (22). Progenitor cells (PCs) home the myocardium in response to ischemia. Cell adhesion markers, integrins play an important role in the trafficking of stem cells to myocardium. In addition, damaged myocardium secretes several chemokines and growth factors that recruit these precursor cells to the heart. This may indicate efficient use of stem cells for therapeutic benefit (23). It was mentioned that PCs exhibit markedly enhanced anti-apoptotic and antioxidative capabilities (24). It was also reported that skeletal myoblast engraftment may induce therapeutic vascularization in patients with myocardial ischemia (25). Intramyocardial delivery of autologous CD34+ cells lead to improvement of functional capacity in patients with refractory angina (26). Cardiac SCs were used as a regenerative substrate to repair the injured myocardium (27).
In SC therapy group, multiple spindle, few cuboidal and branched PB+ve and cuboidal CD105 +ve cells were evident among degenerated muscle fibers, inside and near blood vessels. Few spindle CD105 +ve cells were detected among more or less normal muscle fibers. These findings indicated differentiation of MSCs into repaired cardiac myocytes.
It could be concluded that intestinal I/R induced cardiac muscle degenerative changes. The morphological findings were confirmed by morphometric assessment. Human cord blood mesenchymal stem cell therapy proved definite amelioration of the degenerative changes. A reciprocal relation was recorded between the extent of regeneration and the existance of undifferentiated mesenchymal stem cells.
Fig. 16. Section in the cardiac muscle of a rat in SC therapy group showing few spindle (arrow) CD105 +ve cells among normal muscle fibers (CD105 immunostaining, ×400).
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Belviranli M, Okudan N, Gökbel H, Kiyici A, Öz M, Kumak A. Cytokeratin 18 and h-FABP levels in intestinal ischemia-reperfusion injury: role of coenzyme Q10. Turk J Med Sci. 2013;43:6–11. [Google Scholar]
- 2.Varga J, Tóth S, Staško P, Tóth S Jr, Bilecová-Rabajdová M, Ostró A, Veselá J. Intestinal ischemia-reperfusion injurythe histopathological status of remote vital organs in acute and subacute phases. Ann Transplant. 2012;17:11–20. doi: 10.12659/aot.882631. [DOI] [PubMed] [Google Scholar]
- 3.Fan Q, Chen M, Zuo L, Shang X, Huang MZ, Ciccarelli M, Raake P, Brinks H, Chuprun KJ, Dorn GW 2nd, Koch WJ, Gao E. Myocardial ablation of G Protein-Coupled Receptor Kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PLoS One. 2013;8:e66234. doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardiovascular Cell Therapy Research Network (CCTRN). Szady AD, Pepine CJ, Sharma SV, Cogle CR, Perin EC, Ellis SG, Moyé LA. A critical analysis of clinical outcomes reported in stem cell trials for acute myocardial infarction: some thoughts for design of future trials. Curr Atheroscler Rep. 2013;15:341. doi: 10.1007/s11883-013-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstanty-Kalandyk J, Piątek J, Miszalski-Jamka T, Rudziński P, Walter Z, Bartuś K, Urbańczyk-Zawadzka M, Sadowski J. The combined use of transmyocardial laser revascularisation and intramyocardial injection of bone-marrow derived stem cells in patients with end-stage coronary artery disease: one year follow-up. Kardiol Pol. 2013;71:485–492. doi: 10.5603/KP.2013.0095. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, Sun M, Zhou Y, Wang W, He Z, Tang D, Lu S, Wang X, Li S, Wang W, Li H. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: potential therapeutic application for Parkinson's disease in a rhesus monkey model. PLoS One. 2013;8:e64000. doi: 10.1371/journal.pone.0064000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Lee Y, Kwon ST, Kim JO, Choi ES. Serial MR imaging of intramuscular hematoma: experimental study in a rat model with the pathologic correlation. Korean J Radiol. 2011;12:66–77. doi: 10.3348/kjr.2011.12.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YG, Hwang JW, Park SB, Shin IS, Kang SK, Seo KW, Lee YS, Kang KS. Reduction of liver fibrosis by xenogeneic human umbilical cord blood and adipose tissue-derived multipotent stem cells without treatment of an immunosuppressant. TERM. 2008;5:613–621. [Google Scholar]
- 9.Stocum DL, Zupanc GK. Stretching the limits: stem cells in regeneration science. Dev Dyn. 2008;237:3648–3671. doi: 10.1002/dvdy.21774. [DOI] [PubMed] [Google Scholar]
- 10.Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 12.Haasters F, Prall WC, Anz D, Bourquin C, Pautke C, Endres S, Mutschler W, Docheva D, Schieker M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiernan JA. Histological and Histochemical methods: Theory and Practice. 3rd ed. Arnold Publisher; Landon, New York & New Delhi: 2001. pp. 111–162. [Google Scholar]
- 14.Ellis R. Perls Prussian blue Stain Protocol, Pathology Division. Queen Elizabeth Hospita; South Australia: 2007. [Google Scholar]
- 15.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. doi: 10.1177/0962280209105014. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Tian X, Gou L, Ling X, Wang L, Feng Y, Yin X, Liu Y. Beneficial synergistic effects of concurrent treatment with theanine and caffeine against cerebral ischemia-reperfusion injury in rats. Can J Physiol Pharmacol. 2013;91:562–569. doi: 10.1139/cjpp-2012-0309. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Cha DG, Liu YL, Zhou SF. Differential effects of selective and non-selective nitric oxide synthase inhibitors on the blood perfusion of ischemia-reperfused myocardium in dogs. Med Sci Monit Basic Res. 2013;19:181–186. doi: 10.12659/MSMBR.883964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamareille S, Terwelp M, Amirian J, Felli P, Zhang XQ, Barry WH, Smalling RW. Endothelin-1 release during the early phase of reperfusion is a mediator of myocardial reperfusion injury. Cardiology. 2013;125:242–249. doi: 10.1159/000350655. [DOI] [PubMed] [Google Scholar]
- 20.Jiang C, Xia M, Wang M, Chen S. Dexmedetomidine preconditioning protects isolated rat hearts against ischemia/ reperfusion injuries and its mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:326–330. [PubMed] [Google Scholar]
- 21.Feng Y, Chen F, Xie Y, Wang H, Cona MM, Yu J, Li J, Bogaert J, Janssens S, Oyen R, Ni Y. Lipomatous metaplasia identified in rabbits with reperfused myocardial infarction by 3.0 T magnetic resonance imaging and histopathology. BMC Med Imaging. 2013;13:18. doi: 10.1186/1471-2342-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L, Yang YJ, Dong QT, Qian HY, Gao RL, Qiao SB, Shen R, He ZX, Lu MJ, Zhao SH, Geng YJ, Gersh BJ. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One. 2013;8:e65702. doi: 10.1371/journal.pone.0065702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taghavi S, George JC. Homing of stem cells to ischemic myocardium. Am J Transl Res. 2013;5:404–411. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SH, Kim JY, Yoo SY, Kwon SM. Cytoprotective effect of dieckol on human endothelial progenitor cells (hEPCs) from oxidative stress-induced apoptosis. Free Radic Res. 2013;47:526–534. doi: 10.3109/10715762.2013.797080. [DOI] [PubMed] [Google Scholar]
- 25.Weyers JJ, Schwartz SM, Minami E, Carlson DD, Dupras SK, Weitz K, Simons M, Cox TC, Murry CE, Mahoney WM Jr. Effects of cell grafting on coronary remodeling after myocardial infarction. J Am Heart Assoc. 2013;2:e000202. doi: 10.1161/JAHA.113.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Povsic TJ, Junge C, Nada A, Schatz RA, Harrington RA, Davidson CJ, Fortuin FD, Kereiakes DJ, Mendelsohn FO, Sherman W, Schaer GL, White CJ, Stewart D, Story K, Losordo DW, Henry TD. A phase 3, randomized, double- blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013;165:854–861. doi: 10.1016/j.ahj.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Koudstaal S, Jansen Of Lorkeers SJ, Gaetani R, Gho JM, van Slochteren FJ, Sluijter JP, Doevendans PA, Ellison GM, Chamuleau SA. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med. 2013;2:434–443. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]


