Abstract
Hematopoietic stem cells are of therapeutic interest to the clinicians and researchers due to their promising assistance in management of malignant and inherited hematological conditions. Evaluation of cell surface markers using multiparametric flow cytometry is a well adapted qualitative measure of cells in question for many years. An artillery of these markers has been studied in hematological malignancies and related disorders. However, their role and differential expression on normal hematopoietic stem cells from clinically available sources is not always described carefully. In the present study, we attempted to evaluate expression of CD44, CD90, CD96 and CD123 in three clinically available sources of normal HSCs (Hematopoietic stem cells). Sources of HSCs in the present study involved umbilical cord blood (UCB), normal bone marrow (NBM) and bone marrow from idiopathic thrombocytopenic purpura (ITP) patients (IBM). CD44 is an important homing receptor while CD90 is involved in maintaining stem cell quiescent. CD96 is known to be leukemia specific marker and CD123 is involved in stem cell differentiation and survival. We observed a significant difference in expression CD44, CD90 and CD123 on normal HSCs derived from umbilical cord and ITP marrow. CD96 was highly expressed on HSCs obtained from ITP marrow. Investigating expression of these markers on normal HSCs in different niches will be helpful in correlating their function with niche condition and delineating their ‘abnormal’ expression from the normal.
Keywords: CD44, Thy-1, TACTILE, CD123, Niche, Hematopoietic stem cells
Introduction
Hematopoietic stem cells are cells which while bearing the potentiality of self renewal can also differentiate into specialized blood cells under specific biological requirements or stimulus provided by their niche. In recent years, HSCs have gained huge attention due to their obvious role in immune reconstitution and therapeutics. It is not just the ‘stemness’ of HSCs which is of concern in regenerative medicine but also their interaction with immediate microenvironment or niche that is ultimately responsible for determining their fate. A ‘niche’ is a complex dynamic organization of heterogeneous cell types and extracellular matrix components affecting the stem cells in paracrine manner. The importance of niche in enabling the stem cells to renew and differentiate into specific cell types has been beautifully described by Scadden (1). Site of hematopoiesis changes in humans over the period of gestation from embryonic yolk sac to placenta, to embryonic arota- gonad-mesonephros region (AGM), fetal liver, and finally it appears at bone marrow endosteal region post birth (2). In clinical practice; source of HSCs remain largely confined to mobilized peripheral blood, bone marrow and umbilical cord. We tried to study expression of CD44, CD90, CD96 and CD123 on HSCs form cord blood, normal and ITP bone marrow using multiparametric flow cytometry to understand nuanced commitment of these stem cells towards different niche conditions.
CD44; also known as P-gp1, H-CAM is an 85 kDa transmembrane; single chain glycoprotein; expressed on leukocytes, erythrocytes, epithelial cells and weakly on platelets belongs to an ubiquitously expressed protein family which is involved in cell-cell and cell-matrix adhesion. Primarily, CD 44 is involved in adhesion of cells to their extra cellular matrix through hyaluron. It is also a receptor for osteopontin which is a major cytokine in bone marrow niche, where upon binding; it mediates cell survival in response to IL-3 and GM-CSF through PI3-Akt signaling (3). In recent years, CD 44 is correlated with various tumors and their metastasis (4-7); however its expression on normal HSCs in clinically available sources might give useful information.
Thymocyte differentiation antigen (Thy-1) or CD90 is a small 25∼35 kDa GPI anchored glycoprotein, expressed on the surface of various stem cells and non lymphoid tissues. It is an important regulator of cell-cell and cell-matrix interaction and is involved in inflammation, wound healing and other non immunological functions such as tumor suppression, cell adhesion, migration and apoptosis signaling (8). CD90 is shown to be highly expressed on cord blood primitive cells, especially which are with high proliferation capacity; It is suggested that CD90 is involved in HSC development by mediating a negative signal which inhibits the proliferation of primitive cells (9). It is observed that leukemic stem cells with high proliferation capacity in vivo and in vitro lack the expression of CD90 (10).
CD96; also called TACTILE (T cell activated increased late response) is a type I membrane protein which belongs to immunoglobulin superfamily. CD96 was identified as a T cell receptor which is also expressed on NK cells a decade ago (11) and is known as a leukemic stem cell specific marker in AML but its normal function is yet not established (12).
CD 123or IL3Rα interacts with the common beta chain (CD131) of the IL-3, IL-5 and GM-CSF receptors to form a high affinity IL-3 receptor. Dependent on the cell type, binding of IL-3 to the high affinity IL-3 receptor heterodimer stimulates cell proliferation, differentiation or cell survival (13). CD123 is expressed at high levels only on plasmacytoid dendritic cells and basophils, but at lower levels also on monocytes, eosinophils, myeloid dendritic cells in peripheral blood. It is also expressed on subsets of hematopoietic progenitor cells (multipotent and myeloid precursors, but not lymphoid precursors) and is described as unique stem cell marker in acute myeloid leukemia (14).
Studying expression of these markers in normal stem cells from different sources might be useful in understanding stem cell biology in different niche conditions. Normal expression of these markers on HSCs obtained from clinically available sources will be helpful in differentiating normal from abnormal stem cell. In conditions such as leukemia where difference at m-RNA level has been described previously (15); an overall protein expression level might be a useful diagnostic tool. We evaluated expression of these markers on carefully gated CD45 dim to negative/ CD38-/ Lin-/ CD34+ stem cells, derived from cord blood, normal bone marrow and ITP marrow.
Material and Methods
Samples
Umbilical cord post full term delivery (36∼38 weeks) was used for obtaining cord blood samples (UCB; N=4) from Wadia Hospital. Normal bone marrow samples (NBM; N=6) were collected from discarded ribs during cardiac surgeries and marrow samples from ITP or idiopathic thrombocytopenic purpura patients (IBM; N=6) were collected at the time of routine diagnosis at KEM hospital, Mumbai. Consent was taken as per the guidelines of Institutional ethical committee. Samples were collected in heparin vacutainer in a volume of 2∼4 ml and processed within 6 hours of collection.
Reagents
Fluorochrome labeled monoclonal antibodies were used for all the experiments after proper titration and instrument settings. The list of antibodies used in here is: CD38 (FITC), CD34 (PE-Cy7), CD45 (APC-Cy7) CD44 (PE), CD90 (PE), and CD123 (PE-Cy5) from BD biosciences; human hematopoietic lineage cocktail (FITC) and CD96 (PE) were purchased from E-Biosciences. 0.1% ammonium chloride (Sigma) solution was used for RBC lysing and FACS flow sheath fluid, plain or with 1% BSA was used as washing buffer.
Data acquisition and analysis
FACS Aria version II equipped with 3 lasers and 8 filters was used in combination with FACS Diva software for data acquisition. Analysis of the data was done with the help of FACS Diva, Flowjo, MS-Excel 2007, Garphpad InStat and Dplot softwares. Optimal cytometer settings and compensation for spectral overlaps were ensured before each experiment. Data storage was done in a three step process to prevent excessive memory consumption. Data was analyzed statistically using nonparametric methods; kruskal-wallis and mann whitney tests were run in order to identify difference between all the groups and individual groups respectively. A p-value of 0.05 or less was considered to be significant for defining statistical difference.
Gating strategy and cut off setting
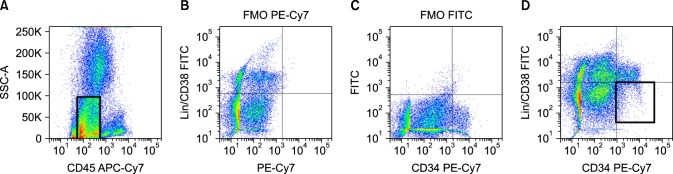
Single cells were gated on CD45 dim to negative after doublet discrimination; these events were further gated on CD34 positive, out of which minimum 300 events of Lineage negative, CD38neagtive cells were evaluated for the expression of these markers. Negative cut off for Lineage/ CD38, CD34 and markers were derived from FMO tubes wherein fluorescence contribution from all other antibodies except the one in question is observed. Gating strategy using dot plots in FACS DiVA software is shown in Fig. 1.
Fig. 1. Gating strategy & setting cut off. Events were initially gated on CD45 dim to negative and cut offs were set on the basis of FMO (fluorescence minus one) tubes. Events were further gated on Lin−/CD38−/CD34+; minimum 400 events were stored in this gate and further analyzed for expression of CD44, CD90, CD96 and CD123.
Data normalization
In order to omit normal day to day variations in instrument settings data was analyzed in the form of signal to noise ratio of median fluorescence intensity. Noise MFI was defined on the basis of unstained or FMO tube for a particular channel wherein MFI in the absence of the marker is noted, signal intensity was noted when optimum volume of a particular antibody is added together with all other gating markers. Signal to noise ratio was calculated as a measure of antigen expression on cell surface and then compared between three clinically available sources of normal HSCs.
Result and Discussion
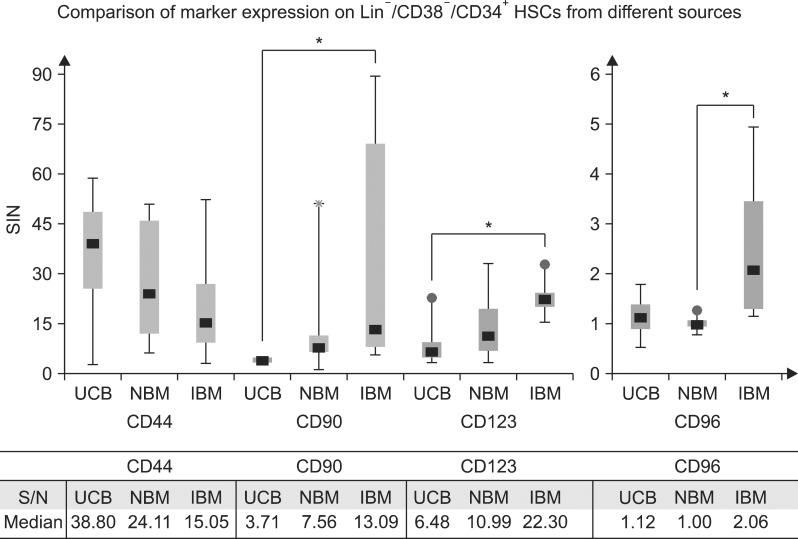
Comparison of marker expression on Lin−/CD38−/CD34+ HCSs from three different sources is shown in Fig. 2. We observed that CD44 expression was slightly higher on cord blood HSCs (median S/N: 39) compared to HSC from normal bone marrow (median S/N: 24) but there was no statistically significant difference in expression of CD44 molecule on HSCs derived from any of the three sources. CD 44 is a receptor for osteopontin which is a cytokine primarily released by osteoblasts in bone marrow niche and is known to be upregulated in vivo after increment in osteopontin concentration (16). However, osteopontin is obviously absent in umbilical cord since there are no osteoblasts and the cytokine functions in a paracrine mode on the HSCs. In this scenario; one might depict a difference in CD44 expression on HSCs derived from different sources due to the difference in niche anatomy but overall protein expression on the HSC cell surface remains statistically similar. CD90 and CD123 were found to be highly expressed on HSCs derived from ITP bone marrow when compared to the counterparts coming from cord blood. Both of these molecules were moderately expressed on HSCs from normal bone marrow samples which showed slightly higher expression than the UCB counterparts but no statistically significant difference was observed. One reason behind higher expression of CD90 and CD123 on ITP marrow HSCs can be correlated with the requirement of compensatory production of megakaryocytes which depends upon the proliferative and differentiating capacity of stem cells. Increase in CD90 expression can be correlated with differentiation of HSCs since it has been observed that lack of CD90 expression was associated with maintenance of long term proliferative capacity or quiescence of the LSCs (10). IL-3 is an important cytokine in hematopoiesis and is required for production of megakaryocytes, erythroid and B lymphoid cells (17). CD123 is a low affinity receptor for IL-3; binding to which is observed to be a crucially important event in receptor activation and downstream signaling (18).
Fig. 2. Comparison of marker expression on HSCs from different sources. Ratio of signal to noise was calculated for median fluorescence intensity for each marker and then was compared in different category of HSC source (UCB: umbilical cord blood; NBM: normal bone marrow; IBM: ITP bone marrow). *A significant difference between two groups (p value is less than 0.05 by Mann Whitney U test).
In the present study all other molecules except CD96 expressed similarly on HSCs from normal and ITP marrow. CD96 was found to be highly expressed on HSCs from ITP marrow when compared to normal marrow HSCs. Function of CD96 is yet not well understood in normal, however; mice experiments suggest an important role in leukemic stem cell engraftment or adhesion to the matrix (12). Further studies involving CD96 and ITP physiology might provide insight of normal CD96 functions. Niche biology plays an important role in an overall fate of stem cell; bone marrow and cord blood are two different microenvironments based on their anatomy, availability of oxygen, cytokines and cell signaling (19). In recent years there are increased efforts in studying UCB progenitors in bone marrow environment due to their obvious ease of availability for therapeutic purpose (20). Studying cell surface markers and their signaling role in these two niches might provide clues for improving area of regenerative medicine.
Potential conflict of interest
The authors have no conflicting financial interest.
Acknowledgments
We sincerely thank Dr. Prashant Mishra, Dr. Supreet and CVTC unit, KEM hospital for providing normal bone marrow samples. Dr. Farah Jijina, Dr. Mallikarjun, Dr. Chandrakala and their team was extremely helpful in providing ITP bone marrow. Dr. Suchitra Surve, Mr. Prasad Taur and staff at Wadia maternity hospital, Mumbai were crucially important in mobilizing cord blood samples. We would like to thank Dr. Sulabha Pathak, Tata Institute of Fundamental Research for providing Dplot software.
References
- 1.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 2.Chotinantakul K, Leeanansaksiri W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012;2012:270425. doi: 10.1155/2012/270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YH, Huang CJ, Chao JR, Chen ST, Lee SF, Yen JJ, Yang-Yen HF. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin- 3 or granulocyte-macrophage colony-stimulating factor. Mol Cell Biol. 2000;20:2734–2742. doi: 10.1128/MCB.20.8.2734-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auvinen P, Tammi R, Kosma VM, Sironen R, Soini Y, Mannermaa A, Tumelius R, Uljas E, Tammi M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int J Cancer. 2013;132:531–539. doi: 10.1002/ijc.27707. [DOI] [PubMed] [Google Scholar]
- 5.Fang YJ, Zhang L, Wu XJ, Lu ZH, Li JB, Ou QJ, Zhang MF, Ding PR, Pan ZZ, Wan DS. Impact of ERβ and CD44 expression on the prognosis of patients with stage II colon cancer. Tumour Biol. 2012;33:1907–1914. doi: 10.1007/s13277-012-0451-y. [DOI] [PubMed] [Google Scholar]
- 6.Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–32824. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RJ, Helbig KJ, Van der Hoek KH, Seth D, Beard MR. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World J Gastroenterol. 2012;18:3389–3399. doi: 10.3748/wjg.v18.i26.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 9.Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410–2417. [PubMed] [Google Scholar]
- 10.Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–3112. [PubMed] [Google Scholar]
- 11.Wang PL, O'Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol. 1992;148:2600–2608. [PubMed] [Google Scholar]
- 12.Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Woodcock JM, Rapoport A, Stomski FC, Korpelainen EI, Bagley CJ, Goodall GJ, Smith WB, Gamble JR, Vadas MA, Lopez AF. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor alpha-chain and functions as a specific IL-3 receptor antagonist. Blood. 1996;87:83–92. [PubMed] [Google Scholar]
- 14.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone A, Najima Y, Ozawa H, Wake A, Taniguchi S, Shultz LD, Ohara O, Ishikawa F. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra9. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marroquin CE, Downey L, Guo H, Kuo PC. Osteopontin increases CD44 expression and cell adhesion in RAW 264.7 murine leukemia cells. Immunol Lett. 2004;95:109–112. doi: 10.1016/j.imlet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwakura I, Inanami O, Abe Y, Satoh K, Takahashi TA, Kuwabara M. Regeneration of megakaryocytopoiesis and thrombopoiesis in vitro from X-irradiated human hematopoietic stem cells. Radiat Res. 2006;166:345–351. doi: 10.1667/RR3595.1. [DOI] [PubMed] [Google Scholar]
- 18.Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Thierfelder WE, Kreider B, Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 19.Dao MA, Creer MH, Nolta JA, Verfaillie CM. Biology of umbilical cord blood progenitors in bone marrow niches. Blood. 2007;110:74–81. doi: 10.1182/blood-2006-08-034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmeister CC, Zhang J, Knight KL, Le P, Stiff PJ. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]