Abstract
A stem cell interacts with the neighboring cells in its environment. To maintain a living organism’s metabolism, either cell-cell or cell-environment interactions may be significant. Usually, these cells communicate with each other through biological signaling by interactive behaviors of primary proteins or complementary chemicals. The signaling intermediates offer the stem cell’s functionality on its metabolism. With the rapid advent of omics technologies, various specific markers by which stem cells cooperate with their surroundings have been discovered and established. In this article, we review several stem cell markers used to communicate with either cancer or immune cells in the human body.
Keywords: Cancer cell, Immune cell, Protein marker, Signaling cross-talk, Stem cell
Stem Cells and Their Adjacent Environments
A stem cell is generally defined as a biological cell that can divide and differentiate into diverse cell types (1). In particular, stem cells possess two distinctive features: potency and self-renewal. In mammals, adults produce some stem cells to repair injured parts of the body, while developing embryos make stem cells for specialized cell differentiation and also to maintain regenerative organs. They are highly potent for any type of tissue regeneration.
There are two species of stem cells: embryonic and adult stem cells. To date, adult cells, such as epithelial cells, are interestingly reprogrammed into stem cells with pluripotent capabilities. They are simply manufactured via an inclusion of some transcription codes (e.g., Oct3/4, Sox2, c-Myc, Klf4, Nanog, or Lin28) to adult cells (2). Table 1 depicts the process that some adult cells undertake to become induced stem cells.
Table 1.
A list of protein markers on some types of stem cells (Reproduced from R&D systemⓇ)
| Name | Species | Specification | Reference |
|---|---|---|---|
|
| |||
| Oct-3/4 | Embryonic stem cell | - POU transcription factor | 61∼65 |
| - It sustains stem cells’self-renewal and pluripotency | |||
| SSEMAs (Stage Specific Embryonic Antigens) | Embryonic stem cell | - Carbohydrate-associated | 66∼73 |
| - It controls cell surface interaction during development | |||
| CD34 | Hematopoietic stem cell | - The most critical marker | 74∼88 |
| - It exclude more primitive stem cells | |||
| CD133 | Hematopoietic stem cell | - An alternative to CD34 for HSC selection and ex vivo expansion | 89∼93 |
| ABCG2 | Hematopoietic stem cell | - ATP-binding cassette superfamily G member | 35, 94∼99 |
| - First identified in a breast cancer cell line | |||
| - It implicate a functional role in developmental stem cell biology | |||
| Sca-1 | Hematopoietic stem cell | - 18 kDa phosphatidylinositol-anchored protein | 100∼111 |
| - It is used to enrich progenitor cell populations and also regulate both B and T cell on activation | |||
| STRO-1 | Mesenchymal/Stromal stem cell | - It is a valuable Ab for the identification, isolation and functional characterization of human bone marrow stromal cell precusors | 112∼116 |
| Nestin | Neural stem cell | - A class VI intermediate filament protein | 117∼130 |
| - Its function is undefined | |||
| PSA-NCAM (polysialic acid-neural cell adhesion molecule) | Neural stem cell | - Critical for many neural developmental processes and highly polysialylated | 131∼139 |
| - It is related to synaptic rearrangement and plasticity | |||
| p75 Neurotrophin R (NTR) | Neural stem cell | - A type I transmembrane protein that belongs to the tumor necrosis factor receptor superfamily | 140∼147 |
| - It enhances responses to neurotrophin | |||
Owing to the unique characteristics that may be represented by cell potency and renewal, stem cells have been topics of great interest in therapeutic and regenerative medicine fields. They may become a main component for next-generation therapies where the injured or diseased organs of patients are replaced by new alternatives grown via stem cell methods. To understand the working mechanism of therapeutic stem cells, it might be helpful to determine the surface markers at the interfaces between stem cells and their neighbors (Fig. 1).
Fig. 1. Schematic representation of stem cell cross-talk with neighboring cells in the environment.
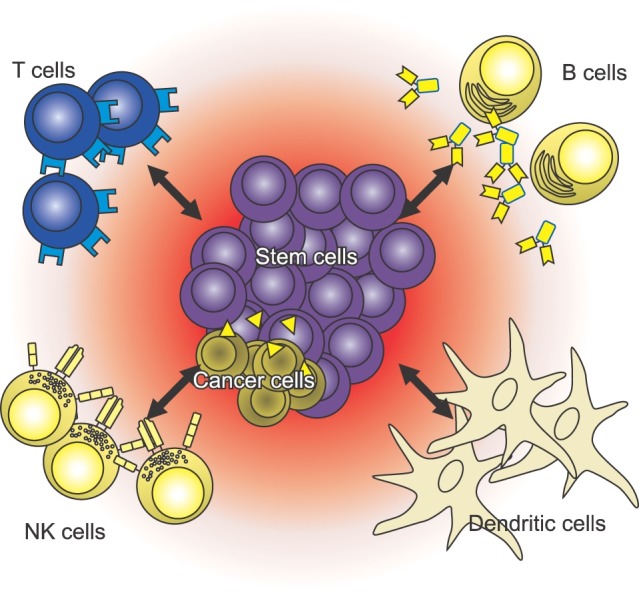
With the advent of genomics and proteomics, a variety of stem cell markers have been identified. Table 1 shows a list of genes and protein products used to identify various stem cells.
In this review, we focused more on the stem cell markers at the interface between a stem cell and its environment, especially in the following two cases: the interaction between stem cells and immune cells in the surroundings of cancerous tumors, and the interaction between stem cells and cancers.
Specific Markers for Stem Cell Cross-Talk at the Moment when the Stem Cells Come in Contact with Cancer
All cells interact with their surrounding microenvironment, including cancer cells (3). Therefore, detecting the interaction of cancer cells with their surroundings has become a powerful theragnostic method. Many studies have been conducted that have focused on the nature of tumorigenesis, which generally falls into more than one of the following mechanisms: self-sufficient proliferation, insensitivity toward growth suppressors, invasion and metastasis, angiogenesis, resistance to apoptosis, and immortality via limitless replication (4). All these mechanisms are closely related with cell-microenvironment interactions where there have been miscommunications initiated by genomic errors.
For example, certain types of cancer cells generate their own growth signals, such as transforming growth factor alpha (TGF-α). Therefore, these cancer cells upregulate the TGF-α gene. Another transforming growth factor, TGF-β, is secreted by metastatic melanoma. TGF-β allows cancer cells to hide from a person’s innate immune system by hindering the activities of natural killer cells and T lymphocytes. As a result, the tumor is not recognized as non-self by the immune system, which makes it difficult to use conventional immunotherapy to treat this type of cancer. Changes that occur in the extracellular matrix also may lead to neoplasia (5, 6).
Angiogenesis is one of many distinct characteristics of cancer cells during tumor formation. At the initial state of tumorigenesis, hypoxia occurs within the cells. Cancer cells extend their vasculature into their surroundings to provide the oxygen-rich nutrients necessary for proliferation and growth. Some studies have claimed that hypoxia leads to transcription of hypoxia-inducible factor-1 (HIF-1), which in turn promotes the expression of angiogenic factors (7, 8). The typical examples of those angiogenic factors include vascular endothelial growth factors (VEGF), fibroblast growth factors (FGF) and placenta-like growth factors (PLGF). A myriad of other factors contribute to vascular formation, even those that are not specific for the vascular endothelium (9).
As described above, no matter how and where the tumorigenesis has been initiated, genomic instability drives the corresponding characteristic gene expression, which can be understood as a way for cells to communicate with their surroundings. Therefore, examining these communication signals makes it possible to observe any differentiation of cancer cells from normal cells and even to evaluate the cancer status; numerous scientists have investigated whether the progression of preneoplasia to cancer can be detected using these signals, which include antibodies, peptides and other chemicals (10). However, these signals are not unique chemicals that only cancer cells exhibit; normal cells, too, release them into their surroundings. The distinctive feature of cancer cells is that they overexpress certain genes compared to the normal cells. This overexpressing characteristic becomes a lighthouse for targeting ligands of drug carriers, which became the core principle in active targeting drug delivery to cancer cells. For example, the luteinizing hormone-releasing hormone (LHRH) receptor is one target that could be bound by LHRH peptide, one of the targeting peptides (11). LHRH receptors are overexpressed by several types of cancer cells, including those of breast, ovarian and prostate cancer (12-14). Therefore, such cancer cells can be selectively bound by LHRH peptide, increasing the specific binding ability of drug carriers that use the LHRH peptide as a targeting ligand. In a similar fashion, SP94, one of the targeting peptides that specifically binds to unknown receptors present on the surface of human hepatocellular carcinoma, has been applied as a ligand in several drug delivery cases (15, 16). The receptor that the SP94 peptide targets is not yet specified-it has only been identified by performing a filamentous phage display, which is a powerful tool for selecting a specific peptide that has a high affinity towards certain cancer cells from a pool of random peptides.
It should be noted that certain types of cancer cells exhibit multiple characteristic signals, and these signals may overlap with those from different cancer cell types. Even cancers from the same origin may exhibit different gene overexpression trends. For example, prostate cancers overexpress LHRH receptors and also androgen receptors (AR) at the same time (17). However, while LNCaP, one of the human prostate adenocarcinomas, is androgen-sensitive, PC3, which is another type of the same cancer, does not show such sensitivity (18). Certain breast cancer cells exhibit an HER2 sensitive phenotype, while others do not.
Consequently, it is necessary to take into account the type of cancer, the degree to which the characteristic overexpression is exhibited and in what combination would multiple overexpressions be expressed to maximize the tumor target specificity when selecting a targeting material. Table 2 displays a list of targeting materials and their targeted tumors.
Table 2.
A list of targeting materials and the targeted tumor
| Target tumor | Targeting material | Target receptor | Reference |
|---|---|---|---|
|
| |||
| Human colon (HCT116) | IFLLWQR (IF7) (peptide) | Annexin 1 (anxa1) | 148 |
| B16 Melanoma | YIGSR (peptide) | Laminin receptor | 149 |
| Ovarian, breast, prostate carcinomas | LHRH peptide | LHRH receptor | 11 |
| Various cancer types | VNTANST (peptide) | Vimentin | 150,151 |
| B16F10 | RGDGW (peptide) | α5β1 integrin | 152 |
| Prostate carcinoma | F77 (mAb) | Prostate specific glycolipid (PCLA) | 153 |
| Hepatocellular carcinoma | SP94 (peptide) | Unknown | 16 |
| Hepatocellular carcinoma | FQHPSFI (HCBP1) (peptide) | Unknown | 154 |
| Lung (H640, A549 and H226) | CSNIDARAC (peptide) | EGF receptor (under research) | 155 |
| Breast cancer | CTCE-9908 | CXCR4 | 156 |
| Metastatic melanoma | SB505124 | TGF-β | 5 |
In 2002, Sooryanarayana Varambally et al. reported that a polycomb group protein enhancer of zeste homolog 2 (EZH2) was overexpressed in hormone-refractory metastatic prostate cancer (19). In addition to simply examining EZH2 overexpression, they also observed increments in the degree of overexpression as the cancer progressed from benign, prostatic atrophy, prostatic intraepithelial neoplasia, clinically localized prostate cancer, and finally metastatic prostate cancer. Therefore, this finding suggests the possibility of predicting the cancer’s progression by examining the correlation between the amounts of EZH2 protein and the aggressiveness of the type of prostate cancer.
The conventional way of delivering drugs to cancer cells has mainly been via a passive targeting method rather than through active targeting drug delivery via the characteristic biochemical signals released by cancer cells. This passive targeting also bases its principle on microenvironment features exhibited by cancer cells. As a tumor grows, angiogenesis progresses and leads to consequent abnormalities in the vasculature. One of the important features of this abnormality is fenestrated vasculature (20). Through the gaps that form from the loosened capillary vessels, small molecules, such as drugs, with sizes of less than a few nanometers may penetrate and accumulate at the tumor sites, which is called an enhanced permeability and retention (EPR) of the tumor. Regardless of how great this EPR effect from leaky vasculature near the cancer is, a majority of the drugs (>95%) still flows to and accumulates in other parts of the body, such as the liver, spleen and lungs (21). Therefore, active targeting needs to be investigated, and adequate selection for a targeting ligand will also be critical.
Specific Markers for Stem Cell Cross-Talk at the Moment When the Stem Cells Come in Contact With Immune Cells
Several diseases arise from the destruction or dysfunction of specific cells. Many attempts have been made to overcome these diseases. For instance, Parkinson’s disease (22, 23), Huntington’s disease (24), amyotrophic lateral sclerosis (25-28), Alzheimer’s disease (29), spinal cord injury (30), brain tumor (31), lysosomal storage diseases (32), liver (33) and heart failure (34), Duchenne’s muscular dystrophy (35), and osteogenesis imperfecta (36) are all target diseases of stem cell therapy. In these cases, treatment with stem cells that can differentiate into the damaged cell types can be effective.
Stem cell therapy mainly uses mesenchymal stem cells (MSC), neural stem cells (NSC) and embryonic stem cells (ESC) (37). When stem cells are delivered to the injury site or the targeted site for substituting functional cells, it is essential that they encounter the immune system of the host, including both the innate and adaptive immune system (38).
When the immune system comes in contact with forward- facing stem cells, some immune cells prepare for defense. Accordingly, T cells, B cells, dendritic cells, and NK cells create adaptive immunity against foreign materials. These immune cells play a part as well in stem cell treatments (38). Stem cells in the treatment area are recognized by activated immune cells as foreign and then become their targets. One of the most important variables is suppressing any immune rejection of stem cells (39) to maintain sufficient stem cell viability in the host system until the therapeutic effects are achieved.
Interestingly, some kinds of stem cells can overcome severe environmental conditions by suppressing the host immune system (39-41). By reducing host immune rejection, stem cells can have enough time to replicate themselves and differentiate into functional target cells. This immune suppression characteristic of stem cells has enormous potential to overcome the aforementioned serious diseases. Therefore, it is important to understand the interactions between the immune system and stem cells.
These interactions can be categorized by two different cell-to-cell communication methods. The first interaction category is based on cytokines. Most immune cells secrete their own cytokines when they run into foreign antigens or suspicious materials. The secreted cytokines warn the environmental cells, which recruit other immune cells. There are many types of cytokines, and their functions are deserving of further study (42). These cytokines affect both immune systems and treated stem cells. In injury situations or graft surgeries, accumulated or treated stem cells are exposed by cytokines of the host’s immune system. By sensing these cytokines, stem cells regulate an immunological rejection (43-47). Cytokines secreted by T cells or other immune cells would be recognized by MSC, NSC and ESC. In the case of human MSC (hMSC), co-cultured B cells are arrested in the G0/G1 phase. B cell arrest is caused by soluble factors produced by the hMSC. In addition, receptors expressed by B cell, CXCR4, CXCR5, and CXCR7 are down-regulated (48). The NSC may be affected by cytokines, such as TNF-α or IL-6 (46, 49-51). With TNF-α, proliferation of NSC depends on the signal pathways. TNF-α signal via TNFR-1 induces apoptosis of NSC. TNFR-2, however, does not inhibit NSC proliferation (41). IL-6 affects NSC in two ways. First, IL-6 mediated via gp130 improves the self-renewal of NSC (52). Moreover, IL-6 treated NSC tends to differentiate into astrocytes (46). ESC also affects cytokines. The MHC expression level of ESC differentiated by 19 days is upregulated in the presence of IFN-γ. With undifferentiated ESC, however, there was no upregulation of MHC expression. ESC can increase its immunogenic profile via differentiation processes; therefore, less immune rejection occurs with the undifferentiated state of ESC (53).
Another category of interaction is based on surface markers expressed on the surface of stem/immune cells (40, 48, 54-57). In an immune response, several interactions are mediated by surface markers, such as T cell activation by dendritic cells (58) or infection recognition by cytotoxic T cells (59). Just like interactions between immune cells, markers expressed on stem cells can reduce immune reactions. In the case of MSC, inflammatory cytokines upregulate the expression of ICAM-1 and VCAM-1. These surface markers make MSC more adhesive to T cells. T cells accumulate around MSC, and the effect of NO produced by MSC (60) becomes more powerful. Thus, these two surface markers are used to suppress T cell activation and T cell apoptosis (54-57). These kinds of interactions are organized and adjusted in Table 3.
Table 3.
The interactions between immune cells and stem cells. Here note that SC designates stem cell and IM does immune cell
| Stem cell | Immune cell | SC marker | IM marker | Cytokine | SC soluble factor | Interaction | Reference |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mesenchymal stem cell | T cell | NO | NO secreted from lisensed MSC, T cell suppression (mouse) | 60 | |||
| CXCR5 | CCR5 | MSC secrets CXCR3 and CCR5 ligand | 54 | ||||
| ICAM-1, VCAM-1 | ICAM-1 and VCAM-1 expression on MSC surface. T cell accumulation. | 55 | |||||
| naïve & memory T cell supression | 157 | ||||||
| ICAM-1, VCAM-1, LFA-3 | ICAM-1, VCAM-1, LFA-3 expression on MSC. | 56 | |||||
| Interacion with T cell | 57 | ||||||
| IDO | MSC secret IDO, T cell suppression | 158 | |||||
| 159 | |||||||
| B cell | CXCR4, CXCR5, CCR7 | B cell CXCR4, CXCR5 and CCR7 down-regulation by soluble factors from hMSC | 48 | ||||
| Dendritic cell | CD83, CD80, CD86, HLA-DR, CD1a | Suppression of CD83, HLA-DR, CD80, CD86 and CD1a of DC by MSC | 40 | ||||
| CCR7, CD49dβ1 | MSC supress CCR7 and CD49dβ1 on DC. Suppress DC immigration to lymph node. | 160 | |||||
| NK cell | NKp30, NKp44, NKG2D | IDO, prostaglandin E2 | NK receptors, NKp30,NKp44 and NKG2D, suppressed by IDO and prostaglandin E2 secreted from MSC | 161 | |||
| IDO | MSC secret IDO. NK cell suppression | 159 | |||||
| Neural stem cell | CCL2, CXCL12 | NSCs can recruit to injury site by CCL2 and CXCL12 | 162 | ||||
| CCR2 | MCP-1 | In site of ischemia, CCR2 recognize MCP-1 and migration to ischemia | 163 | ||||
| TNFR1 | TNF-α | TNF-a make p38 MAPK signaling pathway through TNFR1, cause apoptisis | 49 | ||||
| TNFR1 | TNF-α | TNF-a activate IKK-β by TNFR1, increase NSC proliferation | 50 | ||||
| TNFR1, TNFR2 | TNF-α | Suppress TNFR1, activate TNFR2. Increase neurogenesis | 51 | ||||
| CNTFR, LIFR, gp130 | Renewal of NSC increase by CNTFR/LIFR/gp130-mediated signaling | 52 | |||||
| gp130 | IL-6 | IL-6 treated NSC more differentiate into astrocyte | 46 | ||||
| IL-1βR | IL-1β | IL-1β, produced by stress or directly treated, makes antineurogenetic effect | 47 | ||||
| Embryonic stem cell | HLA-1 | ESC has low level of HLA-1, shows lower | 164 | ||||
| Immunoreject | 165 | ||||||
| HLA-1 | Immunoreject getting larger along with ESC differentiation. | 166 | |||||
| HLA-1 | IFN-γ | In case of differentiated ESC, IFN-γ increases MHC expression and immunoreject | 53 | ||||
| NK cell | Activating NK receptor | NKG2D | ESC expressing NKG2D, activate NK cell and make strong rejection | 167 | |||
Conclusion
Currently, stem-cell medicine has the potential to provide effective treatments for a wide range of human diseases. This expectation has raised a new discipline, representative of either therapeutic or regenerative medicine. To deliver on the promise of stem-cell therapy, there is a need to increase our rudimentary understanding of how stem cells interact with neighboring cells, including immune cells or infected cells.
All of the approaches outlined in this article need to be pursued in parallel; it is likely that an interactive understanding of cells will provide the best result for all situations. There is much to be learned about the immune response to stem cells and cancer infection mechanisms in stem cell areas, and there will undoubtedly be many surprises as our understanding of this area increases.
Potential conflict of interest
The authors have no conflicting financial interest.
Acknowledgments
This study was supported by a grant from the Korea Health technology R&D Project of the Ministry of Health & Welfare of the Republic of Korea (Grant No. A110552).
References
- 1.Bongso A, Lee EH. Stem cells: From Bench top to Bedside. 5. World Scientific; 2005. [Google Scholar]
- 2.Making human embryonic stem cells. The Economist; 2007. pp. 11–22. [Google Scholar]
- 3.Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron. 2008;1:69–83. doi: 10.1007/s12307-008-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor- beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 7.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 8.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 10.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 11.Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, Sinko PJ, Stein S, Farmanfarmaian A, Minko T. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci U S A. 2005;102:12962–12967. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minko T, Dharap SS, Pakunlu RI, Wang Y. Molecular targeting of drug delivery systems to cancer. Curr Drug Targets. 2004;5:389–406. doi: 10.2174/1389450043345443. [DOI] [PubMed] [Google Scholar]
- 13.Dharap SS, Qiu B, Williams GC, Sinko P, Stein S, Minko T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J Control Release. 2003;91:61–73. doi: 10.1016/S0168-3659(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 14.Dharap SS, Minko T. Targeted proapoptotic LHRH-BH3 peptide. Pharm Res. 2003;20:889–896. doi: 10.1023/A:1023839319950. [DOI] [PubMed] [Google Scholar]
- 15.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carroll NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo A, Lin CT, Wu HC. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther. 2008;7:579–589. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 17.Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. A census of amplified and overexpressed human cancer genes. Nat Rev Cancer. 2010;10:59–64. doi: 10.1038/nrc2771. [DOI] [PubMed] [Google Scholar]
- 18.Yeap BB, Wilce JA, Leedman PJ. The androgen receptor mRNA. Bioessays. 2004;26:672–682. doi: 10.1002/bies.20051. [DOI] [PubMed] [Google Scholar]
- 19.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 20.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- 21.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redmond DE Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 25.Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr DA, Lladó J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, Krishnan C, Dike S, Gearhart JD, Rothstein JD. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. 2003;23:5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 28.Garbuzova-Davis S, Willing AE, Milliken M, Saporta S, Zigova T, Cahill DW, Sanberg PR. Positive effect of transplantation of hNT neurons (NTera 2/D1 cell-line) in a model of familial amyotrophic lateral sclerosis. Exp Neurol. 2002;174:169–180. doi: 10.1006/exnr.2002.7860. [DOI] [PubMed] [Google Scholar]
- 29.Sugaya K, Brannen CL. Stem cell strategies for neuroreplacement therapy in Alzheimer's disease. Med Hypotheses. 2001;57:697–700. doi: 10.1054/mehy.2001.1424. [DOI] [PubMed] [Google Scholar]
- 30.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 32.Wynn RF, Wraith JE, Mercer J, O'Meara A, Tylee K, Thornley M, Church HJ, Bigger BW. Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr. 2009;154:609–611. doi: 10.1016/j.jpeds.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 35.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bifari F, Pacelli L, Krampera M. Immunological properties of embryonic and adult stem cells. World J Stem Cells. 2010;2:50–60. doi: 10.4252/wjsc.v2.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:1420–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 41.Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci. 2012;15:1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- 44.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 45.Widera D, Holtkamp W, Entschladen F, Niggemann B, Zänker K, Kaltschmidt B, Kaltschmidt C. MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol. 2004;83:381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- 46.Taga T, Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin Rev Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]
- 47.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 49.Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- 50.Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 54.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine- induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 57.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/S0301-472X(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 58.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 59.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 61.Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 62.Schöler HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989;8:2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 64.Niwa H, Miyazak J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 65.Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 66.Fenderson BA, Eddy EM, Hakomori S. Glycoconjugate expression during embryogenesis and its biological significance. Bioessays. 1990;12:173–179. doi: 10.1002/bies.950120406. [DOI] [PubMed] [Google Scholar]
- 67.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knowles BB, Pan S, Solter D, Linnenbach A, Croce C, Huebner K. Expression of H-2, laminin and SV40 T and TASA on differentiation of transformed murine teratocarcinoma cells. Nature. 1980;288:615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- 69.Fox N, Damjanov I, Martinez-Hernandez A, Knowles BB, Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- 70.Shevinsky LH, Knowles BB, Damjanov I, Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- 71.Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 73.Thomson JA, Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- 74.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 75.Sutherland HJ, Eaves CJ, Eaves AC, Dragowska W, Lansdorp PM. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- 76.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berenson RJ, Andrews RG, Bensinger WI, Kalamasz D, Knitter G, Buckner CD, Bernstein ID. Antigen CD34+ marrow cells engraft lethally irradiated baboons. J Clin Invest. 1988;81:951–955. doi: 10.1172/JCI113409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Civin CI, Trischmann T, Kadan NS, Davis J, Noga S, Cohen K, Duffy B, Groenewegen I, Wiley J, Law P, Hardwick A, Oldham F, Gee A. Highly purified CD34-positive cells reconstitute hematopoiesis. J Clin Oncol. 1996;14:2224–2233. doi: 10.1200/JCO.1996.14.8.2224. [DOI] [PubMed] [Google Scholar]
- 79.Link H, Arseniev L, Bähre O, Kadar JG, Diedrich H, Poliwoda H. Transplantation of allogeneic CD34+ blood cells. Blood. 1996;87:4903–4909. [PubMed] [Google Scholar]
- 80.Shpall EJ, LeMaistre CF, Holland K, Ball E, Jones RB, Saral R, Jacobs C, Heimfeld S, Berenson R, Champlin R. A prospective randomized trial of buffy coat versus CD34-selected autologous bone marrow support in high-risk breast cancer patients receiving high-dose chemotherapy. Blood. 1997;90:4313–4320. [PubMed] [Google Scholar]
- 81.Yabe H, Yabe M, Hattori K, Hinohara T, Morimoto T, Nakamura Y, Noma M, Takei M, Kobayashi N, Tsuji K, Kato S. Successful engraftment of allogeneic CD34-enriched marrow cell transplantation from HLA-mismatched parental donors. Bone Marrow Transplant. 1996;17:985–991. [PubMed] [Google Scholar]
- 82.Sutherland DR, Keating A. The CD34 antigen: structure, biology, and potential clinical applications. J Hematother. 1992;1:115–129. doi: 10.1089/scd.1.1992.1.115. [DOI] [PubMed] [Google Scholar]
- 83.Andrews RG, Singer JW, Bernstein ID. Precursors of colony- forming cells in humans can be distinguished from colony- forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989;169:1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 85.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCIDrepopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 86.Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26:353–360. [PubMed] [Google Scholar]
- 87.Nakamura Y, Ando K, Chargui J, Kawada H, Sato T, Tsuji T, Hotta T, Kato S. Ex vivo generation of CD34(+) cells from CD34(-) hematopoietic cells. Blood. 1999;94:4053–4059. [PubMed] [Google Scholar]
- 88.Sato T, Laver JH, Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- 89.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J. Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 90.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 91.Yu Y, Flint A, Dvorin EL, Bischoff J. AC133-2, a novel isoform of human AC133 stem cell antigen. J Biol Chem. 2002;277:20711–20716. doi: 10.1074/jbc.M202349200. [DOI] [PubMed] [Google Scholar]
- 92.Kobari L, Giarratana MC, Pflumio F, Izac B, Coulombel L, Douay L. CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res. 2001;10:273–281. doi: 10.1089/15258160151134980. [DOI] [PubMed] [Google Scholar]
- 93.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 95.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 96.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rocchi E, Khodjakov A, Volk EL, Yang CH, Litman T, Bates SE, Schneider E. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun. 2000;271:42–46. doi: 10.1006/bbrc.2000.2590. [DOI] [PubMed] [Google Scholar]
- 98.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.V99.2.507. [DOI] [PubMed] [Google Scholar]
- 99.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 102.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 103.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawamoto H, Ohmura K, Katsura Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int Immunol. 1997;9:1011–1019. doi: 10.1093/intimm/9.7.1011. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto Y, Yasumizu R, Amou Y, Watanabe N, Nishio N, Toki J, Fukuhara S, Ikehara S. Characterization of peripheral blood stem cells in mice. Blood. 1996;88:445–454. [PubMed] [Google Scholar]
- 106.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci U S A. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 108.Codias EK, Malek TR. Regulation of B lymphocyte responses to IL-4 and IFN-gamma by activation through Ly-6A/E molecules. J Immunol. 1990;144:2197–2204. [PubMed] [Google Scholar]
- 109.Malek TR, Ortega G, Chan C, Kroczek RA, Shevach EM. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986;164:709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Codias EK, Rutter JE, Fleming TJ, Malek TR. Down-regulation of IL-2 production by activation of T cells through Ly-6A/E. J Immunol. 1990;145:1407–1414. [PubMed] [Google Scholar]
- 111.Flood PM, Dougherty JP, Ron Y. Inhibition of Ly-6A antigen expression prevents T cell activation. J Exp Med. 1990;172:115–120. doi: 10.1084/jem.172.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 113.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- 114.Encina NR, Billotte WG, Hofmann MC. Immunomagnetic isolation of osteoprogenitors from human bone marrow stroma. Lab Invest. 1999;79:449–457. [PubMed] [Google Scholar]
- 115.Oyajobi BO, Lomri A, Hott M, Marie PJ. Isolation and characterization of human clonogenic osteoblast progenitors immunoselected from fetal bone marrow stroma using STRO-1 monoclonal antibody. J Bone Miner Res. 1999;14:351–361. doi: 10.1359/jbmr.1999.14.3.351. [DOI] [PubMed] [Google Scholar]
- 116.Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 117.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-X. [DOI] [PubMed] [Google Scholar]
- 119.Han SJ, Oh MC, Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, Parsa AT. The effect of the 2003 Consensus Reporting Standards on publications describing patients with vestibular schwannoma treated with stereotactic radiosurgery. J Clin Neurosci. 2012;19:1144–1147. doi: 10.1016/j.jocn.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 120.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 121.Frederiksen K, Jat PS, Valtz N, Levy D, McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988;1:439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 122.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 123.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 124.Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 125.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 126.Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881–9890. doi: 10.1074/jbc.274.14.9881. [DOI] [PubMed] [Google Scholar]
- 127.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 128.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin- secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 129.Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. doi: 10.1016/S0006-291X(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 130.Shih CC, Weng Y, Mamelak A, LeBon T, Hu MC, Forman SJ. Identification of a candidate human neurohematopoietic stem-cell population. Blood. 2001;98:2412–2422. doi: 10.1182/blood.V98.8.2412. [DOI] [PubMed] [Google Scholar]
- 131.Kiss JZ, Muller D. Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity. Rev Neurosci. 2001;12:297–310. doi: 10.1515/revneuro.2001.12.4.297. [DOI] [PubMed] [Google Scholar]
- 132.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity- induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/S0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 133.Theodosis DT, Bonfanti L, Olive S, Rougon G. Poulain DA. Adhesion molecules and structural plasticity of the adult hypothalamo-neurohypophysial system. Psychoneuroendocrinology. 1994;19:455–462. doi: 10.1016/0306-4530(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 134.Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/S0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 135.Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci. 1999;19:10228–10236. doi: 10.1523/JNEUROSCI.19-23-10228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Keirstead HS, Ben-Hur T, Rogister B, O'Leary MT, Dubois-Dalcq M, Blakemore WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19:7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci U S A. 2000;97:4315–4320. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kiss JZ, Troncoso E, Djebbara Z, Vutskits L, Muller D. The role of neural cell adhesion molecules in plasticity and repair. Brain Res Brain Res Rev. 2001;36:175–184. doi: 10.1016/S0165-0173(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 140.Barker PA, Murphy RA. The nerve growth factor receptor: a multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol Cell Biochem. 1992;110:1–15. doi: 10.1007/BF02385000. [DOI] [PubMed] [Google Scholar]
- 141.Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/S0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 142.Hapner SJ, Boeshore KL, Large TH, Lefcort F. Neural differentiation promoted by truncated trkC receptors in collaboration with p75(NTR). Dev Biol. 1998;201:90–100. doi: 10.1006/dbio.1998.8970. [DOI] [PubMed] [Google Scholar]
- 143.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–785. doi: 10.1016/0092-8674(92)90393-Q. [DOI] [PubMed] [Google Scholar]
- 144.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/S0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 145.Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS and PNS. Dev Biol. 1998;200:1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- 146.Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G. Mesenchymal cell precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol Reprod. 2001;64:464–472. doi: 10.1095/biolreprod64.2.464. [DOI] [PubMed] [Google Scholar]
- 147.Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- 148.Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y, Ohyama C, Fukuda M, Fukuda MN. Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc Natl Acad Sci U S A. 2011;108:19587–19592. doi: 10.1073/pnas.1105057108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sarfati G, Dvir T, Elkabets M, Apte RN, Cohen S. Targeting of polymeric nanoparticles to lung metastases by surface-attachment of YIGSR peptide from laminin. Biomaterials. 2011;32:152–161. doi: 10.1016/j.biomaterials.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 150.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther. 2011;19:1468–1477. doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Work LM, Büning H, Hunt E, Nicklin SA, Denby L, Britton N, Leike K, Odenthal M, Drebber U, Hallek M, Baker AH. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 152.Samanta S, Sistla R, Chaudhuri A. The use of RGDGWKlipopeptide to selectively deliver genes to mouse tumor vasculature and its complexation with p53 to inhibit tumor growth. Biomaterials. 2010;31:1787–1797. doi: 10.1016/j.biomaterials.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 153.Zhang G, Zhang H, Wang Q, Lal P, Carroll AM, de la Llera-Moya M, Xu X, Greene MI. Suppression of human prostate tumor growth by a unique prostate-specific monoclonal antibody F77 targeting a glycolipid marker. Proc Natl Acad Sci U S A. 2010;107:732–737. doi: 10.1073/pnas.0911397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang B, Zhang Y, Wang J, Zhang Y, Chen J, Pan Y, Ren L, Hu Z, Zhao J, Liao M, Wang S. Screening and identification of a targeting peptide to hepatocarcinoma from a phage display peptide library. Mol Med. 2007;13:246–254. doi: 10.2119/2006-00115.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He X, Na MH, Kim JS, Lee GY, Park JY, Hoffman AS, Nam JO, Han SE, Sim GY, Oh YK, Kim IS, Lee BH. A novel peptide probe for imaging and targeted delivery of liposomal doxorubicin to lung tumor. Mol Pharm. 2011;8:430–438. doi: 10.1021/mp100266g. [DOI] [PubMed] [Google Scholar]
- 156.Hassan S, Buchanan M, Jahan K, Aguilar-Mahecha A, Gaboury L, Muller WJ, Alsawafi Y, Mourskaia AA, Siegel PM, Salvucci O, Basik M. CXCR4 peptide antagonist inhibits primary breast tumor growth, metastasis and enhances the efficacy of anti-VEGF treatment or docetaxel in a transgenic mouse model. Int J Cancer. 2011;129:225–232. doi: 10.1002/ijc.25665. [DOI] [PubMed] [Google Scholar]
- 157.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 158.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenasemediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 159.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 160.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 162.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 164.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, Ferber I, Lebkowski J, Martin T, Madrenas J, Bhatia M. Human embryonic stem cells possess immune- privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 165.Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 166.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 167.Dressel R, Schindehütte J, Kuhlmann T, Elsner L, Novota P, Baier PC, Schillert A, Bickeböller H, Herrmann T, Trenkwalder C, Paulus W, Mansouri A. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS One. 2008;3:e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]


