Abstract
Background
Plasmodium knowlesi is the fifth Plasmodium species that can infect humans. The Plasmodium merozoite surface protein-142 (MSP-142) is a potential candidate for malaria vaccine. However, limited studies have focused on P. knowlesi MSP-142.
Methods
A ~42 kDa recombinant P. knowlesi MSP-142 (pkMSP-142) was expressed using an Escherichia coli system. The purified pkMSP-142 was evaluated with malaria and non-malaria human patient sera (n = 189) using Western blots and ELISA. The immunogenicity of pkMSP-142 was evaluated in mouse model.
Results
The purified pkMSP-142 had a sensitivity of 91.0% for detection of human malaria in both assays. Specificity was 97.5 and 92.6% in Western blots and ELISA, respectively. Levels of cytokine interferon-gamma, interleukin-2, interleukin-4, and interleukin-10 significantly increased in pkMSP-142-immunized mice as compared to the negative control mice. pkMSP-142-raised antibody had high endpoint titres, and the IgG isotype distribution was IgG1 > IgG2b > IgG3 > IgG2a.
Conclusions
pkMSP-142 was highly immunogenic and able to detect human malaria. Hence, pkMSP-142 would be a useful candidate for malaria vaccine development and seroprevalence studies.
Keywords: Plasmodium knowlesi, Merozoite surface protein, Recombinant expression, Immunogenicity
Background
Malaria is one of the important infectious diseases that causes high global mortality and morbidity. Plasmodium knowlesi has recently been recognized as the fifth Plasmodium species that can cause malaria in humans [1,2]. Plasmodium knowlesi replicates every 24 hours, which is the most rapid replication rate among all human Plasmodium species. Quoditian fever, hyperparasitaemia, life-threatening complications and death may occur if the patient remains untreated [3].
Proteins expressed on the surface of Plasmodium merozoites are promising targets for malaria vaccine development. Merozoite surface protein 1 (MSP-1) is a high molecular mass protein which undergoes two proteolytic steps to produce several fragments. Primary processing occurs during maturation of merozoites, and the secondary processing occurs during the invasion of merozoites into erythrocytes [4-6]. Proteolytic processing of MSP-1 has been intensively studied in Plasmodium falciparum. During the first processing, the P. falciparum MSP-1 precursor polypeptide is cleaved into four major fragments of ~83 kDa (MSP-183), 30 kDa (MSP-130), 38 kDa (MSP-138), and 42 kDa (MSP-142) in size. The secondary processing further cleaves the MSP-142 into two fragments, MSP-133 and MSP-119. The soluble MSP-133 sheds from the merozoite surface [7-9], whereas the membrane-bound MSP-119 remains associated with merozoites and is carried into the new erythrocyte during invasion [10,11].
MSP-142 is one of the leading candidates for blood-stage malaria vaccines as it is able to induce protective immune responses [12-14]. Antibodies directed against MSP-142 and MSP-119 can interrupt merozoite invasion in vitro[15-17]. Children with naturally acquired immune response to Plasmodium MSP-119 are significantly associated with resistance towards malarial infection and clinical manifestations [18], while pregnant women with anti-MSP-119 antibodies are protected against placental infection and infection in infants [19]. Immunization studies using MSP-142 and MSP-119 in animal models such as rodents, mice and primates [20-24] found that protective immune response is elicited during challenge with life Plasmodium parasites.
MSP-119-mediated protective responses are mainly responsible for humoral immunity. Low prevalence of T cell responses to MSP-119 is due to limited T cell epitopes on this fragment. Protective T cell responses, on the other hand, are induced by epitopes on MSP-133[25-27]. MSP-133 regulates cell mediated responses inducing effector T cells which help in protective B cells response, cytokines production and antiparasitic activity regulation against Plasmodium in an antibody-independent manner [28,29]. It is thus more appropriate to include both MSP-119 and MSP-133 fragments in the malaria vaccine design in order to elicit both humoral and cell mediated responses. Therefore, MSP-142 which has both immunodominant B and T cell epitopes, is considered an important and potential vaccine candidate [30,31].
To date, most of the efforts for development of malaria vaccines and human trials are still focus on P. falciparum. Phase I human vaccine studies by using P. falciparum MSP-142 in USA [32,33], western Kenya [34] and Mali [35] showed high safety, tolerability and immunogenicity, which protective cytokines and antibody responses were detected in the volunteers. However, the raised anti-MSP-142 antibodies were insufficient to inhibit parasite growth up to protection level [36,37] and in a Phase II human trial with Kenyan children, the overall vaccine efficacy was considerably low [38]. Nonetheless, the low level protection elicited by this single antigen vaccine could be enhanced and overcome by multi-antigens vaccine development or addition of other immunostimulants.
Considerable amount of studies on MSP-142 have been carried out on several Plasmodium sp. but not much is known about P. knowlesi MSP-142, particularly about its immunogenicity. In the present study, a recombinant MSP-142 of P. knowlesi (pkMSP-142) was produced and evaluated using ELISA and Western blot assays. Immunogenicity was assessed using the mouse model. Cytokine levels in pkMSP-142-immunized mice were determined and antibody responses were characterized.
Methods
Ethics statement
Animal ethic and experiment procedures were approved by University of Malaya Institutional Animal Care And Use Committee (PAR/28/09/2011/CFW). Human ethic was approved by University of Malaya Medical Centre Medical Ethics Committee (MEC Ref. No: 817.18).
Construction of recombinant plasmid pkMSP-142
Plasmodium knowlesi genomic DNA was extracted from a P. knowlesi-infected patient blood sample using blood extraction kit (QIAGEN, Hilden, Germany). The MSP-1 42 gene was amplified by polymerase chain reaction (PCR) using primer pair MSP142_F: 5′ - CGCGGATCCGAGAATCACGTGGCTGCATTCA −3′ and MSP142_R: 5′ - CGCGGATCCCTAGCTGGAGGAGCTACAGAA −3′ based on the sequence of P. knowlesi H strain (GenBank accession number XM_002258546). The amplification conditions were as follows: initial denaturing step at 95°C for 4 minutes; 35 cycles at 95°C for 45 seconds, 55°C for 45 seconds, and 72°C for 1 minute; final elongation step at 72°C for 10 minutes. The PCR product was purified and cloned into pCR 2.1-TOPO plasmid vector (Invitrogen, USA), followed by verification using sequence analysis. Restriction enzyme BamHI was used to digest the plasmid at 37°C for three hours, and the digested fragment was ligated with expression vector pRSET A (Invitrogen, USA) at 4°C overnight. The recombinant plasmid was transformed into expression host Escherichia coli strain BL21 (DE3) pLysS.
Expression of pkMSP-142
BL21 (DE3) pLysS cells containing recombinant plasmid pkMSP-142 was propagated in Luria-Bertani (LB) broth containing ampicillin (100 μg/ml) and chloramphenicol (35 μg/ml) at 37°C with shaking until the optical density at 600 nm (OD600) reached 0.4-0.6. The culture was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and allowed to grow for another two hours. The cells were harvested by centrifugation at 5,000 × g for 10 minutes.
Purification of pkMSP-142
pkMSP-142 was purified by using nickel-NTA agarose resin which has a high binding affinity towards the N-terminal polyhistidine tag of the recombinant pkMSP-142. ProBondTM purification system (Invitrogen, USA) with hybrid condition was used. Cell pellet from a 50 ml pkMSP-142 culture was dissolved and suspended in denaturing buffer (6 M guanidinium lysis buffer, pH 7.8) by using 16 ml guanidinium lysis buffer per gram of cell pellet. Cell lysate was sonicated on ice with three five-second pulses at high intensity for cell wall disruption. Purification column containing agarose resin was prepared under denaturing condition. The protein lysate was added to the column, washed twice with denaturing binding buffer (pH 7.8) containing 8 M urea and twice with denaturing wash buffer (pH 6.0) containing 8 M urea. Renaturation was carried out by washing four times using native wash buffer (pH 8.0) which contains 20 mM imidazole. The purified recombinant pkMSP-142 was finally eluted with native elution buffer (pH 8.0) containing 250 mM imidazole. Concentration of purified pkMSP-142 was determined by using Bradford Assay kit (Bio-Rad, USA).
SDS-PAGE, Coomassie brilliant blue staining and Western blot
Non-purified and purified pkMSP-142 were resolved by 12% SDS-PAGE under reducing and non-reducing conditions, and stained with Coomassie brilliant blue (Bio-Rad, USA). The separated proteins were also electrophorectically transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA) and blocked overnight in tris buffered saline (TBS) containing 5% skimmed milk at 4°C. The membranes were probed with anti-XpressTM antibody (1:5000 dilution) with TBS containing 2.5% skimmed milk for one hour. The membranes were washed three times with TBS-T (TBS containing 0.2% Tween-20) and treated with biotin-labelled goat anti-mouse IgG (1:2,500 dilution) for one hour, followed by streptavidin-AP (1:2500 dilution) for one hour. Finally, the membranes were developed by chromogenic substrate NBT/BCIP. The colour was allowed to develop at room temperature in dark.
Evaluation of purified pkMSP-142 using serum samples in Western blot assay
The purified pkMSP-142 was evaluated by Western blot assay using 189 sera of patients infected with P. knowlesi (n = 38), non-P. knowlesi malaria parasites (n = 29), non-malarial parasites (n = 47) and healthy donor (n = 75). PVDF membrane strips containing 60 ng of blotted pkMSP-142 were incubated with the different serum samples, followed by biotin-labelled goat anti-human IgM + IgG + IgA (1:2500 dilution), streptavidin-AP, and finally NBT/BCIP.
Evaluation of purified pkMSP-142 by using serum samples in ELISA
The same 189 sera used in the Western blot assay were used in ELISA. Purified pkMSP-142, 10 μg/ml, was coated on 96-well microtiter plates using 0.05 M sodium carbonate buffer, pH 9.6 at 4°C overnight. The wells were blocked with phosphate buffered saline (PBS) containing 1% bovine serum albumin for two hours at 37°C. The wells were washed three times with 0.1% PBS-T. Patient serum (1:80 dilution) was separately added into each well and incubated for one hour at 37°C. The wells were washed five times and peroxidase-labelled goat anti-human IgM + IgG + IgA (1:2,500 dilution) was added followed by one hour incubation at 37°C. The wells were washed five times with PBS-T and incubated with 3, 3′, 5, 5′-Tetramethyl Benzidine, TMB (Amresco, USA) for 30 minutes in dark. Stop solution 2 N H2SO4 was added to stop the reaction and absorbance at OD450 was measured. Samples were run in duplicates. The cut-off value was set at MN + 2σ of the healthy donor serum group, where MN is the mean absorbance (OD450) and σ is the standard deviation. Samples with absorbance values higher than MN + 2σ were considered positive.
Mice immunization
Six to eight-week old female BALB/c mice were used for immunization (pkMSP-142-immunized group and negative control group, n = 5 per group). Purified pkMSP-142, 30 μg, was mixed with adjuvant in a volume of 1:1 ratio and the mixture was injected into mice. Complete Freund’s Adjuvant (CFA) (Sigma, USA) was used in the prime boost and Incomplete Freund’s Adjuvant (IFA) (Sigma, USA) was used in the subsequent boosters. Booster was given on days 14 and 21 post-immunization. All injections were given subcutaneously. Serum of each mouse was collected at day 0, 7, 14, 21 and 31 post-immunization. Mice in the negative control group were injected with purified non-recombinant protein pRSET A.
Measurement of cytokine levels in mice
Mice were sacrificed ten days after the second booster and their spleen cells were harvested and purified. The cells were grown in tissue culture grade, flat-bottom, 96-well microtitre plates (TPP, Switzerland), with total cells of 2 × 105 per well. Stimulator purified pkMSP-142 (30 μg/ml) was added. The plates were placed in 5% carbon dioxide (CO2) incubator at 37°C and the cells were allowed to grow for 65 hours. The plates were then centrifuged at 2,000 rpm for 20 minutes. Cell supernatants were collected and the levels of cytokine interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-10 (IL-10) and interferon-gamma (IFN-γ) in the supernatants were determined using ELISA kits (Thermo Scientific, USA) following the manufacturer’s instruction. Mann–Whitney statistical test was performed to determine whether increase of cytokine levels was significant.
Antibody characterization and IgG subclass distribution
pkMSP-142-immunized mice sera were analysed by Western blot assay for detection of antibody against pkMSP-142. Purified pkMSP-142, 350 ng, was blotted on PVDF membrane strips and incubated with mice sera collected at different time points. Level of IgM and IgG, IgG isotype distribution and endpoint titre of mice sera were determined by ELISA using purified pkMSP-142 as coating antigen. Biotin-labelled anti-mouse IgM and IgG were used to determine the IgM and IgG level, respectively. Peroxidase-labelled anti-mouse IgG1, IgG2a, IgG2b and IgG3 were used for determination of IgG subclass distribution. For antibody endpoint titre determination, serial dilution was performed on the mice sera (1:400 – 1:819200 dilution) and detected by peroxidase-labelled anti-mouse IgM + IgG + IgA with TMB as substrate. Mice sera from the negative control group were used to determine the cut-off value as described before.
Results
Cloning, expression and purification of recombinant pkMSP-142
The P. knowlesi MSP-1 42 gene (969 bp) was amplified from genomic DNA by PCR. The amplified fragment was confirmed as P. knowlesi MSP-1 42 gene through nucleotide sequencing and the deduced amino acid sequence. The recombinant plasmid pkMSP-142 showed protein expression with molecular mass of ~42 kDa (Figure 1A, lanes 6 and 7). pkMSP-142 was expressed as inclusion bodies which were solubilized under denaturing conditions by using denaturing guanidinium lysis buffer. Purified pkMSP-142 showed a distinct band of ~42 kDa (Figure 1A, lanes 8 and 9 and Figure 1B, lane 3) which was absent in the purified plasmid control (Figure 1A, lanes 3 and 4 and Figure 1B, lane 1). The purified pkMSP-142 was observed to have a molecular mass of ~42 kDa under reducing (Figure 1C, lanes 2 and 3) and ~39 kDa under non-reducing conditions (Figure 1C, lanes 4 and 5). The concentration of the purified pkMSP-142 obtained was 1.0 mg/ml.
Figure 1.
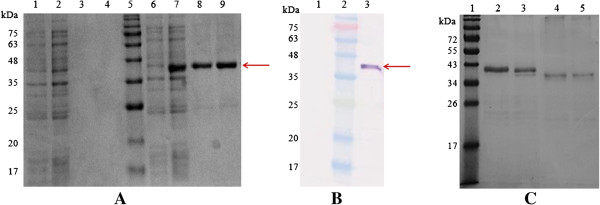
Recombinant pkMSP-142 produced in Escherichia coli expression system and purified with ProBondTM purification system. The pkMSP-142 with a size of ~42 kDa (arrows) was detected in (A) Coomassie brilliant blue stained SDS gel, and (B) Western blot probed with anti-XpressTM antibody. (A) Lanes 1 and 2, pRSET A at 0 and 2 hour respectively; lanes 3 and 4, purified pRSET A; lanes 6 and 7, recombinant pkMSP-142 at 0 and 2 hour respectively; lane 8 and 9, purified pkMSP-142. (B) Lane 1, purified pRSET A; lane 3, purified pkMSP-142. (C) Lanes 2 and 3, purified pkMSP-142 under reducing condition with sample boiling and without boiling respectively; lanes 4 and 5, purified pkMSP-142 under non-reducing condition with sample boiling and without boiling respectively. Lane 5 in (A), lane 2 in (B) and lane 1 in (C) were Prestained Broad Range Protein Marker.
Evaluation of purified pkMSP-142 by using Western blot assay and ELISA
In the Western blot assay (Additional file 1), pkMSP-142 reacted with 33/38 (86.8%) of knowlesi malaria serum samples and 28/29 (96.6%) of non-knowlesi malaria serum samples. Therefore, the overall sensitivity of pkMSP-142 for malaria detection was 61/67 (91.0%). Three of the 122 non-Plasmodium parasitic infection and healthy donor sera reacted with pkMSP-142, giving a specificity of 119/122 (97.5%). In ELISA, pkMSP-142 reacted with 34/38 knowlesi malaria serum samples, thus giving sensitivity of 89.5% for knowlesi malaria detection. Twenty-seven of the 29 (93.1%) non-knowlesi malaria serum samples reacted with pkMSP-142. Therefore, the overall sensitivity for detection of malarial infection was 61/67 (91.0%). The specificity of ELISA was 113/122 (92.6%). Evaluation of pkMSP-142 with patient sera by using Western blot assay and ELISA is summarized in Table 1.
Table 1.
Evaluation of pkMSP-1 42 with patient sera in Western blot assay and ELISA
|
Human sera group |
Number of sera tested |
Western blot |
ELISA |
||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
|
A.
P. knowlesi
|
38 |
33 |
5 |
34 |
4 |
|
B. Non-
P. knowlesi
human malaria |
|
|
|
|
|
| i. P. vivax |
15 |
14 |
1 |
14 |
1 |
| ii. P. falciparum |
13 |
13 |
0 |
12 |
1 |
| iii. P. ovale |
1 |
1 |
0 |
1 |
0 |
|
C. Non-malarial parasitic infection |
|
|
|
|
|
| i. Filariasis |
4 |
1 |
3 |
0 |
4 |
| ii. Amoebiasis |
16 |
2 |
14 |
4 |
2 |
| iii. Cysticercosis |
13 |
0 |
13 |
2 |
11 |
| iv. Toxoplasmosis |
11 |
0 |
11 |
1 |
10 |
| v. Toxocarasis |
3 |
0 |
3 |
1 |
2 |
| D. Healthy donor | 75 | 0 | 75 | 1 | 74 |
Cytokine profiles in mouse
Cytokine secretion profiles of mice immunized with pkMSP-142 were determined by ELISA. From the results, IFN-γ, IL-2, IL-4 and IL-10 levels of pkMSP-142-immunized mice group were all significantly higher than those of the negative control group (P < 0.05) (Table 2).
Table 2.
Cytokine profiles of mice immunized with pkMSP-1 42
| Antigen | IL-2 | IL-4 | IL-10 | IFN-γ |
|---|---|---|---|---|
| pRSET A |
75.8 (60.0-86.1) |
3.4 (0.7-6.8) |
89.9 (82.9-131.8) |
165.7 (0.0-411.9) |
| pkMSP-142 | 280.0 (268.1-287.0)* P = 0.008 | 91.0 (56.6-146.2)* P = 0.008 | 156.7 (136.8-250.8)* P = 0.016 | 2262.0 (1,515.0-2,437.0)* P = 0.008 |
Values shown are median (interquartile range). IL-2, interleukin-2; IL-4, interleukin-4; IL-10, interleukin-10; IFN-γ, interferon-gamma. Concentration of cytokines in pg/ml. *P < 0.05.
Antibody characterization and IgG isotype distribution
Antibody responses in mice towards pkMSP-142 at different time points were analysed. Western blot strips showed that antibody against pkMSP-142 was detected one week after prime boost. pkMSP-142 reacted with pkMSP-142-immunized mice sera at day 7, 14, 21 and 31 post-immunization. No reactivity was observed in the negative control mice sera (Figure 2). ELISA results indicated that both IgM and IgG were detected in pkMSP-142-immunized mice sera one week after prime boost. IgM level slightly decreased from day 14 until day 31 post-immunization. IgG level was relatively lower than IgM at first week after prime boost, yet high response was detected at day 14 post-immunization and continued to rise until day 31 post-immunization (Figure 3A). The predominant IgG isotype was IgG1, followed by IgG2b, IgG3, and IgG2a (Figure 3B). pkMSP-142 induced high antibody response with the endpoint titre ranging between 1:204,800 and 1:819,200.
Figure 2.
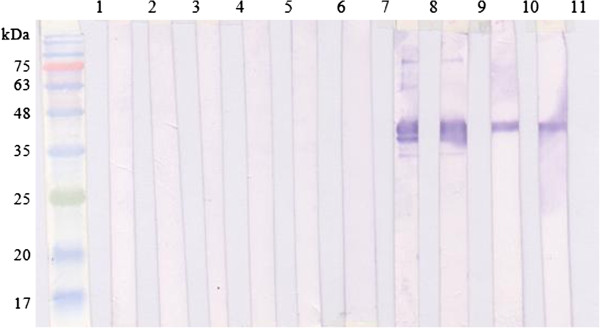
Anti-pkMSP-142 antibody detected in pkMSP-142-immunized mice. Lanes 2–6, sera of mice injected with non-recombinant protein pRSET A at day 0, 7, 14, 21 and 31 post-immunization; lanes 7–11, sera of mice injected with purified pkMSP-142 at day 0, 7, 14, 21 and 31 post-immunization. Lane 1 contained protein size standards.
Figure 3.
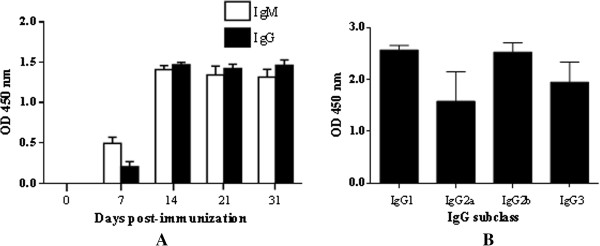
Immune responses in pkMSP-142-immunized mice. (A) IgM and IgG levels in pkMSP-142-immunized mice. Both IgM and IgG were detected at day 7 post-immunization and the levels increased throughout the whole immunization period. (B) IgG isotype-specific antibody levels. The IgG isotype distribution in pkMSP-142-immunized mice was IgG1 > IgG2b > IgG3 > IgG2a.
Discussion
MSP-142 of several Plasmodium sp. has been demonstrated to be immunogenic and able to elicit protective immunity [12,39]. MSP-142 is non-glycosylated and this is crucial for its immunogenicity, as glycosylated form of milk-derived MSP-142 secreted by transgenic mice does not confer protection against malaria during Plasmodium challenge [40]. The E. coli expression system was chosen in the present study due to its simplicity of techniques, cost-effectiveness and high efficiency of expression of non-glycosylated protein [31,41]. Hybrid condition was chosen for protein purification to preserve the protein structure and activity. The pkMSP-142, which was expressed as inclusion bodies, was solubilized under denaturing buffer, then washed and eluted with native wash buffer and native elution buffer respectively to refold the protein. Purified pkMSP-142 appeared to have a smaller molecular mass in non-reducing condition compared to reducing condition. The shift in mobility upon reduction in SDS-PAGE indicated the presence of disulfide linkages in pkMSP-142 and hence pkMSP-142 was likely refolded after purification.
In the present study, data showed high sensitivity (> 90%) of pkMSP-142 to detect malarial infection in both Western blot and ELISA. Hence, this suggests that pkMSP-142 is suitable as antigen in both assays for serodetection of malarial infection. The specificity of ELISA was relatively lower than that of the Western blot assay. This discrepancy might be due to the borderline activity of some patients’ sera in the ELISA. Suchankova et al. obtained positive results in ELISA but yet negative in Western blot when comparing the two assays for detection of human papillomavirus antibody. They suggested that the borderline activity of the sera, in which some of the serum sample OD absorbance values fall just above the cut-off value, led to the discrepancies in results [42]. Similarly, in the present study, some of the non-Plasmodium and healthy donor sera had OD absorbance values just slightly higher than the cut-off value, thus giving false positive results. Nonetheless, ELISA is useful as it can measure the titre of antibody compared to the qualitative detection of Western blot assay.
A few of the malaria infected patient sera did not react with pkMSP-142 in Western blot and/or ELISA and this could be explained by the genetic diversity of MSP-1. Plasmodium MSP-1 exhibits extensive sequence diversity among isolates and host immune selective pressure could be one of the reasons that lead to the polymorphism [43,44]. Plasmodium knowlesi MSP-1 comprises of five conserved and four variable domains, which the conserved domains subjected to nucleotide substitutions and exhibited allelic dimorphism, while three of four variable domains contained complex repetitive sequence motifs which lead to extensive sequence and size variation. Besides, microheterogeneity comprising amino acid substitutions causing different alleles was observed in P. knowlesi MSP-133 epitopes [45]. Presence of sequence diversity in these epitopes may alter the immunological recognition of the epitopes and hence benefit the parasite survival by evasion of host immune response. For instance, Bergmann-Leitner et al. demonstrated that the P. falciparum anti-MSP-119 antibodies were allele-specific during inhibition of merozoite invasion and parasite growth [46]. Therefore, antibodies in some of the malaria infected patient sera in the present study could be unable to detect the variant epitopes on pkMSP-142, which led to negative reactivity.
Plasmodium knowlesi MSP-133 (pkMSP-133) was evaluated with similar patient serum samples in the previous study [47]. The sensitivity of pkMSP-142 for detection of malarial infection was similar with pkMSP-133 in Western blot (both >90%), but higher compared to pkMSP-133 in ELISA. pkMSP-142 consisted of both MSP-133 and MSP-119 regionss. Previous studies showed that human sera from malaria-endemic areas demonstrated strong MSP-119 reactivity [48] and MSP-119 fragment consists of several immunodominant B cell epitopes which are important to induce protective anti-MSP-119 antibodies [49]. Therefore, these epitopes could be recognized by specific anti-MSP-119 antibodies in the sera of malaria-infected patients when pkMSP-142 was used as antigen, but not pkMSP-133.
pkMSP-142 reacted with most of the non-knowlesi malaria sera and this could be due to serological cross-reactivity. Previous studies demonstrated that serum cross-reactivity could occur among malaria patients [50-55]. Furthermore, almost similar level of protection could be induced in immunized animals during challenge with heterologous Plasmodium parasite due to their high homology of antigens [56]. Sera from patients infected with P. falciparum and Plasmodium malariae cross-reacted with recombinant Plasmodium vivax MSP-1 which has 42% sequence similarity with P. falciparum MSP-1 [57]. Plasmodium knowlesi MSP-142 shares high amino acid similarity with MSP-142 of P. vivax (84%), P. falciparum (59%) and Plasmodium ovale (70%). Hence, P. knowlesi MSP-142 may share certain common B-cell epitopes with human Plasmodium species, leading to cross-reactivity.
It has been reported that previous infection with P. vivax could be one of the reasons for reactivity of recombinant P. vivax AMA-1 with P. falciparum-infected patient sera [58]. In the present study, pkMSP-142 reacted with some non-knowlesi human malaria and non-Plasmodium parasitic infection sera. This could probably due to the patients’ previous exposure to P. knowlesi. It is known that antibodies generated against knowlesi infection can persist up to five years or longer [59].
In the present study, high IFN-γ and IL-2 levels in pkMSP-142-immunized mice group indicated that Th1-driven immune response has been stimulated. IFN-γ is a key molecule in human anti-malarial host defense. It activates macrophages to kill malarial blood stage parasites by reactive oxygen and nitrogen intermediates, and induces macrophages to secrete monokines such as IL-1, IL-6 and TNF [60,61]. IFN-γ, which regulates the pro-inflammatory and Th1 responses, was detected during primary P. knowlesi infection in rhesus macaques [62]. IL-2 functions as T cell growth factor and promotes the functional properties of natural killer cells, B cells and macrophages.
The high level of IL-4, IL-10 and predominant IgG1 production in the pkMSP-142-immunized mice group showed that Th2 response has also been stimulated. IL-10 is an anti-inflammatory cytokine which is secreted by activated Th2 cells. It down-regulates the production of pro-inflammatory IFN-γ and limits the potentially harmful inflammatory responses during malarial blood stage parasites infection in mouse [63,64]. A study on P. knowelsi-inoculated olive baboons reported association between increased levels of IL-4, IL-10, IgM and IgG with increased protection against knowlesi-infection [65]. In natural human infection, Cox-Singh et al. reported increase in the IL-10 level in knowlesi malaria patients with considerable parasitaemia. Therefore, they postulated that this anti-inflammatory cytokine plays a role in modulating the expected immune surge during merozoite reinvasion [66].
Cytokines produced by each subset promote the polarization process, which Th1 cells-produced cytokines that will down-regulate Th2 response, and vice versa[67]. The concentrations of IFN-γ and IL-10 have been noted to increase in P. vivax-infected individuals during natural infection [68]. Therefore, stimulation of Th1 and Th2 subsets upon pkMSP-142 immunization is important as homeostasis between Th1/Th2 cells could achieve a balance regulation between pro-inflammatory and anti-inflammatory actions in the immune response.
The high titre of anti-pkMSP-142 antibodies suggests that pkMSP-142 was highly immunogenic. Four isotypes of IgG were detected in the pkMSP-142-immunized mice. These IgG isotypes help to activate effector responses in different manners. Murine IgG1 binds to mast cell, subtypes IgG2a and IgG2b play a role in complement binding and antibody opsonization, while IgG3 is responsible for carbohydrate epitope recognition [69]. IgG2a is the dominant IgG isotype for modulating murine malaria parasitaemia [70]. Both cell-mediated and humoral immunity were elicited by pkMSP-142, and these findings support P. knowlesi MSP-142 as a potential blood stage vaccine candidate. Similar results were reported in studies involving mice immunized with recombinant P. falciparum and P. vivax MSP-142[71,72].
Conclusion
Results from the present study suggest that E. coli-expressed pkMSP-142 can be useful in general seroprevalence and seroepidemiological screening. Moreover, pkMSP-142 was highly immunogenic and both T cell and B cell responses were elicited in mice. Therefore, pkMSP-142 can serve as a candidate for malaria vaccine design, although further evaluation needs be carried out to validate its potential and limitations.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FWC carried out the expression and immunogenicity assays, and drafted the manuscript. RM performed microscopic analysis of infected blood samples. MYF and YLL performed the data analysis. All authors read and approved the final manuscript.
Supplementary Material
Purified pkMSP-1 42 was detected by patient sera infected with knowlesi malaria and non-knowlesi malaria. Western Blot strips containing 60 ng of the purified recombinant pkMSP-142 were tested with selected sera from different categories. Lanes 2–5, sera from patients infected with malaria: P. knowlesi (lanes 2 and 3), P. falciparum (lane 4), P. vivax (lane 5). Lanes 6–10, sera of patients infected with non-malarial parasites: filariasis (lane 6), amoebiasis (lane 7), toxoplasmosis (lane 8), cysticercosis (lane 9), toxocarasis (lane 10). Lane 11, healthy donor serum which served as negative control. Lane 1 contained protein size standards.
Contributor Information
Fei Wen Cheong, Email: fwcheong18@gmail.com.
Mun Yik Fong, Email: fongmy@um.edu.my.
Yee Ling Lau, Email: lauyeeling@um.edu.my.
Rohela Mahmud, Email: rohela@um.edu.my.
Acknowledgements
The study was supported by UM High Impact Research Grant UM-MOHE (UM.C/625/1/HIR/MOHE/MED/09) from the Ministry of Higher Education Malaysia and UM Postgraduate Research Grant (PV030/2011A). Special thanks to Department of Parasitology Diagnostic Laboratory, Faculty of Medicine, University of Malaya and University of Malaya Medical Centre for providing patient blood and serum samples.
References
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;12:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- Bronner U, Divis PC, Farnert A, Singh B. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malar J. 2009;12:15. doi: 10.1186/1475-2875-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PC, Singh B. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;12:852–860. doi: 10.1086/605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder AA, Blackman MJ, Burghaus PA, Chappel JA, Ling IT, McCallum-Deighton N, Shai S. A malaria merozoite surface protein (MSP1)-structure, processing and function. Mem Inst Oswaldo Cruz. 1992;12(Suppl 3):37–42. doi: 10.1590/s0074-02761992000700004. [DOI] [PubMed] [Google Scholar]
- Holder AA, Freeman RR. Protective antigens of rodent and human bloodstage malaria. Philos Trans R Soc Lond B Biol Sci. 1984;12:171–177. doi: 10.1098/rstb.1984.0117. [DOI] [PubMed] [Google Scholar]
- McBride JS, Heidrich HG. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987;12:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Whittle H, Holder AA. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;12:35–44. doi: 10.1016/0166-6851(91)90128-S. [DOI] [PubMed] [Google Scholar]
- Blackman MJ. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 1994;12:213–220. doi: 10.1016/s0091-679x(08)61853-1. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Holder AA. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992;12:307–315. doi: 10.1016/0166-6851(92)90228-C. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Ling IT, Nicholls SC, Holder AA. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;12:29–33. doi: 10.1016/0166-6851(91)90127-R. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Dennis ED, Hirst EM, Kocken CH, Scott-Finnigan TJ, Thomas AW. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;12:229–239. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- Quin SJ, Langhorne J. Different regions of the malaria merozoite surface protein 1 of Plasmodium chabaudi elicit distinct T-cell and antibody isotype responses. Infect Immun. 2001;12:2245–2251. doi: 10.1128/IAI.69.4.2245-2251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MF. Towards a blood-stage vaccine for malaria: are we following all the leads. Nat Rev Immunol. 2001;12:117–125. doi: 10.1038/35100540. [DOI] [PubMed] [Google Scholar]
- Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;12:377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;12:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AF, Burghaus P, Druilhe P, Holder AA, Riley EM. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;12:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- Chang SP, Gibson HL, Lee-Ng CT, Barr PJ, Hui GS. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;12:548–555. [PubMed] [Google Scholar]
- Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, Takacs B, Schonfeld HJ, Holder AA, Greenwood BM. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;12:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Branch OH, Udhayakumar V, Hightower AW, Oloo AJ, Hawley WA, Nahlen BL, Bloland PB, Kaslow DC, Lal AA. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg. 1998;12:211–219. doi: 10.4269/ajtmh.1998.58.211. [DOI] [PubMed] [Google Scholar]
- Chang SP, Case SE, Gosnell WL, Hashimoto A, Kramer KJ, Tam LQ, Hashiro CQ, Nikaido CM, Gibson HL, Lee-Ng CT, Barr PJ, Yokota BT, Hut GS. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;12:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darko CA, Angov E, Collins WE, Bergmann-Leitner ES, Girouard AS, Hitt SL, McBride JS, Diggs CL, Holder AA, Long CA, Barnwell JW, Lyon JA. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect Immun. 2005;12:287–297. doi: 10.1128/IAI.73.1.287-297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renia L, Ling IT, Marussig M, Miltgen F, Holder AA, Mazier D. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun. 1997;12:4419–4423. doi: 10.1128/iai.65.11.4419-4423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling IT, Ogun SA, Holder AA. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;12:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Daly TM, Long CA. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;12:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udhayakumar V, Anyona D, Kariuki S, Shi YP, Bloland PB, Branch OH, Weiss W, Nahlen BL, Kaslow DC, Lal AA. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J Immunol. 1995;12:6022–6030. [PubMed] [Google Scholar]
- Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;12:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers AW, Cioce V, Shimp RL, Lawson M, Hui G, Muratova O, Kaslow DC, Robinson R, Long CA, Miller LH. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect Immun. 2001;12:1536–1546. doi: 10.1128/IAI.69.3.1536-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipasa J, Hirunpetcharat C, Mahakunkijcharoen Y, Xu H, Elliott S, Good MF. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J Immunol. 2002;12:944–951. doi: 10.4049/jimmunol.169.2.944. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Goodman AL, Biswas S, Forbes EK, Moore AC, Gilbert SC, Hill AV. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe. 2009;12:95–105. doi: 10.1016/j.chom.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Ware LA, Barbosa A, Ockenhouse CF, Lanar DE. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect Immun. 2001;12:5464–5470. doi: 10.1128/IAI.69.9.5464-5470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp C, Kauth CW, Bujard H, Lutz R. Expression and purification of Plasmodium falciparum MSP-1(42): A malaria vaccine candidate. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;12:61–72. doi: 10.1016/S1570-0232(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Angov E, Kester KE, Diggs C, Soisson L, Cummings JF, Stewart AV, Palmer DR, Mahajan B, Krzych U, Tornieporth N, Delchambre M, Vanhandenhove M, Ofori-Anyinam O, Cohen J, Lyon JA, Heppner DG. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine. 2006;12:3009–3017. doi: 10.1016/j.vaccine.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Otsyula N, Angov E, Bergmann-Leitner E, Koech M, Khan F, Bennett J, Otieno L, Cummings J, Andagalu B, Tosh D, Waitumbi J, Richie N, Shi M, Miller L, Otieno W, Otieno GA, Ware L, House B, Godeaux O, Dubois MC, Ogutu B, Ballou WR, Soisson L, Diggs C, Cohen J, Polhemus M, Heppner DG Jr, Ockenhouse CF, Spring MD. Results from tandem Phase 1 studies evaluating the safety, reactogenicity and immunogenicity of the vaccine candidate antigen Plasmodium falciparum FVO merozoite surface protein-1 (MSP1(42)) administered intramuscularly with adjuvant system AS01. Malar J. 2013;12:29. doi: 10.1186/1475-2875-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoute JA, Gombe J, Withers MR, Siangla J, McKinney D, Onyango M, Cummings JF, Milman J, Tucker K, Soisson L, Stewart VA, Lyon JA, Angov E, Leach A, Cohen J, Kester KE, Ockenhouse CF, Holland CA, Diggs CL, Wittes J, Heppner DG Jr. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine. 2007;12:176–184. doi: 10.1016/j.vaccine.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Sagara I, Dicko A, Diemert DJ, Heppner DG Jr, Stewart VA, Angov E, Soisson L, Leach A, Tucker K, Lyke KE, Plowe CV. Safety and allele-specific immunogenicity of a malaria vaccine in Malian adults: results of a phase I randomized trial. PLoS Clin Trials. 2006;12:e34. doi: 10.1371/journal.pctr.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin E, Long CA, Stowers AW, Zou L, Singh S, MacDonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB. Phase 1 study of two merozoite surface protein 1 (MSP1(42)) vaccines for Plasmodium falciparum malaria. PLoS Clin Trials. 2007;12:e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaman MC, Martin LB, Malkin E, Narum DL, Miller LH, Mahanty S, Long CA. Ex vivo cytokine and memory T cell responses to the 42-kDa fragment of Plasmodium falciparum merozoite surface protein-1 in vaccinated volunteers. J Immunol. 2008;12:1451–1461. doi: 10.4049/jimmunol.180.3.1451. [DOI] [PubMed] [Google Scholar]
- Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, Tucker K, Waitumbi JN, Diggs C, Wittes J, Malkin E, Leach A, Soisson LA, Milman JB, Otieno L, Holland CA, Polhemus M, Remich SA, Ockenhouse CF, Cohen J, Ballou WR, Martin SK, Angov E, Stewart VA, Lyon JA, Heppner DG, Withers MR. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One. 2009;12:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Kumar S, Kaslow DC, Miller LH. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;12:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers AW, Chen Lh LH, Zhang Y, Kennedy MC, Zou L, Lambert L, Rice TJ, Kaslow DC, Saul A, Long CA, Meade H, Miller LH. A recombinant vaccine expressed in the milk of transgenic mice protects Aotus monkeys from a lethal challenge with Plasmodium falciparum. Proc Natl Acad Sci U S A. 2002;12:339–344. doi: 10.1073/pnas.012590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Kennedy MC, Long CA, Saul AJ, Miller LH, Stowers AW. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect Immun. 2003;12:6766–6774. doi: 10.1128/IAI.71.12.6766-6774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova A, Ritterova L, Krcmar M, Krchnak V, Vagner J, Jochmus I, Gissmann L, Kanka J, Vonka V. Comparison of ELISA and western blotting for human papillomavirus type 16 E7 antibody determination. J Gen Virol. 1991;12(Pt 10):2577–2581. doi: 10.1099/0022-1317-72-10-2577. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Mackay M, Goman M, Scaife JG. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;12:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Putaporntip C, Jongwutiwes S, Sakihama N, Ferreira MU, Kho WG, Kaneko A, Kanbara H, Hattori T, Tanabe K. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc Natl Acad Sci U S A. 2002;12:16348–16353. doi: 10.1073/pnas.252348999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaporntip C, Thongaree S, Jongwutiwes S. Differential sequence diversity at merozoite surface protein-1 locus of Plasmodium knowlesi from humans and macaques in Thailand. Infect Genet Evol. 2013;12:213–219. doi: 10.1016/j.meegid.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Bergmann-Leitner ES, Duncan EH, Angov E. MSP-1p42-specific antibodies affect growth and development of intra-erythrocytic parasites of Plasmodium falciparum. Malar J. 2009;12:183. doi: 10.1186/1475-2875-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong FW, Lau YL, Fong MY, Mahmud R. Evaluation of recombinant Plasmodium knowlesi merozoite surface protein-133 for detection of human malaria. Am J Trop Med Hyg. 2013;12:835–840. doi: 10.4269/ajtmh.12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;12:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- Hui GS, Nikaido C, Hashiro C, Kaslow DC, Collins WE. Dominance of conserved B-cell epitopes of the Plasmodium falciparum merozoite surface protein, MSP1, in blood-stage infections of naive Aotus monkeys. Infect Immun. 1996;12:1502–1509. doi: 10.1128/iai.64.5.1502-1509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Collins WE, Millet P. Immunologic characterization of Plasmodium vivax antigens using Plasmodium cynomolgi liver stage-primed immune sera. Am J Trop Med Hyg. 1994;12:365–371. doi: 10.4269/ajtmh.1994.51.365. [DOI] [PubMed] [Google Scholar]
- Kamboj KK, Barnwell JW, Nussenzweig RS, Cochrane AH. Characterization of cross-reactive blood-stage antigens of the Plasmodium cynomolgi complex using anti-Plasmodium vivax monoclonal antibodies. J Parasitol. 1988;12:403–408. [PubMed] [Google Scholar]
- Valderrama-Aguirre A, Quintero G, Gomez A, Castellanos A, Perez Y, Mendez F, Arevalo-Herrera M, Herrera S. Antigenicity, immunogenicity, and protective efficacy of Plasmodium vivax MSP1 PV200l: a potential malaria vaccine subunit. Am J Trop Med Hyg. 2005;12:16–24. doi: 10.4269/ajtmh.2005.73.16. [DOI] [PubMed] [Google Scholar]
- Diggs CL, Sadun EH. Serological cross reactivity between Plasmodium vivax and Plasmodium falciparum as determined by a modified fluorescent antibody test. Exp Parasitol. 1965;12:217–223. doi: 10.1016/0014-4894(65)90046-9. [DOI] [PubMed] [Google Scholar]
- Kumar N, Folgar JP, Lubega P. Recognition of Plasmodium falciparum asexual stage antigens by antibodies in sera from people exposed to Plasmodium vivax. Am J Trop Med Hyg. 1992;12:422–428. doi: 10.4269/ajtmh.1992.47.422. [DOI] [PubMed] [Google Scholar]
- Miller LH, Johnson JG, Schmidt-Ullrich R, Haynes JD, Wallach DF, Carter R. Determinants on surface proteins of Plasmodium knowlesi merozoites common to Plasmodium falciparum schizonts. J Exp Med. 1980;12:790–798. doi: 10.1084/jem.151.4.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Kaushal DC, Ware LA, Puri SK, Kaushal NA, Narula A, Upadhyaya DS, Lanar DE. Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys. Infect Immun. 2005;12:5936–5944. doi: 10.1128/IAI.73.9.5936-5944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Hwang HA, Yun WS, Kim SI, Lee KW, Park SK, Lee YJ, Kim TK, Wongsrichanalai C, Sakanari JA, Park H. Efficacy of the merozoite surface protein 1 of Plasmodium vivax as an antigen for ELISA to diagnose malaria. Yonsei Med J. 2004;12:129–134. doi: 10.3349/ymj.2004.45.1.129. [DOI] [PubMed] [Google Scholar]
- Haghi AM, Khoramizade MR, Nateghpour M, Mohebali M, Edrissian GH, Eshraghian MR, Sepehrizadeh Z. A recombinant Plasmodium vivax apical membrane antigen-1 to detect human infection in Iran. Korean J Parasitol. 2012;12:15–21. doi: 10.3347/kjp.2012.50.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;12:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner PG. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect Immun. 1998;12:6040–6044. doi: 10.1128/iai.66.12.6040-6044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA, Hunt NH. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983;12:1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praba-Egge AD, Montenegro S, Cogswell FB, Hopper T, James MA. Cytokine responses during acute simian Plasmodium cynomolgi and Plasmodium knowlesi infections. Am J Trop Med Hyg. 2002;12:586–596. doi: 10.4269/ajtmh.2002.67.586. [DOI] [PubMed] [Google Scholar]
- Linke A, Kuhn R, Muller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;12:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;12:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa B, Jenneby M, Esther KA, Hastings OS, Michael GM. Immunity to Plasmodium knowlesi H strain malaria in olive baboons. Int J Integr Biol. 2010;12:147–152. [Google Scholar]
- Cox-Singh J, Singh B, Daneshvar C, Planche T, Parker-Williams J, Krishna S. Anti-inflammatory cytokines predominate in acute human Plasmodium knowlesi infections. PLoS One. 2011;12:e20541. doi: 10.1371/journal.pone.0020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;12:223–246. [PubMed] [Google Scholar]
- Medina TS, Costa SP, Oliveira MD, Ventura AM, Souza JM, Gomes TF, Vallinoto AC, Povoa MM, Silva JS, Cunha MG. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar J. 2011;12:264. doi: 10.1186/1475-2875-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. Selective increases in antibody isotypes and immunoglobulin G subclass responses to secreted antigens in tuberculosis patients and healthy household contacts of the patients. Clin Diagn Lab Immunol. 1995;12:726–732. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WI, Evans CB, Taylor DW. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991;12:3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S, Ahmad G, Malhotra P, Mukherjee P, Chauhan VS. Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli. Infect Immun. 2004;12:5775–5782. doi: 10.1128/IAI.72.10.5775-5782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S, Mohmmed A, Dasaradhi PV, Crabb BS, Katyal A, Malhotra P, Chauhan VS. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine. 2006;12:2007–2016. doi: 10.1016/j.vaccine.2005.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purified pkMSP-1 42 was detected by patient sera infected with knowlesi malaria and non-knowlesi malaria. Western Blot strips containing 60 ng of the purified recombinant pkMSP-142 were tested with selected sera from different categories. Lanes 2–5, sera from patients infected with malaria: P. knowlesi (lanes 2 and 3), P. falciparum (lane 4), P. vivax (lane 5). Lanes 6–10, sera of patients infected with non-malarial parasites: filariasis (lane 6), amoebiasis (lane 7), toxoplasmosis (lane 8), cysticercosis (lane 9), toxocarasis (lane 10). Lane 11, healthy donor serum which served as negative control. Lane 1 contained protein size standards.


