Abstract
The precise orchestration of two opposing protein complexes - one in the cytoplasm (β-catenin destruction complex) and the other at the plasma membrane (LRP6 signaling complex) – is critical for controlling levels of the transcriptional co-factor, β-catenin, and subsequent activation of the Wnt/β-catenin signal transduction pathway. The Wnt pathway component, Axin, acts as an essential scaffold for assembly of both complexes. How the β-catenin destruction and LRP6 signaling complexes are modulated following Wnt stimulation remains controversial. A recent study in Science by He and colleagues reveals an underlying logic for Wnt pathway control in which Axin phosphorylation toggles a switch between the active and inactive states. This mini-review focuses on this and two other recent studies that provide insight into the initial signaling events triggered by Wnt exposure. We emphasize regulation of the β-catenin destruction and LRP6 signaling complexes and propose a framework for future work in this area.
Keywords: Wnt signal transduction, β-catenin destruction complex, Axin, LRP6 signaling complex, PP1
Introduction
Throughout metazoan development, the evolutionarily conserved Wnt signal transduction pathway directs diverse cellular processes, including proliferation, fate determination, differentiation, and survival [1,2]. In adult organisms, Wnt signaling controls stem cell self-renewal and tissue homeostasis [3,4]. Mutations in Wnt pathway components underlie numerous developmental disorders and cancers in humans [1,5]. For example, the vast majority of colorectal carcinomas are triggered by aberrant Wnt pathway activation [6-8]. New small molecule inhibitors that target Wnt pathway components have recently entered pre-clinical studies and phase I clinical trials [9-14]. These promising strategies for targeted therapy against Wnt-dependent diseases highlight the importance of fundamental research that elucidates critical regulatory nodes in Wnt signaling. Herein, we analyze recent studies from the He lab published in Science, as well as studies from the Clevers and Kirschner labs published in Cell and Science, respectively, that provide insight into the initial signaling events triggered by Wnt stimulation [15-17].
A tale of two complexes: “Destruction” and “LRP6 Signaling”
Levels of β-catenin, the key transcriptional co-factor in the canonical Wnt pathway, are tightly controlled within cells [1,2]. Excess β-catenin leads to constitutive pathway activation in the absence of the Wnt ligand. Conversely, insufficient levels of β-catenin impede the response to Wnt stimulation and also disrupt the second essential role of β-catenin in the formation and maintenance of adherens junctions, which are crucial for epithelial tissue integrity [18-21]. β-catenin protein synthesis is constant under physiological conditions in most cellular systems. In order to maintain steady-state levels, β-catenin is targeted for proteolysis by a dedicated cytoplasmic “β-catenin destruction complex” (or simply “destruction complex”) composed of multiple proteins, including two tumor suppressors, Axin and Adenomatous polyposis coli (APC), and two enzymes, casein kinase 1α (CK1α) and glycogen synthase kinase 3 (GSK3) (Figure 1A). Following sequential phosphorylation by CK1α and GSK3, β-catenin is targeted for ubiquitination by the F-box E3 ubiquitin ligase β-TrCP, with subsequent proteasomal degradation [22-25]. Axin, which has distinct binding sites for APC, GSK3, CK1α, and β-catenin, catalyzes this reaction by providing an essential scaffold for destruction complex assembly [26-29].
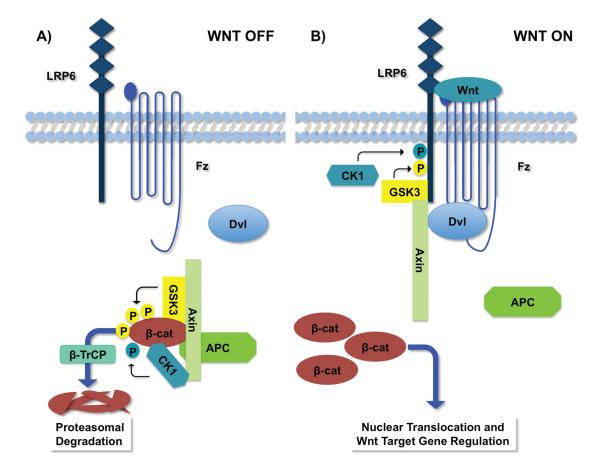
Figure 1. Wnt/β-catenin Signaling.
A) The β-catenin destruction complex. In the absence of Wnt stimulation, steady-state levels of β-catenin are maintained via its constitutive synthesis and proteolysis. The Axin scaffold facilitates the association of β-catenin, GSK3, CK1α , and APC. Phosphorylation of β-catenin by CK1α and GSK3 promotes its recognition by the E3 ubiquitin ligase β-TrCP, targeting β-catenin for proteasomal degradation. B) The LRP6 signaling complex. Wnt exposure induces formation of a receptor complex between Fz, LRP6, and Wnt, and recruitment of Dvl to Fz. Formation of this complex triggers phosphorylation of LRP6 by GSK3 and CK1, and subsequent recruitment of Axin and GSK3 to phospho-LRP6, which results in increased LRP6 phosphorylation. Formation of the LRP6 signaling complex results in inactivation of the destruction complex, leading to β-catenin stabilization, nuclear translocation, and a Wnt-specific transcriptional program.
Wnt stimulation inhibits β-catenin proteolysis, allowing accumulated cytoplasmic β-catenin to enter the nucleus, engage TCF/LEF transcription factors, and regulate Wnt target gene expression [1,2]. Signaling is initiated when Wnt ligands bind their transmembrane co-receptors, Frizzled and low-density lipoprotein receptor-related protein 5/6 (herein referred to as LRP6 for simplicity)(Figure 1B). Upon engagement of the co-receptors by Wnt, phosphorylation of LRP6 recruits Axin, forming a membrane-associated “LRP6 signaling complex,” which includes GSK3 and the cytoplasmic protein, Dishevelled (Dvl) [30-36]. In the prevailing model, recruitment of Axin to the LRP6 signaling complex inhibits the activity of the destruction complex (possibly via its disassembly), thereby stabilizing β-catenin [2].
Axin: double agent in dueling complexes
Axin is a pivotal player in the central switch that regulates Wnt-induced stabilization of β-catenin. The concentration of Axin is several orders of magnitude lower than that of other destruction complex components [37,38]. Because Axin is a required scaffold for the destruction complex, its limiting concentration dictates the amount of β-catenin that is targeted for proteolysis [39,40]. Upon Wnt stimulation, Axin switches roles to provide a scaffold for the LRP6 signaling complex, a function facilitated by its binding to LRP6, Dvl, and GSK3 (Figure 1B). By bringing GSK3 in proximity with LRP6, Axin promotes further LRP6 phosphorylation, creating a positive feedback loop [35]. Phospho-LRP6 subsequently inhibits β-catenin degradation to promote signaling [41-43].
The mechanism by which the destruction complex is inhibited in response to Wnt stimulation has been controversial [1,2]. Previous studies have long supported a model in which the LRP6 signaling complex inhibits the phosphorylation of β-catenin, thereby blocking its recognition by the E3 ligase, β-TrCP [1,2,22,44]. A recent study by Clevers and colleagues published in Cell, however, has called into question the fundamental tenets of this prevailing model for β-catenin regulation (see below) [16]. The crucial roles played by Axin in both the destruction and LRP6 signaling complexes suggested that it might hold the key to unlocking this mystery.
Coupling Axin phosphorylation state to Wnt pathway activation
GSK3-mediated phosphorylation of Axin increases its interaction with other components in the destruction complex, thereby enhancing β-catenin phosphorylation and subsequent degradation [39,45]. Axin phosphorylation increases both its activity in the destruction complex and its stability [46]. Following Wnt stimulation, Axin is dephosphorylated, which occurs concurrently with decreased β-catenin-Axin interaction and increased β-catenin stabilization [46,47]. Protein phosphatase 1 (PP1), a ubiquitous serine/threonine phosphatase, dephosphorylates Axin [48]. The phosphorylation and dephosphorylation of Axin are believed to be highly regulated processes. Due to low levels of endogenous Axin, however, previous studies have relied largely on overexpression to analyze Axin activity and in vitro assays to examine Axin phosphorylation. How changes in Axin phosphorylation modulate Wnt signaling under physiological conditions has not been adequately explored.
To explore this important issue, He and colleagues demonstrated that the timing of Wnt-dependent Axin dephosphorylation at certain phosphorylation sites is coincident with Wnt-dependent β-catenin stabilization, suggesting a functional link between these two processes [15]. Critical for their studies was the generation of an antibody that recognizes phosphorylation of two serine residues in Axin, S497 and S500, which were shown previously to be in vitro sites of GSK3-mediated phosphorylation [15,45]. Notably, S497 and S500 are distinct from the phosphorylation sites known to regulate Axin stability [46]. The He group found that endogenous Axin is dephosphorylated at S497/500 within 15 to 30 minutes of Wnt stimulation, concurrent with the initial stabilization of β-catenin. These intriguing observations raised the immediate question of whether Axin dephosphorylation not only coincides with, but also is necessary for, β-catenin stabilization.
A balancing act between GSK3 and PP1 controls Axin scaffold function
Insight into how the phosphorylation state of Axin controls the degradation of β-catenin came from the identification by the He group of the phosphatase PP1 and its negative regulator, Inhibitor-2 (I2) [15]. These two proteins were shown to alter the activity of Axin in response to Wnt activation via their effects on the association of Axin with both the β-catenin destruction complex and the LRP6 signaling complex [15]. Starting with an overexpression screen to identify proteins that promote Wnt signaling, they identified the gamma isoform of PP1c, one of three human genes that encode the catalytic subunit of PP1. The He group demonstrated that PP1c gamma inhibited GSK3-dependent Axin phosphorylation and promoted Axin dephosphorylation following Wnt exposure. PP1c likely has an evolutionarily conserved role in Wnt signaling, as a previous RNAi screen for Wingless pathway components in Drosophila S2 cells had also identified PP1c [48].
The phosphatase activity of PP1 towards Axin was itself shown by He and colleagues to be regulated by the PP1c inhibitor, I2 [15]. Distinct PP1c-binding proteins confer specificity on PP1c towards its many substrates. I2, which was the first PP1 regulator identified, inactivates PP1 by blocking its catalytic site [49,50]. The He group demonstrated that endogenous I2 prevented aberrant activation of Wnt signaling in cultured human cells and Xenopus embryos. Furthermore, overexpression of I2 inhibited Wnt signaling, Wnt-mediated Axin dephosphorylation, and β-catenin stabilization. By elucidating the important roles of PP1 and I2 in regulating Wnt signaling, the He group has provided crucial evidence that Axin dephosphorylation is important for the stabilization of β-catenin in response to Wnt stimulation.
Using a combination of pharmacological and genetic studies, He and colleagues have advanced our understanding of how the phosphorylation state of Axin alters its activity and association with other components in the Wnt pathway. They found that, in the absence of Wnt signaling, GSK3-dependent Axin phosphorylation increased the association of Axin with LRP6 and with β-catenin; conversely, upon Wnt activation, PP1-dependent Axin dephosphorylation decreased both of these interactions. Therefore, the phosphorylation state of Axin, which is regulated by Wnt signaling, determines its availability as a scaffold for both the destruction and LRP6 signaling complexes.
An intramolecular interaction inactivates Axin
How does the phosphorylation state of Axin control its scaffold function? He and colleagues proposed that the various phosphorylation states of Axin are associated with distinct structural conformations that alter its scaffolding activity [15] (Figure 2). In the absence of Wnt, GSK3-mediated phosphorylation of Axin promotes an “open” conformation that facilitates the association of Axin with β-catenin and its availability for engagement with LRP6 following Wnt exposure. Following Wnt stimulation, Axin binds to LRP6 and is dephosphorylated by PP1. The dephosphorylated form of Axin subsequently undergoes an intramolecular association to form a “closed” conformation, which inhibits the association of Axin with both β-catenin and LRP6 (Figure 2). Three independent findings from the He group support this new concept [15]. (i) The centrally located β-catenin binding domain (BCD) of Axin forms an intramolecular association with its carboxy-terminal DIX domain (the binding site for Dvl) [1,51-54]. This interaction is decreased by GSK3-mediated Axin phosphorylation, but increased following Wnt stimulation (Figure 2). (ii) The DIX domain of Axin competes with β-catenin for binding to the BCD domain of Axin. (iii) An inverse correlation exists between the strength of intramolecular interaction of the BCD-DIX domain of Axin and its capacity to inhibit Wnt signaling. Thus, regulated phosphorylation and dephosphorylation within the BCD domain of Axin controls its conformation and, as a consequence, its scaffold function.
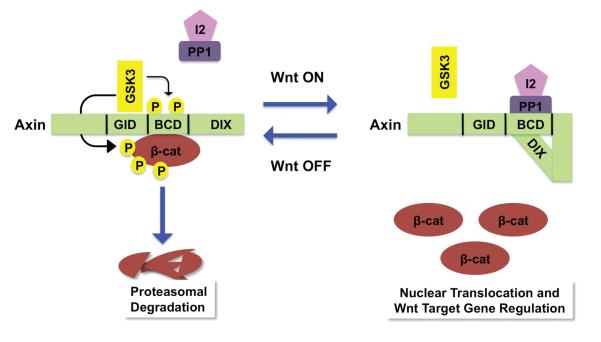
Figure 2. The Axin conformation controls its scaffold function.
Left. In the absence of Wnt, Axin is phosphorylated by GSK3 at the S497/500 sites, located within the central β-catenin binding domain (BCD). Axin phosphorylation results in an “open” conformation that promotes its interaction with β-catenin, targeting β-catenin for ubiquitin-dependent proteolysis. The open conformation also primes Axin for interaction with LRP6. GID: GSK3 interaction domain; DIX: Dishevelled interaction domain. Right. In the presence of Wnt, Axin is dephosphorylated by PP1 at the S497/500 sites. Following its dephosphorylation, the BCD of Axin associates with its carboxy-terminal DIX domain, resulting in a “closed” conformation. This intramolecular association prevents the interaction of Axin with both LRP6 and β-catenin, thereby inactivating Axin.
Based on these findings, He and colleagues proposed a new model in which Axin has a central role in the Wnt signaling pathway, not only as a scaffold for the destruction and LRP6 signaling complexes, but also as a conformation-dependent switch toggled by Wnt-induced dephosphorylation to activate signaling (Figure 3) [15]. This model provides a mechanistic explanation as to how Axin dephosphorylation results in β-catenin stabilization following Wnt stimulation. The prediction from this model is that dissociation of Axin from LRP6 renders LRP6 available for multiple rounds of engagement with Axin, thereby allowing LRP6 to act in a catalytic fashion to sustain activation of the pathway. Additionally, the conformation of Axin could serve as a feedback sensor of β-catenin levels. When present at high levels, β-catenin would inhibit the intramolecular association of Axin, promote the Axin-β-catenin interaction, and thereby facilitate its own degradation.
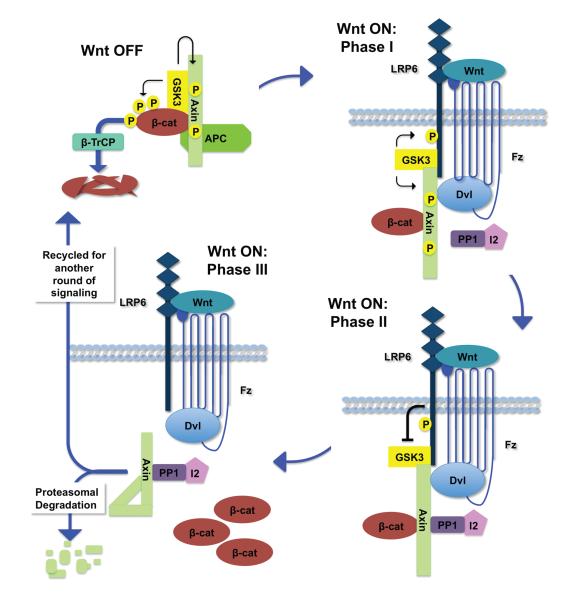
Figure 3. The Axin phosphorylation/dephosphorylation cycle.
Top left, in the absence of Wnt. Axin is phosphorylated by GSK3, maintained in an open conformation, engaged in the destruction complex, and primed for interaction with LRP6. Top right, Phase I following Wnt exposure. LRP6 undergoes phosphorylation. Phospho-Axin is recruited from the destruction complex to the LRP6 signaling complex through its interaction with phospho-LRP6. Bottom right, Phase II following Wnt exposure. The LRP6 signaling complex inhibits GSK3, thereby tipping the balance towards Axin dephosphorylation by PP1. Bottom left, Phase III following Wnt exposure. Dephosphorylation of Axin by PP1 results in a closed conformation, thereby disengaging Axin from LRP6 and β-catenin. Phospho-LRP6 becomes free for additional rounds of engagement with phospho-Axin, leading to further dephosphorylation and inactivation of Axin. Dephosphorylated Axin is subsequently targeted for proteasomal degradation or recycled for destruction complex assembly.
Revisiting the regulation of β-catenin following Wnt stimulation
How does the new mechanism for Axin control proposed by He and colleagues fit into the bigger picture of Wnt pathway activation? The prevailing model for β-catenin regulation within the destruction complex is founded on the crucial scaffold role of Axin, which brings β-catenin in close proximity to GSK3 to promote its phosphorylation and proteolysis. In the widely accepted model of Wnt pathway activation, β-catenin phosphorylation is decreased through physical dissociation and/or inactivation of the destruction complex following Wnt stimulation [2,22,44,47].
Clevers and colleagues recently proposed a fundamentally different model for β-catenin regulation in which they posited that Wnt stimulation inhibits β-catenin degradation, not by inhibiting its phosphorylation by GSK3 within the destruction complex, but rather by inhibiting its ubiquitination by β-TrCP [16]. The Clevers group used an Axin antibody to perform co-immunoprecipitations to examine destruction complex components in the absence or presence of Wnt exposure. They concluded that both the Axin-β-catenin interaction and β-catenin phosphorylation by GSK3 within the destruction complex are unaltered following Wnt exposure. Instead, they concluded that Wnt stimulation results in the dissociation of β-TrCP from β-catenin. Their new model posits that phosphorylated β-catenin accumulates and saturates the intact destruction complex upon Wnt stimulation. As a consequence, the saturated destruction complex is incapable of degrading newly synthesized β-catenin, thereby resulting in β-catenin accumulation and subsequent Wnt pathway activation. As these provocative new findings contrasted markedly with the long-standing model for β-catenin regulation upon Wnt stimulation, they have necessitated re-examination of the core mechanisms that underlie the activation of Wnt signaling.
The question of whether Wnt stimulation and the subsequent stabilization of β-catenin is due to inhibition of β-catenin phosphorylation (long-standing model) or inhibition of β-catenin ubiquitination (alternative model) within the destruction complex was tackled directly by Kirschner and colleagues [17]. Their studies have provided compelling support for the prevailing model that Wnt stimulation inhibits β-catenin phosphorylation via dissociation and/or inactivation of the destruction complex. The Kirschner group examined phospho-β-catenin levels before and after Wnt stimulation, coupling their quantitative measurements with kinetic modeling to gain insight into the temporal course of β-catenin regulation. They observed a rapid decrease in phosphorylated β-catenin levels within 30 minutes of Wnt exposure (similar to the He group’s observations) that parallels an increase in total β-catenin levels. Significantly, when GSK3-phosphorylated β-catenin levels were at a minimum, the rate of accumulation of total β-catenin was at a maximum. By 2 hours and beyond, while the rate of β-catenin synthesis remained the same, GSK3-phosphorylated β-catenin levels returned to pre-Wnt stimulation levels, and a higher steady-state level of β-catenin was observed. This return to pre-Wnt stimulation rates of β-catenin degradation can be simply explained by the increased flux through the degradation system as the concentration of β-catenin rises.
Whereas Clevers and colleagues hypothesized that newly synthesized, nonphosphorylated β-catenin is stabilized following saturation of the destruction complex [16], the Kirschner group demonstrated that the destruction complex is not saturated when β-catenin levels reach a new steady state following Wnt exposure [17]. Therefore, the Kirschner group concluded that partial inhibition of β-catenin phosphorylation alone (possibly from destruction complex disassembly) fully accounts for the entire temporal course of β-catenin regulation following Wnt stimulation. The Clevers and Kirschner groups approached the question of how Wnt stimulation inhibits β-catenin degradation in different ways: the Clevers group assessed the status of the destruction complex, whereas the Kirschner group assessed changes in total levels of pathway constituents. Thus, it is possible that the opposite conclusions reached by these two groups reflect their differing experimental approaches.
To address these contradictory conclusions, He and colleagues reassessed the phosphorylation state of β-catenin following Wnt exposure using an experimental approach that was similar to that of the Clevers group [15]. Their new findings have provided compelling evidence that inhibition of β-catenin phosphorylation causes subsequent β-catenin stabilization and that this is the major consequence of Wnt stimulation. He and colleagues focused on the interaction between Axin and β-catenin and tested whether phosphorylated β-catenin accumulates in the destruction complex following Wnt exposure. They analyzed both the kinetics of β-catenin accumulation and the affinity of the Axin-β-catenin interaction by examining changes in the dissociation rate following Wnt exposure. As observed by Clevers and colleagues [16], the He group found that increased levels of β-catenin are associated with Axin within 2 hours of Wnt stimulation; however, careful measurements of cytoplasmic β-catenin levels revealed that the apparent affinity between β-catenin and Axin actually decreased following Wnt stimulation [15]. Similarly, the He group found a parallel decrease in the rate of β-catenin phosphorylation within the Axin complex in response to Wnt stimulation [15].
Given that the Clevers and the He groups used similar experimental approaches and made some similar observations regarding phospho-β-catenin levels and the association of phospho-β-catenin with Axin, how did the two groups reach opposite conclusions? A major difference between these two studies was that the He group made careful measurements of cytoplasmic β-catenin levels and the total amount of β-catenin that associated with Axin, thereby allowing quantification of the changes in the β-catenin phosphorylation rate and the affinity of Axin-phospho-β-catenin interaction following Wnt exposure. Based on these calculations, He and colleagues concluded that the rate of GSK3-dependent β-catenin phosphorylation is the primary determinant of β-catenin stability following Wnt stimulation and that this rate is dependent on Axin activation and inactivation through GSK3 and PP1, respectively.
Conclusions and future directions
He and colleagues have uncovered an elegant regulatory mechanism that underlies the initial signaling events triggered by Wnt exposure. Their discovery that the Axin phosphorylation state controls its conformation, and thereby determines its availability as a scaffold, sheds light on how the destruction and LRP6 signaling complexes are regulated following Wnt stimulation. Furthermore, the studies of He, Kirschner, and colleagues have provided compelling support for the recently challenged but long-standing model by which Wnt stimulation stabilizes β-catenin.
These new findings also raise interesting questions for future studies. (i) Is PP1 activated by Wnt stimulation, and, if so, is activated PP1 directed to the pool of phospho-Axin associated with phospho-LRP6? Is PP1 activation controlled through regulation of I2? (ii) Axin dephosphorylation following Wnt stimulation controls its degradation, although it occurs at phosphorylation sites that are distinct from those identified by He and colleagues [46,47]. Because Axin degradation occurs more than 2 hours after Wnt stimulation, it is unlikely to affect the initial response to Wnt exposure; alternatively, it may modulate the duration and/or level of signaling. How are the distinct Axin phosphorylation sites differentially regulated to direct early and late Wnt responses, and how are these dephosphorylation events coordinated? (iii) Because Axin dephosphorylation occurs only after its association with phospho-LRP6, what initial changes decrease the interaction of Axin with the β-catenin destruction complex? Do modifications in Axin and/or its binding partners facilitate this Wnt-dependent shift? (iv) Would direct structural analyses confirm the conformational changes in Axin? In the proposed feedback model, increased β-catenin levels “compete” with the intramolecular closed confirmation of Axin to drive Axin-β-catenin interactions, thereby blocking further rises in β-catenin levels. Are the elevated β-catenin levels that result from Wnt stimulation sufficient to overcome interaction between the Axin BCD and DIX domains, or is this process facilitated?
The recent studies from the He, Kirschner, and Clevers labs have provided a major advance in our mechanistic understanding and have raised new questions that will guide future studies of Wnt signal transduction, a pathway that plays critical roles in human development and disease.
Acknowledgments
We thank Laurie Lee and Ai Tian for critical reading of the manuscript. Our work is supported by Emerald Foundation (Y.A.), Norris Cotton Cancer Center (Y.A.), and the National Institutes of Health (RO1CA105038 to Y.A.; R01GM081635 and R01GM103926 to E. L.).
References
- 1.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 4.Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–64. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Laine CM, Joeng KS, Campeau PM, Kiviranta R, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–16. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin PJ, Sparks AB, Korinek V, Barker N, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, He X. Destruction of a destructor: a new avenue for cancer therapeutics targeting the Wnt pathway. J Mol Cell Biol. 2010;2:70–3. doi: 10.1093/jmcb/mjp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lum L, Clevers H. Cell biology. The unusual case of Porcupine. Science. 2012;337:922–3. doi: 10.1126/science.1228179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau T, Chan E, Callow M, Waaler J, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–44. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, Dodge ME, Tang W, Lu J, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SM, Mishina YM, Liu S, Cheung A, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 14.Waaler J, Machon O, Tumova L, Dinh H, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72:2822–32. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- 15.Kim SE, Huang H, Zhao M, Zhang X, et al. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–70. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li VS, Ng SS, Boersema PJ, Low TY, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–56. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez AR, Klein AM, Kirschner MW. Kinetic responses of beta-catenin specify the sites of Wnt control. Science. 2012;338:1337–40. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 18.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 19.Rudloff S, Kemler R. Differential requirements for beta-catenin during mouse development. Development. 2012;139:3711–21. doi: 10.1242/dev.085597. [DOI] [PubMed] [Google Scholar]
- 20.Muller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–63. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol. 1996;134:133–48. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Li Y, Semenov M, Han C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 23.Amit S, Hatzubai A, Birman Y, Andersen JS, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart M, Concordet JP, Lassot I, Albert I, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–10. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–6. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 26.Hedgepeth CM, Deardorff MA, Rankin K, Klein PS. Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin. Mol Cell Biol. 1999;19:7147–57. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagotto F, Jho E, Zeng L, Kurth T, et al. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–56. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt J, Ferandin Y, Meijer L. Purification of CK1 by affinity chromatography on immobilised axin. Protein Expr Purif. 2007;54:101–9. doi: 10.1016/j.pep.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Spink KE, Polakis P, Weis WI. Structural basis of the Axin adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–9. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao J, Wang J, Liu B, Pan W, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 31.Tamai K, Zeng X, Liu C, Zhang X, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–56. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 32.Bilic J, Huang YL, Davidson G, Zimmermann T, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–22. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 33.Zeng X, Tamai K, Doble B, Li S, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson G, Wu W, Shen J, Bilic J, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–72. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 35.Zeng X, Huang H, Tamai K, Zhang X, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–75. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120:2402–12. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 37.Lee E, Salic A, Kruger R, Heinrich R, et al. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–32. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda S, Kishida S, Yamamoto H, Murai H, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behrens J, Jerchow BA, Wurtele M, Grimm J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 41.Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proc Natl Acad Sci U S A. 2008;105:8032–7. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piao S, Lee SH, Kim H, Yum S, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu G, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valvezan AJ, Zhang F, Diehl JA, Klein PS. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J Biol Chem. 2012;287:3823–32. doi: 10.1074/jbc.M111.323337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jho E, Lomvardas S, Costantini F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Kishida S, Kishida M, Ikeda S, et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 47.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–73. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo W, Peterson A, Garcia BA, Coombs G, et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–21. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurley TD, Yang J, Zhang L, Goodwin KD, et al. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007;282:28874–83. doi: 10.1074/jbc.M703472200. [DOI] [PubMed] [Google Scholar]
- 50.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–45. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Kishida S, Yamamoto H, Hino S, Ikeda S, et al. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–22. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng L, Fagotto F, Zhang T, Hsu W, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 53.Julius MA, Schelbert B, Hsu W, Fitzpatrick E, et al. Domains of axin and disheveled required for interaction and function in wnt signaling. Biochem Biophys Res Commun. 2000;276:1162–9. doi: 10.1006/bbrc.2000.3607. [DOI] [PubMed] [Google Scholar]
- 54.Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–66. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]