Abstract
Purpose
To evaluate the clinical activity of sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive AML and monitor the emergence of secondary FLT3 tyrosine kinase domain (TKD) mutations during treatment.
Experimental Design
Six children with relapsed/refractory AML were treated with sorafenib in combination with clofarabine and cytarabine, followed by single-agent sorafenib if not a candidate for transplantation. Sunitinib was initiated after sorafenib relapse. Bone marrow samples were obtained for assessment of FLT3 TKD mutations by deep amplicon sequencing. The phase of secondary mutations with ITD alleles was assessed by cloning and sequencing of FLT3 exons 14 through 20. Identified mutations were modeled in Ba/F3 cells and the effect of kinase inhibitors on FLT3 signaling and cell viability was assessed.
Results
Four patients achieved complete remission, but 3 receiving maintenance therapy with sorafenib relapsed after 14–37 weeks. Sunitinib reduced circulating blasts in 2 patients and marrow blasts in 1. Two patients did not respond to sorafenib combination therapy or sunitinib. FLT3 mutations at residues D835 and F691 were observed in sorafenib resistance samples on both ITD-positive and –negative alleles. Deep sequencing revealed low-level mutations and their evolution during sorafenib treatment. Sunitinib suppressed leukemic clones with D835H and F691L mutations, but not D835Y. Cells expressing sorafenib-resistant FLT3 mutations were sensitive to sunitinib in vitro.
Conclusions
Sunitinib has activity in patients that are resistant to sorafenib and harbor secondary FLT3 TKD mutations. The use of sensitive methods to monitor FLT3 mutations during therapy may allow individualized treatment with the currently available kinase inhibitors.
Keywords: acute myeloid leukemia, sorafenib, sunitinib, FLT3, mutations
INTRODUCTION
FMS-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) activating mutations are seen in leukemic cells of approximately 30% of adults and 15% of children with acute myeloid leukemia (AML) at diagnosis, and are associated with a poor prognosis. A variety of small molecule tyrosine kinase inhibitors (TKIs) that target FLT3 are in various stages of preclinical and clinical development in AML (1–3). The multikinase inhibitors sorafenib and sunitinib demonstrated potent activity against leukemia cells with FLT3-ITD mutations in preclinical studies (4, 5) and are now being used to treat patients with FLT3-ITD-positive AML.
Most patients with FLT3-ITD-positive AML show an initial favorable response to FLT3 inhibitors, followed by the development of resistance (6–9). A variety of resistance mechanisms to FLT3 inhibitors have been described, including increased FLT3 receptor and ligand expression, upregulation of compensatory signaling pathways, acquired mutations in the tyrosine kinase domain (TKD) of FLT3, mutations in other kinase genes, and upregulation of anti-apoptotic proteins (2, 10–13). One of the most common mechanisms of resistance to FLT3 inhibitors is the acquisition of secondary FLT3 TKD mutations (14–21). These primarily consist of point mutations in the activation loop of TKD2 and in the ATP-binding pocket of TKD1. Numerous preclinical studies have shown that FLT3 TKIs display non-overlapping profiles of resistance to FLT3 mutations (14–21), and it has been suggested that this information may guide the selection of TKI therapy for individual patients, administered either sequentially or in combination.
In this study, we report the clinical activity of sequential therapy with sorafenib and sunitinib in children with FLT3-ITD-positive AML and the emergence of polyclonal secondary FLT3 TKD mutations during TKI therapy as identified by deep amplicon sequencing. We observed prominent mutations at residues D835 and F691 in blast samples at sorafenib resistance. Deep sequencing revealed low-level mutations in resistance samples as well as the evolution of mutations during sorafenib treatment. Subsequent sunitinib therapy suppressed leukemic clones with F691L and D835H mutations, but not those containing D835Y mutations.
MATERIALS AND METHODS
Patients
From November 2009 to October 2012, six children with relapsed or refractory FLT3-ITD-positive AML received initial therapy with sorafenib 200 mg/m2 twice daily alone or in combination with clofarabine plus cytarabine as described previously (22). Patients not eligible for hematopoietic stem cell transplantation (HSCT) received subsequent treatment with sorafenib monotherapy if they responded initially, followed by sunitinib at the time of resistance to sorafenib. The rationale for sequential therapy with sunitinib was based on in vitro data showing that sorafenib and sunitinib have different inhibitory potencies towards a variety of drug-resistant FLT3 mutations in binding assays and cell models (20, 23). Bone marrow samples were obtained before and during treatment and at the time of subsequent relapse for assessment of FLT3 mutations. Bone marrow samples were enriched for leukemic blasts by ficoll purification. Trough concentrations of sorafenib and the metabolite sorafenib N-oxide were measured in plasma during sorafenib treatment using a validated analytical method based on liquid chromatography-tandem mass spectrometry (24). All treatments and assessments were approved by the institutional review board and informed consent was obtained from all patients or their legal guardians.
FLT3 mutation analysis
To assess for FLT3 mutations, FLT3 exons 13 to 23 were sequenced in paired blast samples that were obtained pre-treatment and at TKI resistance. If a mutation was not clearly detected from direct sequencing, PCR products of individual exons were cloned and sequenced. To determine if FLT3 point mutations were on ITD-positive or ITD–negative alleles, FLT3 exons 14 through 20 were amplified from cDNA. Details of the PCR primers, reaction conditions, and cloning and sequencing procedures are summarized in the Supplemental Materials.
Deep amplicon sequencing
Analysis of mutations in FLT3 exons 17 and 20 was performed by deep amplicon sequencing. Details of the PCR primers and reaction conditions are summarized in the Supplemental Materials. For deep sequencing analysis, PCR amplicons (1 ng input DNA) were fragmented, tagged with adapters, and libraries were prepared using the Nextera XT DNA Sample Preparation Kit following manufacturer’s instructions (Illumina, San Diego, CA). Libraries were normalized and pooled using manufacturer’s protocol then sequenced on an Illumina MiSeq System (Illumina) with 150-bp paired-end reads. Image analyses and base calling were performed using MiSeq Control Software versions 1.5.15.1 or 2.2 and Real Time Analysis (RTA) versions 1.13.148 or 1.17.28. After removing the adapter sequences, high-quality reads (Phred-like score Q25 or greater) with at least 50 nucleotides were aligned to human FLT3 reference sequence (UCSC hg19). Mutations were detected and the frequencies of mutation were determined using CLC Genomics Workbench v5.5 (CLC Bio, Denmark). As a control, PCR amplicons of gDNA from three individual healthy subjects were included in each sequencing run and subjected to the same analysis as those of the patient samples, and the results were used to generate empirical thresholds for making significant calls for mutation observation. The minimum frequency of mutation detection at each genomic location was set with a threshold at the upper limit of the 99.99% confidence interval from the maximum sequencing error rate, generated from three independent sequencing runs for each of the three normal samples (Supplemental Table 2). These thresholds represent the platform sensitivities for detecting the low frequency allele with corresponding substitution.
Cell culture and reagents
Mouse Ba/F3 cells were obtained from DSMZ (Brunswick, Germany) and maintained in RPMI 1640 + L-Glutamine (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA) and 10 ng/mL IL-3 (PeproTech, Rocky Hill, NJ). Retroviral supernatants were produced by using ecotropic Phoenix packaging cells (Allele Biotechnology, San Diego, CA) and were maintained in DMEM (Gibco) supplemented with 10% FBS. Antibodies against FLT3 (C-20), β-actin (C4), and phosphotyrosine (pY99) were obtained from Santa Cruz Biotechnology (Dallas, TX); phospho-STAT5 (pY694) antibody was purchased from Cell Signaling Technology (Danvers, MA).
Generation of FLT3-expressing Ba/F3 cells
A MSCV-IRES-GFP vector containing FLT3-ITD was a generous gift from Dr. Scott Lowe (Memorial Sloan-Kettering). Point mutations were introduced by site-directed mutagenesis as previously described (25), and mutations were confirmed by DNA sequencing. Subsequently, Eco-HEK293 cells were transfected with FLT3 vectors using FuGENE HD Transfection Reagent (Promega, Madison, WI) according the manufacturer’s instructions and viral supernatant was collected over a 36-hr period. Viral supernatant was added to retronectin-coated plates and centrifuged at 2100 × g for 2 h. Ba/F3 cells (1 × 106) were then transduced with virus for 48 hr followed by GFP FACS analysis. GFP-positive cells were selected for IL3-independent cell growth over a period of 7 days.
Western Blot Analysis
Cells were collected, washed with phosphate-buffered saline, and lysed using radioimmunoprecipitation assay buffer (without SDS; 150 mM NaCl, 9.1 mM Na2HPO4, 1.7 mM NaH2PO4, 1% NP-40, and 0.5% deoxycholic acid, pH 7.4) supplemented with protease inhibitors (Roche, Indianapolis, IN) and phosphatase inhibitors (Merck KGaA, Darmstadt, Germany). For FLT3 immunoprecipitation, 200 μg total cell lysate was incubated with FLT3 antibodies and protein A/G agarose (Santa Cruz Biotechnology) for 2 h at 4°C with constant agitation. Total cell lysate (30–50 μg) or FLT3 immunoprecipitation eluent was separated by SDS-polyacrylamide gel electrophoresis according to the manufacturer’s instructions (Invitrogen) and transferred to PVDF membranes followed by immunoblot analysis using the indicated antibodies as previously described (25).
Structural modeling of kinase inhibitor interaction with FLT3
A model of FLT3 interaction with sorafenib and sunitinib was constructed by superimposing the crystal structures of c-Kit bound to sunitinib (Protein Data Bank (PDB) code 3G0E) (26) and B-Raf bound to sorafenib (PDB 1UWH) (27) on FLT3 (PDB 1RJB) (11). Images were generated and analyzed using Pymol software (Schrodinger, San Diego, CA).
RESULTS
Patients and treatment outcome
The characteristics and treatment outcomes of 6 patients with relapsed/refractory FLT3-ITD-positive AML are summarized in Table 1. After treatment with sorafenib alone, or in combination with clofarabine and cytarabine, 4 patients achieved morphological complete remission (reduction of bone marrow blasts to < 5%). Three of the 4 patients received subsequent monotherapy with sorafenib followed by sunitinib (Figure 1) and the fourth patient received a HSCT. Two patients had persistent disease after receiving sorafenib in combination with clofarabine and cytarabine as well as with subsequent sunitinib monotherapy. The clinical course of each patient is described in detail below.
Table 1.
Patient characteristics and treatment outcome.
| Patient Number | Sex/Age | Prior therapy | Karyotype at enrollment | Initial therapy | BBMR | Subsequent therapy | Blasts in relapse sample (%) | New FLT3 mutation at relapse* |
|---|---|---|---|---|---|---|---|---|
| 1 | 14/M | SJCRH AML02; matched Sib HSCT | 47,XY,+8[20] | Sorafenib, Clo/Ara-C | CR (d 43) | Sorafenib (19 weeks) Sunitinib (10 weeks) |
93 (Sorafenib) --- |
D835H (Sorafenib) --- |
| 2 | 10/M | As per COG AAML0531 |
46,XY[20] | Sorafenib, Clo/Ara-C, | CRi (d 116) | Sorafenib (37 weeks) Sunitinib (9 weeks) |
57 (Sorafenib) --- |
D835H (Sorafenib) --- |
| 3 | 12/M | SJCRH AML08; HLA- identical mother HSCT | 46,XY,t(1;7)(q32;q36),t(1;1) (p13;q12),t(15;17)(q26;q23, t(18;20)(q11.2;q13.3)[20] | Sorafenib | CR (d 53) | Sorafenib (14 weeks) Sunitinib (13 weeks) |
59 (Sorafenib) 70 (Sunitinib) |
D835H (Sorafenib) D835H/Y (Sunitinib) |
| 4 | 12/M | COG AAML0531 | 47,XY,+8[12]/46,XY[8] | Sorafenib, Clo/Ara-C | NR (d 50) | Sunitinib (NR) | --- | --- |
| 5 | 12/F | As per COG AAML0531; matched Sib HSCT |
46,XX[20] | Sorafenib, Clo/Ara-C | CRi (d 58) | HSCT | --- | --- |
| 6 | 11/F | As per COG AAML0531: matched Sib HSCT | 46,XX,t(6;9)(p23;q24)[20] | Sorafenib, Clo/Ara-C | NR (d 36) | Sunitinib (NR) | --- | --- |
Mutation detected by cloning and/or direct sequencing.
Abbreviations: BBMR, best bone marrow response; Clo/Ara-C; Clofarabine plus Cytarabine; COG, Children’s Oncology Group protocol; CR, complete response; Cri, CR with incomplete count recovery; d, day; FC, flow cytometry; HSCT, hematopoietic stem cell transplantation; NR, no response; Sib, Sibling; SJCRH, St. Jude Children’s Research Hospital protocol.
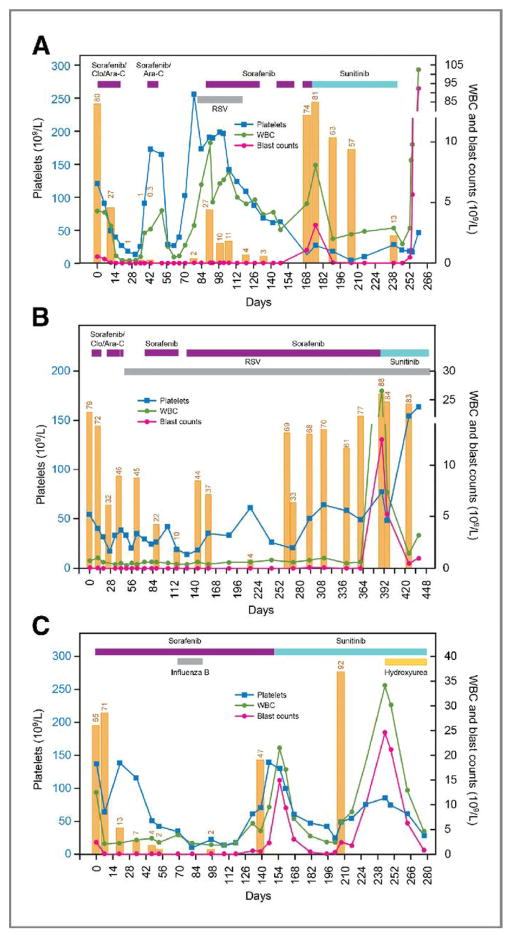
Figure 1.
Clinical course of three patients treated sequentially with sorafenib and sunitinib. Day 0 indicates the initiation of treatment with sorafenib. Duration of treatment is indicated by the horizontal bars at the top of the graph (purple, sorafenib ± clofarabine and/or cytarabine; blue, sunitinib; orange, hydroxyurea). Vertical orange bars represent percentage of bone marrow blast cells assessed by flow cytometry. Abbreviations: Ara-C, cytarabine; Clo, clofarabine; RSV, respiratory syncytial virus; WBC, white blood cell.
Patient 1 was a 14-year-old male who presented with relapsed AML 23 months after HSCT. He achieved a remission with 0.3% minimal residual disease (MRD) 43 days after receiving sorafenib 200 mg/m2 twice daily in combination with clofarabine and cytarabine and received a second course of sorafenib and cytarabine while waiting for second HSCT, but developed respiratory syncytial virus (RSV) infection. While waiting for clearance of the RSV infection, the bone marrow blast percentage increased to 27%. Single-agent sorafenib 200 mg/m2 once daily was given for 6 weeks, and the patient obtained a remission with 4% MRD on day 120 and clearance of the RSV infection. Sorafenib adminstration was temporarily interrupted due to thrombocytopenia. However, the patient’s bone marrow blast percentage increased to 74% and there was no response to a subsequent 7-day course of sorafenib 200 mg/m2 administered twice daily. Sunitinib 50 mg daily was started with disappearance of circulating blasts and decrease in marrow blasts. Due to the development of skin abscesses, sunitinib was stopped after 10 weeks of therapy and the peripheral blood blasts rapidly increased.
Patient 2 was a 10-year-old male with AML with myelodysplasia-related changes, which was refractory to 5 previous regimens. He received 2 courses of sorafenib 200 mg/m2 twice daily in combination with cytarabine and clofarabine. He developed RSV infection and received single-agent sorafenib 200 mg once daily. The patient achieved a morphological remission with incomplete blood count recovery and 10% MRD. Thereafter, he received single-agent sorafenib for 8 months. Although bone marrow had variable percentages of leukemia cells detected by flow cytometry, peripheral blood blasts were not detected. Due to the persistent marrow infiltration with leukemic cells, the sorafenib dose was increased to 200 mg twice daily for 6 weeks. However, his bone marrow blast percentage increased to 88% with a rapid increase in peripheral blood blast count to 12.5×109/L. Sunitinib 25 mg daily was started and a decline in the peripheral blast count to 0.5×109/L was observed. Due to the development of pneumonia, sunitinib was stopped after 9 weeks of therapy.
Patient 3 was a 12-year-old male with relapsed AML, 15 months after HSCT from HLA identical mother. Cytogenetic analysis showed a complex karyotype. Due to chronic graft-versus-host disease, sorafenib 200 mg/m2 once daily was initiated. The patient obtained a remission with 2% MRD, which was maintained for 4 months. The bone marrow blast percentage rose to 47% on day 139, and sorafenib was increased to 200 mg/m2 twice daily for 2 weeks with no response. Treatment was started with sunitinib 25 mg once daily with a subsequent decline in the peripheral blast count. The patient responded to sunitinib for 8 weeks, until a rapid increase in the bone marrow and peripheral blood blasts were observed, at which time hydroxyurea was added. Although peripheral blasts decreased, the patient deceased due to multi-organ failure.
Patients 4 (12-year-old boy with refractory AML) and 6 (11-year-old girl with relapsed AML after HSCT) did not show response to combination therapy with sorafenib (200mg/m2 twice daily) plus clofarabine and cytarabine followed by single-agent sorafenib therapy up to day 51 and 39, respectively (blast percentage 69% to 64% for patient 4 and 79% to 28% for patient 6). Sorafenib was switched to sunitinib for both patients but no responses were seen. Patient 5 (12-year-old girl with relapsed AML after HSCT) did not show immediate response to combination therapy of sorafenib (200mg/m2 twice daily) plus clofarabine and cytarabine, however, the patient achieved complete remission with incomplete count recovery on day 58 with single-agent sorafenib (blast percentage 34%, 27% and 3%, on pre-treatment, day 36, and day 58, respectively). The patient subsequently received cord blood transplantation.
Plasma concentrations of sorafenib and the active metabolite sorafenib N-oxide were measured during therapy, and for patients 2 and 3, up until the time of sorafenib resistance (Supplemental Table 3). Drug concentrations at sorafenib relapse were comparable to those at the initiation of therapy indicating that the observed relapse was not due to lack of drug adherence.
Identification of FLT3 TKD mutations
FLT3 mutations were assessed in bone marrow blast samples obtained pre-treatment and at the time of TKI resistance in 3 patients (Table 2). In all cases, expression of the FLT3-ITD mutation persisted at sorafenib resistance (Supplemental Figure S1). The p.Asp835His (D835H) mutation, which lies in the activation loop of TKD2, was observed in all samples at sorafenib resistance but not in paired pre-treatment samples (Supplemental Figure S1). In patient 3, more than 1 mutation was observed at both sorafenib and sunitinib resistance. The p.Phe691Leu (F691L) mutation, which resides in the ATP-binding domain of TKD1, was observed along with the D835H mutation at sorafenib resistance; whereas at sunitinib resistance, the D835H mutation remained and the p.Asp835Tyr (D835Y) mutation was detected (Supplemental Figure S1).
Table 2.
FLT3 kinase domain mutations observed in patients during sequential therapy with sorafenib and sunitinib by next generation sequencing.
| Patient Number | Sample | Blasts (%) | Mutation frequency
(%)a
|
Coverage D835H/Y/F691L (number of reads x 1000) | ||
|---|---|---|---|---|---|---|
| D835H | D835Y | F691L | ||||
| 1 | Pre-treatment | 80 | ND | ND | ND | 109/103 |
| During sorafenib (day 11) | 38 | ND | ND | ND | 163/94 | |
| During sorafenib (day 91) | 39 | ND | ND | ND | 138/83 | |
| Sorafenib resistance (day 176) | 93 | 33.1 | ND | ND | 30/44 | |
| During sunitinib (day 29) | 35 | 14.4 | ND | ND | 5.4/61 | |
|
| ||||||
| 2 | Pre-treatment | 77 | ND | ND | ND | 122/82 |
| Sorafenib resistance (day 389) | 57 | 35.7 | 0.63 | 0.16 | 29/79 | |
| During sunitinib (day 36) | 53 | 28.2 | 1.03 | ND | 46/142 | |
|
| ||||||
| 3 | Pre-treatment | 48 | ND | ND | ND | 123/57 |
| During sorafenib (day 53) | 2.1b | 0.023 | ND | ND | 110/73 | |
| During sorafenib (day 97) | 2.1b | 0.029 | ND | 0.075 | 86/82 | |
| Sorafenib resistance (day 158) | 59 | 11.0 | 4.0 | 18.2 | 27/55 | |
| During sunitinib (day 57) | 92 | 19.2 | 16.2 | 3.9 | 76/132 | |
| Sunitinib resistance (day 99) | 70 | 13.5 | 28.5 | 2.9 | 28/87 | |
|
| ||||||
| 4 | Pre-treatment | 62 | ND | ND | ND | 96/66 |
| During sorafenib (day 50) | 25 | ND | ND | ND | 95/80 | |
|
| ||||||
| 5 | Pre-treatment | 34 | ND | ND | ND | 85/83 |
| During sorafenib (day 36) | 27 | ND | 0.10 | ND | 107/75 | |
|
| ||||||
| 6 | Pre-treatment | 75 | ND | ND | ND | 35/160 |
| During sorafenib (day 36) | 25 | ND | ND | ND | 146/188 | |
Abbreviations: ND, not detected.
Minimal residual disease sample.
Activating point mutations in the TKD of FLT3, primarily at residue D835, have been observed at diagnosis in approximately 5–10% of patients with AML (28, 29). TKD mutations usually occur independent of the ITD mutation, but can appear together on the same or opposite allele (29, 30). To determine the phase of D835H/Y and F691L mutations on FLT3-ITD alleles in TKI resistance samples, FLT3 exons 14 through 20 (spanning the ITD region through TKD2) were cloned and sequenced from cDNA. In patient 1, the ITD allele burden was 100% at sorafenib resistance, and thus all D835H mutations were found in ITD-positive clones. In patient 2, the D835H mutation was detected in both ITD-positive and –negative clones at sorafenib resistance with an ITD allele burden of 36%. In patient 3, the ITD allele burden was 41% at sorafenib resistance, with the D835Y mutation observed only in ITD-positive clones but the F691L mutation observed in both ITD-positive and –negative clones. Likewise, at sunitinib resistance, the D835H and D835Y mutations were observed in both ITD-positive and –negative clones (Supplemental Table 4).
We next assessed if the double TKD mutations (D835H/F691L and D835H/D835Y) observed at sorafenib and sunitinib resistance in patient 3 were polyclonal (existing on distinct alleles) or compound (existing on the same allele). Cloning and sequence analysis revealed that the mutations were predominantly polyclonal (Supplemental Table 4). Only 1 clone at sorafenib resistance harbored both the F691L and D835H mutation (1/4 observations; 25% of FLT3-ITD-positive alleles).
Deep amplicon sequencing reveals emergence of FLT3 TKD mutations
We extended the FLT3 mutation analysis to deep sequencing of exons 17 and 20, containing the F691L and D835H/Y mutations, respectively, using an assay that provided an average effective depth coverage across the targeted region of greater than 80,000. For this analysis, we included additional samples that were obtained during treatment (Table 2). FLT3 TKD mutations were not detected in pre-treatment samples. Likewise, no mutations were observed during the first course of treatment with sorafenib and chemotherapy, including the 2 patients that did not respond to therapy; therefore, the presence of FLT3 TKD mutations is not a mechanism of primary drug resistance in these patients. In patient 5, the D835Y mutation was observed at a frequency 0.10% on day 36 of treatment; however, this was very close to our defined threshold of mutation detection at this genomic location of 0.085%, and thus may represent a false positive. Assessment of mutations in patient 3, who received continuous monotherapy with sorafenib for 14 weeks prior to developing resistance, revealed the evolution of clones harboring FLT3 TKD mutations. At day 53 of therapy, the D835H mutation was observed at a frequency of 0.023%. At day 97 of treatment, the F691L mutation (0.075%) was detected along with the D835H mutation (0.029%). At day 158 (sorafenib resistance), the D835Y mutation (4.0%) was observed along with an increase in frequency of the D835H and F691L mutations (11.0% and 18.2%, respectively). In patient 2, in addition to the predominant D835H mutation observed at sorafenib resistance on day 389, low-level D835Y and F691L mutations were also detected (0.63% and 0.16%, respectively).
In patients receiving subsequent monotherapy with sunitinib, we were able to monitor the effect of treatment on clones harboring the various FLT3 TKD mutations (Table 2). In patient 1, the frequency of the D835H mutation decreased by approximately 50% between sorafenib relapse and day 29 of sunitinib treatment (from 33.1% to 14.4%), and remained about the same in patient 2 after 36 days of sunitinib treatment (from 35.7% to 28.2%) and in patient 3 after 99 days of treatment (from 11.0% to 13.5%). In contrast, the D835Y mutation increased in patients 2 and 3 (from 0.63% to 1.03% and 4.0% to 28.5%, respectively) and the F691L mutation decreased (from 0.16% to not detected and 18.2% to 2.9%, respectively) during sunitinib treatment.
Allele selection of the FLT3 D835H mutation
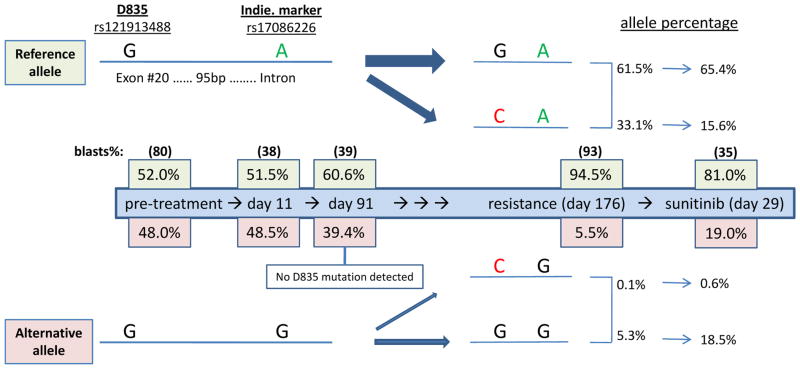
In the amplicon for FLT3 exon 20, an additional variant was detected in patient 1. The variant consisted of an A to G nucleotide substitution located in the intron region, 95 base pairs downstream of residue D835. This common substitution (rs17086226), with an A/G allele frequency at 0.78/0.22 out of 4,542 alleles reported in dbSNP build 137, is not likely to direct the occurrence of the D835H mutation in AML. By monitoring the rs17086226 variant during treatment, we were able to assess the allele selection of the D835H mutation. As shown in Figure 2, there is evidence of allele selection between days 11 and 91 of sorafenib treatment prior to detection of the D835H mutation. On day 11, 51.5% of alleles contained the reference sequence at both positions, whereas by day 91, alleles with the reference sequence increased to 60.6%. On day 176 of sorafenib treatment, the D835H mutation was detected primarily on alleles with the reference versus variant sequence for rs17086226 (33.1% versus 0.1%). This provides evidence for allele-preferred selection of the D835H mutation and suggests that emerging resistant populations arise through clonal expansion under selective pressure during sorafenib treatment.
Figure 2.
Allele selection of the FLT3 D835H mutation in patient 1 during treatment with sequential sorafenib and sunitinib. The percentage of blasts samples that contained the reference allele (top) or alternative allele (bottom) for the FLT3 D835 mutation in exon 20 (rs121913488) and the rs17086226 variant in the intron downstream of exon 20 were monitored during treatment with sorafenib and sunitinib using deep amplicon sequencing.
Sorafenib-resistant FLT3 mutations are sensitive to sunitinib in vitro
Each FLT3 TKD point mutation (D835H/Y or F691L) was modeled in Ba/F3 cells in the absence or presence of the ITD mutation, described as single and double mutants, respectively. Single and double mutants containing D835H/Y as well as the double mutant with F691L conferred IL3-independent cell growth and increased STAT5 phosphorylation, whereas the single F691L mutant did not transform cells (Supplemental Figure S2). Unlike D835 mutations, which have been considered to be primary transformative events in AML (31), mutation at F691 is a secondary mutation thought to hinder drug binding and, as our data suggest, may not have transformative potential in vitro.
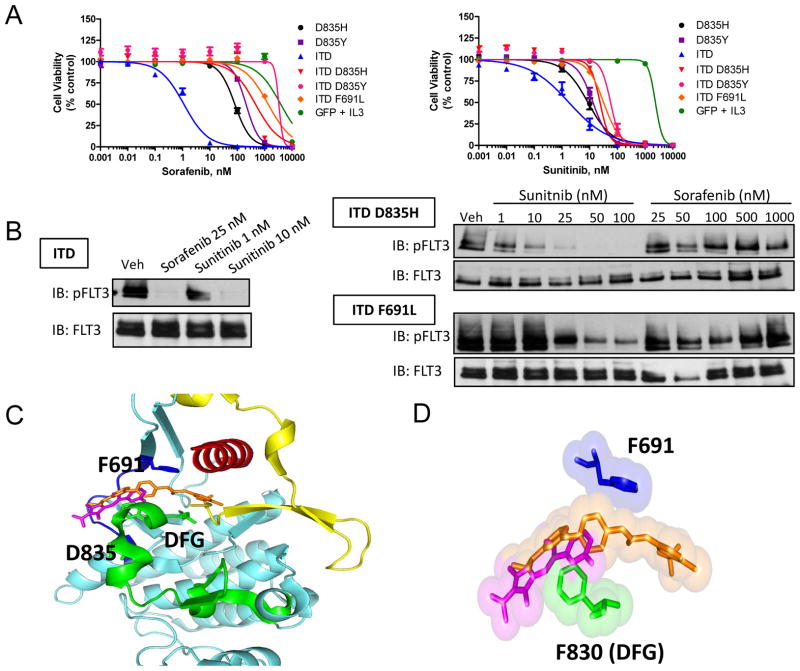
Ba/F3 cells expressing single or double FLT3 mutants were resistant to sorafenib-dependent inhibition of cell viability (Figure 3A). Cells with single D835H/Y mutants were resistant to sorafenib (IC50, 81 and 210 nM) in comparison to cells with the ITD only (IC50, 1.1 nM) (Table 3). Cells with double mutants containing ITD-D835H/Y or ITD-F691L were highly resistant to sorafenib (IC50, 460 – 3300 nM). In contrast, sunitinib showed moderate activity against cells with single D835H/Y mutants (IC50, 8.7 and 13 nM) and those with double mutants containing ITD-D835H/Y and ITD-F691L (IC50, 24–57 nM) (Figure 3A, Table 3). The relative sensitivity of the ITD, ITD-D835H, and ITD-F691L mutants to sorafenib and sunitinib in the cell viability experiments correlated with a loss of FLT3 phosphorylation (Figure 3B), further indicating that sunitinib has superior potency to inhibit FLT3 double mutants.
Figure 3.
Sorafenib-resistant FLT3 mutations are sensitive to sunitinib treatment.
A) BaF3 cells were treated with sorafenib (left) or sunitinib (right) for 72 hr and cell viability was measured. Data represent the mean +/− SEM of 2–4 independent experiments (n = 12–24 replicates). B) BaF3 cells were treated for 1 hr with sorafenib or sunitinib. FLT3 was then immunoprecipitated from cell lysate, and Western blot analysis was performed for phosphorylated and total FLT3 (IB, immunoblot). C) Structure model of sorafenib and sunitinib interaction with wild-type FLT3 (adapted from PDB IDs 1RJB, 3G0E, and 1UWH using Pymol software) (blue, hinge region; green, activation loop; red, αC helix; yellow, juxtamembrane domain; orange, sorafenib; magenta, sunitinib). D) Surface and stick representation of sorafenib, sunitinib, and residues F691 and F830 (DFG motif).
Table 3.
FLT3 inhibitor IC50 values in Ba/F3 cells expressing FLT3 mutations.
| Mutation | IC50 (nM)a
|
|||
|---|---|---|---|---|
| Sorafenib | Sunitinib | Quizartinib | Midostaurin | |
| GFP + IL3 | 3900 | 2400 | 13,000 | 330 |
| D835H | 81 | 8.7 | 1.9 | 1.2 |
| D835Y | 210 | 13 | 11 | 1.5 |
| ITD | 1.1 | 1.8 | 0.15 | 4.5 |
| ITD D835H | 460 | 24 | 21 | 24 |
| ITD D835Y | 3300 | 57 | 150 | 33 |
| ITD F691L | 1300 | 32 | 210 | 19 |
Concentration inhibiting 50% of growth in a cell viability assay.
Modeling of TKI interaction with FLT3
In accordance with the type II kinase inhibitor designation (26, 27), homology modeling of sorafenib and sunitinib interaction with FLT3 suggests that these drugs contact regions within the ATP-binding pocket (hinge region) as well as the conserved catalytic DFG residues within the activation loop (Figure 3C). Interestingly, the inhibitor-FLT3 interaction model suggests that, unlike sorafenib, sunitinib does not make direct contacts with the F691 residue (Figure 3D). This model supports the hypothesis that the F691L mutation would maintain sensitivity to sunitinib. In agreement, Ba/F3 cells expressing the ITD and F691L were more sensitive to sunitinib compared to sorafenib as measured by loss of cell viability and FLT3 phosphorylation.
The D835 residue is positioned on the activation loop of FLT3 away from the drug binding region (Figure 3C). It is thought that mutation of this residue will destabilize the inactive conformation and shift the pool of kinase to the active, “DFG-in” conformation. This could indirectly affect the binding of type II inhibitors that make contact with the inactive, “DFG-out” confirmation. This hypothesis has been proposed from modeling the effects of FLT3 D835 mutations on the interaction of quizartinib, a type II inhibitor, with FLT3 (9). Thus, a type I inhibitor, which can bind effectively to the active, “DFG-in” kinase conformation, may be active against FLT3 D835 mutations. Therefore, we evaluated the in vitro activity of midostaurin, a FLT3 inhibitor in phase III evaluation that was previously shown to exhibit type I inhibitor properties and retain activity against cells with FLT3 activation loop mutations (15, 32). Midostaurin displayed high potency against cells containing single D835H/Y mutations (IC50, 1.2 and 1.5 nM), and showed moderate activity in cells with double ITD-D835H/Y mutations (IC50, 24 and 33 nM) (Table 3, Supplemental Figure S2). Interestingly, cells with the ITD-F691L double mutation were also moderately sensitive to midostaurin (IC50, 19 nM). Consistent with previous reports (9, 33), cells harboring double ITD-D835H/Y or ITD-F691L mutations were highly resistant to quizartinib (IC50, 21–210 nM) in comparison to cells with the ITD only (IC50, 0.15 nM). We also showed that cells with single D835H/Y mutations were resistant to quizartinib (IC50, 1.9 and 11 nM) relative to those with the ITD. Our data with midostaurin supports the use of type I TKIs, which do not discriminate between active and inactive kinase, as a potential strategy to overcome drug resistance due to FLT3 mutations at residue D835.
DISCUSSION
Clinical activity of sorafenib monotherapy in FLT3-ITD-positive adult AML, including the induction of complete remissions, has been described (6–8, 34, 35). However, clinical responses have not been durable in most patients. We describe 4 out of 6 children with FLT3-ITD-positive AML who initially achieved complete remission with sorafenib in combination with chemotherapy, but 3 of the 4 who went on to receive maintenance therapy with single-agent sorafenib developed clinical resistance after 14 to 37 weeks. We show that sequential therapy with sunitinib resulted in reductions in leukemic burden. With direct sequencing, we identified leukemic blasts harboring FLT3 D835H/Y and F691L TKD mutations at sorafenib relapse that were not present in pre-treatment samples. Deeper coverage with next-generation sequencing allowed more precise detection and monitoring of FLT3 mutations during TKI therapy. Although FLT3 mutations were not detected in pre-treatment samples or after a course of sorafenib combination therapy, leukemic blasts at sorafenib resistance harbored the D835H mutation. In addition, several cases acquired low-level D835Y and F691L mutations at sorafenib relapse. Subsequent treatment with sunitinib decreased the percentage of leukemic cells harboring F691L mutations, suppressed the expansion of clones with D835H, but showed limited activity against cells with D835Y. Our data highlight the ability of sensitive methods to monitor the emergence of FLT3 drug-resistant mutations, which may ultimately guide drug selection and the timing of TKI therapy in patients with FLT3-ITD-positive AML.
Recently, several groups have identified FLT3 TKD mutations in adults with FLT3-ITD-positive AML with acquired resistance to quizartinib and sorafenib. In paired pre-treatment and relapse blast samples from 8 patients that initially achieved complete remission with quizartinib monotherapy, Smith et al. observed mutations at residue D835 with tyrosine, valine, or phenylalanine amino acid exchanges in 6 of 8 relapse samples, and the F691L mutation in 2 patients at relapse (9). Similarly, in paired blast samples from 6 patients that initially responded then progressed during sorafenib treatment, Man et al. observed mutations at residue D835 with tyrosine and histidine exchanges in resistant samples from 4 patients (6). Both studies were unable to detect FLT3 mutations in pre-treatment samples using methods including direct sequencing (6) or single molecule real-time sequencing (9). Likewise, we were unable to detect FLT3 TKD mutations in pre-treatment samples using targeted amplicon sequencing that provided an average effective coverage of greater than 80,000; it is possible that a rare clone harboring FLT3 TKD mutations is present in pre-treatment samples but currently available methods are not sensitive enough to detect rare clones if they exist. To address this, Man et al. expanded leukemia initiating cells obtained before sorafenib treatment in NOD/SCID mice. The D835Y mutation that was only detected in sorafenib resistant blast samples from 3 patients was now observed in clones derived from paired pre-treatment samples that were expanded in murine xenografts. This suggests that FLT3 secondary mutations reside in pre-existing clones that are too small to be detected by current sequencing methods and emerge under selective pressure during TKI therapy.
A model for clonal evolution of FLT3 alleles in MOLM13 cell lines treated with the FLT3 inhibitor tandutinib has recently been proposed (19). In this model, FLT3 TKD mutations are acquired specifically on ITD-positive alleles. However, we observed FLT3 D835H/Y or F691L mutations on both ITD-positive and -negative alleles in sorafenib and sunitinib resistance samples in 2 of 3 patients. Consistent with our observations, FLT3 D835 mutations were reported on both ITD-positive and -negative alleles in 6 of 8 patients that relapse during quizartinib therapy; F691L mutations that were observed in the other 2 patients were on FLT3-ITD-positive alleles (36). Our in vitro drug sensitivity data demonstrated that Ba/F3 cells harboring FLT3 single TKD or double ITD-TKD mutations are resistant to sorafenib and quizartinib, whereas sunitinib and midostaurin retained activity. We speculate that effective targeting of drug resistance will require selection of FLT3 inhibitors with activity against FLT3 TKD mutations on both ITD-positive and –negative alleles. Despite TKD mutations being observed on the same or opposite allele than the ITD in clinical samples, we demonstrated allele-preferred selection of the D835H mutation in 1 patient through deep amplicon sequencing and dual monitoring of the FLT3 D835H mutation and rs17086226 variant during sequential treatment with sorafenib and sunitinib. As this was observed in only 1 patient, the results are preliminary and require investigation in a larger population More studies are required to define the allele-specificity and clonal evolution of secondary FLT3 mutations during TKI therapy in FLT3-ITD-positive AML.
Based on the few studies reported thus far in patients treated with sorafenib and quizartinib (6, 9), including our own, substitutions of D835 are the most commonly observed secondary FLT3 TKD mutations in resistance samples. Previous homology modeling of FLT3 with inhibitors predicts that exchange of the aspartate residue would destabilize the inactive kinase conformation by disrupting the hydrogen bond formed with the main-chain amide group of S838; in turn, this would hinder the binding of type II inhibitors, like sorafenib and quizartinib, which make contact with the inactive, “DFG-out” confirmation of the activation loop (9). We showed that midostaurin, which exhibits type I inhibitor properties (32), retains activity against cells with FLT3 D835 single and double mutants. Interestingly, although sunitinib has been described as a type II inhibitor (26, 37), it was active against cells with FLT3 D835 single and double mutants in vitro, and demonstrated clinical activity in our 3 patients who relapse during treatment with sorafenib and harbored a D835H mutation. Our data are consistent with a previous investigation showing that sunitinib inhibits FLT3 phosphorylation in cells harboring a single D835H mutation with an IC50 < 10 nM (4, 5). Sunitinib has also been shown to exhibit type I inhibitor properties, which may explain its activity against some FLT3 activation loop mutations (38, 39). Homology modeling predicts that histidine substitution at D835 may retain partial ability to make a hydrogen bond with S838 to stabilize the inactive kinase conformation; whereas, a tyrosine exchange would entirely disrupt hydrogen bond formation. Therefore, a tyrosine substitution may be relatively more destabilizing than histidine and could more severely alter sunitinib binding; however, further structural analysis is necessary. We also demonstrated that sunitinib has activity against the F691L mutation in vitro and in vivo, which is supported by a recent case report showing transient clinical activity of sunitinib in an adult with AML harboring a FLT3 ITD-F691L mutation after previously relapsing on sorafenib and quizartinib therapy (33). Although secondary FLT3 TKD mutations are associated with FLT3 inhibitor resistance, it is unknown if the mutations are the initiating event leading to drug resistance; this possibility will require further study.
Our data support recent findings of the development of secondary FLT3 TKD mutations during the evolution of FLT3 TKI resistance. We demonstrated that sunitinib has anti-leukemic activity in patients who have become resistant to sorafenib, suggesting that sequential therapy with FLT3 inhibitors with different resistance profiles may provide clinical benefit. The application of sensitive methods to detect and monitor drug resistant leukemic clones during therapy may allow individualized treatment with the currently available kinase inhibitors. As individual TKIs may select for unique secondary FLT3 mutations, future investigations should also be aimed at identifying effective drug combinations with FLT3 inhibitors that suppress the emergence of drug-resistant leukemic clones.
Supplementary Material
Translational Relevance.
Most patients with acute myeloid leukemia (AML) and FLT3-internal tandem duplication (ITD) mutations show an initial favorable response to FLT3 inhibitors, followed by the development of resistance. One of the most common mechanisms of resistance to FLT3 inhibitors in preclinical models is the acquisition of secondary FLT3 tyrosine kinase domain (TKD) mutations. Since the FLT3 inhibitors sorafenib and sunitinib display non-overlapping profiles of resistance to cells expressing FLT3 TKD mutations, we hypothesized that sunitinib would have clinical activity in patients that developed resistance during sorafenib monotherapy. We demonstrate that sunitinib has anti-leukemic activity in patients who became resistant to sorafenib, indicating that sequential therapy with different FLT3 inhibitors may provide clinical benefit. Deep amplicon sequencing revealed low-level FLT3 TKD mutations at residues D835 and F691 and their evolution during sorafenib treatment. Subsequent sunitinib therapy preferentially suppressed leukemic clones with D835H and F691L mutations. The application of sensitive methods to detect and monitor leukemic clones with drug-resistant FLT3 mutations during therapy may allow individualized treatment with the currently available FLT3 inhibitors.
Acknowledgments
Acknowledgment of research support: This study was supported by the American Lebanese Syrian Associated Charities (ALSAC), National Institutes of Health Cancer Center Support Grant P30 CA021765 and R01 CA138744.
Footnotes
Conflicts of interest: All authors declare no potential conflict of interest.
Disclosure of Potential Conflict of Interest
The authors declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- 1.Fathi AT, Chabner BA. FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations. Oncologist. 2011;16:1162–74. doi: 10.1634/theoncologist.2011-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knapper S. The clinical development of FLT3 inhibitors in acute myeloid leukemia. Expert Opin Investig Drugs. 2011;20:1377–95. doi: 10.1517/13543784.2011.611802. [DOI] [PubMed] [Google Scholar]
- 3.Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia. 2012;26:2176–85. doi: 10.1038/leu.2012.114. [DOI] [PubMed] [Google Scholar]
- 4.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 6.Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119:5133–43. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 7.Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder T, Zohren F, Saure C, Bruns I, Czibere A, Safaian NN, et al. Sorafenib treatment in 13 patients with acute myeloid leukemia and activating FLT3 mutations in combination with chemotherapy or as monotherapy. Acta Haematol. 2011;124:153–9. doi: 10.1159/000320173. [DOI] [PubMed] [Google Scholar]
- 9.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12:8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Molecular cell. 2004;13:169–78. doi: 10.1016/s1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 12.Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–92. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117:3286–93. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagrintseva K, Schwab R, Kohl TM, Schnittger S, Eichenlaub S, Ellwart JW, et al. Mutations in the tyrosine kinase domain of FLT3 define a new molecular mechanism of acquired drug resistance to PTK inhibitors in FLT3-ITD-transformed hematopoietic cells. Blood. 2004;103:2266–75. doi: 10.1182/blood-2003-05-1653. [DOI] [PubMed] [Google Scholar]
- 15.Barry EV, Clark JJ, Cools J, Roesel J, Gilliland DG. Uniform sensitivity of FLT3 activation loop mutants to the tyrosine kinase inhibitor midostaurin. Blood. 2007;110:4476–9. doi: 10.1182/blood-2007-07-101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–9. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- 17.Grundler R, Thiede C, Miething C, Steudel C, Peschel C, Duyster J. Sensitivity toward tyrosine kinase inhibitors varies between different activating mutations of the FLT3 receptor. Blood. 2003;102:646–51. doi: 10.1182/blood-2002-11-3441. [DOI] [PubMed] [Google Scholar]
- 18.Kancha RK, Grundler R, Peschel C, Duyster J. Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations. Exp Hematol. 2007;35:1522–6. doi: 10.1016/j.exphem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Moore AS, Faisal A, Gonzalez de Castro D, Bavetsias V, Sun C, Atrash B, et al. Selective FLT3 inhibition of FLT3-ITD+ acute myeloid leukaemia resulting in secondary D835Y mutation: a model for emerging clinical resistance patterns. Leukemia. 2012;26:1462–70. doi: 10.1038/leu.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Bubnoff N, Engh RA, Aberg E, Sanger J, Peschel C, Duyster J. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlapping profile of resistance mutations in vitro. Cancer Res. 2009;69:3032–41. doi: 10.1158/0008-5472.CAN-08-2923. [DOI] [PubMed] [Google Scholar]
- 21.Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2012;27:48–55. doi: 10.1038/leu.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29:3293–300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Zhao M, Navid F, Pratz K, Smith BD, Rudek MA, et al. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3033–8. doi: 10.1016/j.jchromb.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman EI, Dollins CM, Crawford M, Grant S, Nana-Sinkam SP, Richards KL, et al. Lyn kinase-dependent regulation of miR181 and myeloid cell leukemia-1 expression: implications for drug resistance in myelogenous leukemia. Molecular pharmacology. 2010;78:811–7. doi: 10.1124/mol.110.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106:1542–7. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 28.Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–61. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Jones D, Medeiros LJ, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br J Haematol. 2005;130:726–8. doi: 10.1111/j.1365-2141.2005.05666.x. [DOI] [PubMed] [Google Scholar]
- 31.Whitman SP, Ruppert AS, Radmacher MD, Mrozek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–9. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg E, Roesel J, Furet P, Bold G, Imbach P, Florsheimer A, et al. Antileukemic Effects of Novel First- and Second-Generation FLT3 Inhibitors: Structure-Affinity Comparison. Genes Cancer. 2011;1:1021–32. doi: 10.1177/1947601910396505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albers C, Leischner H, Verbeek M, Yu C, Illert AL, Peschel C, et al. The secondary FLT3-ITD F691L mutation induces resistance to AC220 in FLT3-ITD(+) AML but retains in vitro sensitivity to PKC412 and Sunitinib. Leukemia. 2013 doi: 10.1038/leu.2013.14. [DOI] [PubMed] [Google Scholar]
- 34.Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96:62–8. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollig C, Brandts C, Shaid S, Hentrich M, Kramer A, Junghanss C, et al. Survey and analysis of the efficacy and prescription pattern of sorafenib in patients with acute myeloid leukemia. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.637210. [DOI] [PubMed] [Google Scholar]
- 36.Smith CC, Chin J, Lasater E, Paguirigan AL, Lin K, Stewart W, et al. Constitutively Activating Mutations At the FLT3 Activation Loop Residue D835 Are Associated with Clinical Resistance to AC220. Blood (ASH Annual Meeting Abstracts) 2012;120:674. [Google Scholar]
- 37.McTigue M, Murray BW, Chen JH, Deng YL, Solowiej J, Kania RS. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A. 2012;109:18281–9. doi: 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutach AK, Villasenor AG, Lam D, Belunis C, Janson C, Lok S, et al. Crystal structures of IL-2-inducible T cell kinase complexed with inhibitors: insights into rational drug design and activity regulation. Chem Biol Drug Des. 2010;76:154–63. doi: 10.1111/j.1747-0285.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 39.Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, et al. Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol. 2010;17:1241–9. doi: 10.1016/j.chembiol.2010.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.