Abstract
Antibody dependent cellular cytotoxicity [ADCC] has been suggested to play an important role in control of Human Immunodeficiency Virus-1 [HIV-1] viral load and protection from infection. ADCC antibody responses have been mapped to multiple linear and conformational epitopes within the HIV-1 envelope glycoproteins gp120 and gp41. Many epitopes targeted by antibodies that mediate ADCC overlap with those recognized by antibodies capable of virus neutralization. In addition, recent studies conducted with human monoclonal antibodies derived from HIV-1 infected individuals and HIV-1 vaccine-candidate vaccinees have identified a number of antibodies that lack the ability to capture primary HIV-1 isolates or mediate neutralizing activity, but are able to bind to the surface of infected CD4+ T cells and mediate ADCC. Of note, the conformational changes in the gp120 that may not exclusively relate to binding of the CD4 molecule are important in exposing epitopes recognized by ADCC responses. Here we discuss the HIV-1 envelope epitopes targeted by ADCC antibodies in the context of the potential protective capacities of ADCC.
Keywords: AIDS vaccines, antibody dependent cellular cytotoxicity, epitope, HIV-1, humoral responses, monoclonal antibodies.
INTRODUCTION
A primary goal of HIV-1 vaccine development is to induce protective antibody responses that can prevent transmission. However, to date, broadly neutralizing antibodies (BNAbs) and antibodies with the ability to neutralize Tier 2 primary isolates have been difficult to induce in humans with experimental HIV-1 vaccines [1]. In contrast, some HIV-1 vaccines can induce antibodies that bind to the surface of virus-infected CD4+ T cells and mediate antibody dependent cellular cytotoxicity (ADCC) [2-6].
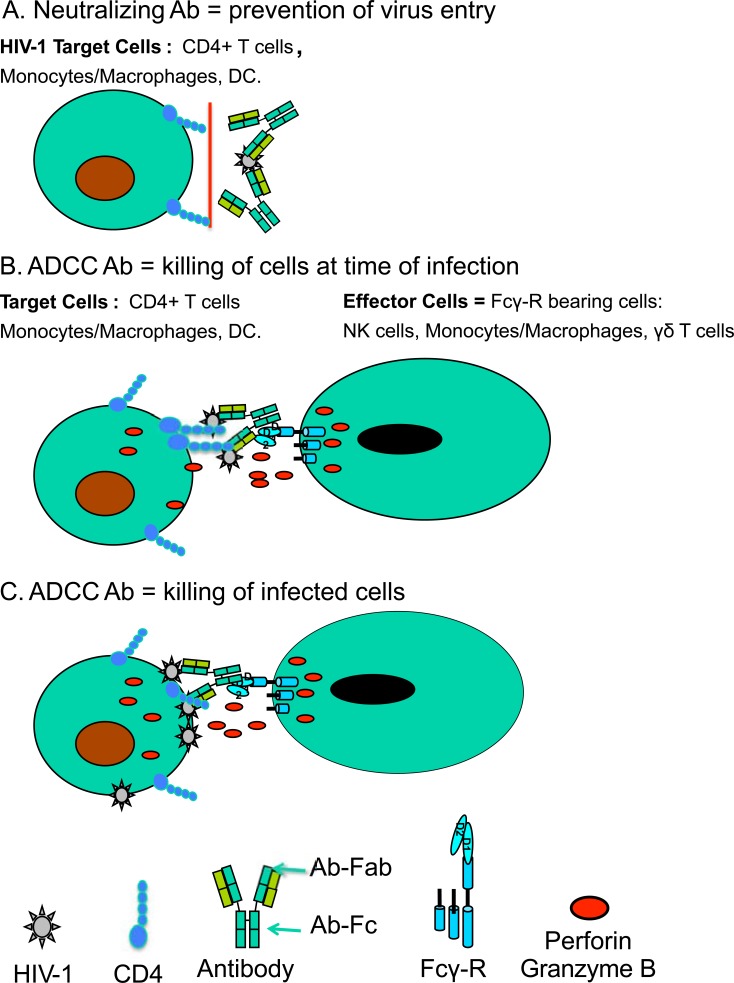
The complexity of HIV-1 antibody (Ab) responses that can protect from HIV-1 infection has been discussed elsewhere [1,7]. The role of neutralizing Ab responses has been clearly defined as preventing cells from becoming infected (Fig. 1A). ADCC Ab can either recognize cells targeted by HIV-1 at the time of virus entry, while the virions bind to the CD4 receptor (Fig. 1B), or at the time of virus budding on the surface of infected cells (Fig. 1C). As discussed in this review, the neutralizing and ADCC activity of HIV-1 Ab responses are not always mutually exclusive; both can contribute to the prevention of cellular infection by HIV-1, and ADCC Ab can provide additional help by eliminating HIV-1 infected cells.
Fig. (1).
Ab, Ag, and cellular interactions involved in virus neutralization and ADCC. (A) Virus neutralization requires HIV-specific Abs that bind infectious HIV-1 virions in a manner that prevents productive infection of the cells targeted by HIV-1 (target cells) including CD4+ T lymphocytes, monocytes, or dendritic cells. Major Env targets of neutralizing Abs include the CD4 binding site, the co-receptor binding site, and regions involved in post-attachment membrane fusion such as the gp41 membrane proximal external region. (B, C) ADCC is an immune effector function that requires the recognition of HIV-1 Ags on the surface of target cells by specific Ab, the binding of the constant region of the Ab by Fc receptors on the surface of suitable effector cells (NK cells, monocytes, macrophages, PMN), and the induction of a signaling cascade by the Ag–Ab–Fc receptor complex. The signal cascade results in the release of soluble effector molecules including perforin and granzymes that induce killing of the target cell. Abs that can bind HIV virions during the process of entry into target cells (B) have the potential to mediate ADCC and prevent productive infection. Abs that can bind HIV Ags or virons expressed on the surface of infected cells prior to or during virus budding (C) have the potential to mediate ADCC and limit the release of progeny virus.
Several studies have indicated that ADCC Ab responses correlate with the control of HIV-1 infection and the delay of
overt disease [8-12]. Associations have also been made between ADCC responses and protection from HIV-1 infection. The presence of ADCC Ab responses in breast milk has been correlated with prevention of HIV-1 transmission from infected mothers to their newborn infants [13]. Additionally, analysis of the RV144 HIV-1 vaccine clinical trial conducted in Thailand that provided an estimated 31.2% protection from infection showed a significantly lower risk of infection in individuals with low level anti-HIV-envelope (Env) specific IgA plasma responses and high ADCC responses, findings that have given rise to the hypothesis of a protective role for ADCC in this trial [14].
Although these studies suggest a role for ADCC in mediating protection from HIV-1 disease progression and infection, definitive evidence that ADCC responses alone can prevent HIV-1 transmission has not yet been demonstrated. ADCC responses require a harmonized interaction of Abs with appropriate epitope specificities and glycosylation profiles [15] with Fcγ-Receptor-bearing effector cell populations [16,17]. In this review we will discuss the complexity of the epitopes recognized by ADCC responses in HIV-1 infected individuals and candidate vaccine recipients.
TARGETING OF HIV-1 ANTIGENS BY ADCC-MEDIATING ANTIBODIES
The first reported and most studied HIV-1 antigen targets of ADCC responses are those directed against envelope glycoproteins gp120 and gp41[18-20]. These responses have been confirmed using a variety of target cell populations, including cells coated with recombinant gp120[18] and gp140[21] as well as HIV-1-infected cells [20,22-24]. Chung et al. have provided a useful review of the most commonly used ADCC assays [25], and recently Pollara [23] and Alpert [26] have described two novel approaches. The latter assay was utilized to identify the correlation between high ADCC responses and lower risk of infection the RV144 clinical trial [14]. In addition to the different target cells and assays, a number of human monoclonal Abs (mAbs) have been used to identify ADCC epitopes within defined regions of the envelope glycoproteins (Table 1).
Table 1.
Defined Regions of the HIV-Envelope Glycoprotein Targeted by Human mAbs with ADCC Activity
| HIV-1 Envelope Glycoprotein | Envelope Region | mAb | Discontinuous or Linear Epitope | Neutralizing or Non-Neutralizinga | ADCC References |
|---|---|---|---|---|---|
| gp41 | Cluster I | 246-D | Linear | Non-neutralizing | [34, 36] |
| 4B3 | Linear | Non-neutralizing | [36] | ||
| 98-43 | Linear | Non-neutralizing | [22] | ||
| 50-69 | Discontinuous | Non-neutralizing | [22] | ||
| Cluster II (HR2) | 98-6 | Discontinuous | Non-neutralizing | [22, 34] | |
| 126-50 | Discontinuous | Non-neutralizing | [22] | ||
| Not defined | 31710B | Not defined | [35] | ||
| MPER | 120-16 | Linear | Non-neutralizing | [22, 34] | |
| 2F5 | Linear | Neutralizing | [36, 86] | ||
| 4E10 | Linear | Neutralizing | [36] | ||
| gp120 | CD4i C1 region (Cluster A) | A32 | Discontinuous | Non-neutralizing | [24] |
| CD4i CoRBS (Cluster C) | 17B | Discontinuous | Neutralizing | [24] | |
| CD4i | CH08 | Discontinuous | Neutralizing | [87] | |
| CD4i Cluster A | C11, L9-i1, N5-i5, L9-i2, N12-i3, N26-i1 | Discontinuous | Non-neutralizing | [33] | |
| CD4i Cluster B | N12-i15 | Discontinuous | Non-neutralizing | [33] | |
| CD4i Cluster C.1 | L9-i3, N5-i1, N5-i3, N5-i4, N5-i8, N10-i1.1, N10-15.3, N12-i1, N12-i2, N12-i4, N12-i5, N12-i7, N12-i8 | Discontinuous | Neutralizing | [33] | |
| CD4i Cluster C.2 | N12-i10, N12-i17, N12-i18, N12-i19 | Discontinuous | Neutralizing | [33] | |
| CD4i Cluster C.3 | N5-i2, N5-i6, N5-i9, N5-i14, N5-i7, N5-i12, N10-i3.1, N12-i12, N12-i9, N12-i11 | Discontinuous | 9/10 Neutralizing | [33] | |
| CD4i Cluster C.4 | L9-i4, N5-i10.1, N5-i13, N10-i2, N12-i14, N12-i16 | Discontinuous | 2/6 Neutralizing | [33] | |
| CD4i | N10-i4, N10-i6.1 | Discontinuous | Non-neutralizing | [33] | |
| CD4i C1 region | CH20, CH29, CH38, CH40, CH49, CH51, CH52, CH53, CH54, CH55, CH57, CH77, CH78, CH80, CH81, CH90, CH91, CH92, CH94 | Discontinuous | Non-neutralizing | [32] | |
| C2, C3, C4, V4 glycosylation sites | 2G12 | Discontinuous | Neutralizing | [76, 24] | |
| C5 | 670-D | Discontinuous | Neutralizing | [34] | |
| 750-D | Not defined | Non-neutralizing | [34] | ||
| 42F, 43F | Linear | Non-neutralizing | [88, 35] | ||
| CD4 binding site | 15e | Discontinuous | Neutralizing | [55] | |
| F105 | Discontinuous | Neutralizing | [89] | ||
| 448-D | Discontinuous | Neutralizing | [34] | ||
| 1125H, 5145A | Discontinuous | Neutralizing | [35] | ||
| b12 | Discontinuous | Neutralizing | [90] | ||
| VRC01 | Discontinuous | Neutralizing | [24] | ||
| V2 | CH58, CH59, HG107, HG120 | Linear | Neutralizing | [6] | |
| PG9 | Discontinuous | Neutralizing | [52] | ||
| V3 | 694/98D | Linear | Neutralizing | [34] | |
| 4117C, 41148D | Linear | Neutralizing | [35] | ||
| CH22, CH23 | Linear | Neutralizing | [32] |
Neutralization activity against lab-adapted or primary HIV-1 isolates with no consideration of breadth or potency.
Although the HIV-1 envelope represents the major target of ADCC responses, it should be noted that investigators have recently reported that Pol [27], Nef [28], and Vpu [29,30] antigenic regions can also be recognized by ADCC-mediating Abs. However, it is still poorly understood if these epitopes can be recognized on the surface of HIV-1 infected cells by ADCC-mediating Abs, and investigators are currently studying the relevance of these responses following vaccination or infection.
NATURE OF THE HIV-1 EPITOPES RECOGNIZED BY ADCC RESPONSES
The epitopes recognized by human ADCC responses directed against HIV-1 envelope glycoproteins (Env) have been reported as either linear or conformational/ discontinuous (Table 1). Most of the identified linear epitopes are within the conserved regions [24,31-33], the variable region 3 (V3) loop of gp120 [34,35] and the ectodomain of gp41 [36]. The conformational epitopes are distributed among several regions of the envelope, appear mainly within gp120, and include the inner domain of gp120 [24,33,37], the variable region 2 (V2) [6], and the CD4 binding site (CD4bs) [38] (Fig. 2). For both linear and conformational ADCC Env epitopes, the key characteristic is that they must be accessible to the Abs at the time of virus entry or maturation of Env on the membrane of infected cells.
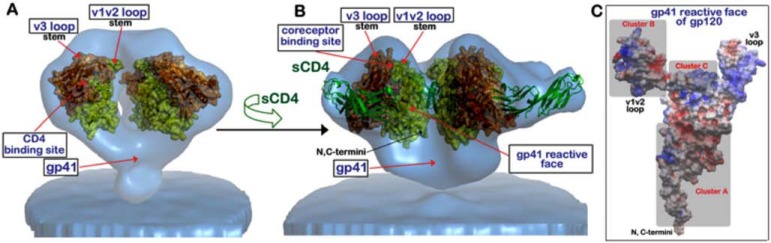
Fig. (2).
Co-localization of HIV-1 epitopes recognized by ADCC Ab responses within Env trimer and monomeric gp120. (A) Unliganded and (B) CD4-triggered trimeric Env from native HIV-1 BaL as derived by cryo electron tomography [40,41]. X-ray coordinates of (A) the unliganded gp12093TH057 coree (PDB:3TGT) and (B) the gp120HXBc2core/s CD4 subset of gp120HXBc2core/CD4/17b complex (PDB:1GC1) were fitted into tomograms using Chimera [85]. Transparent molecular surfaces are displayed over gp120 molecules with inner and outer domain colored orange and yellow, respectively. Regions of the HIV-envelope glycoprotein defined to be targeted by mAbs with ADCC activity are indicated by red arrows. (C) Model of full-length gp120 monomer shown with the gp41 reactive face up and ADCC epitope Clusters A-C as described in highlighted in grey [33]. Electrostatic potential is shown over the surface of gp120 displayed as negative in red, positive in blue, and apolar in white. Model was created by loop modeling of the missing V1/V2 region using the ICM software routines (MolSoft, LaJolla, CA) and gp120 coordinates from crystal structures PDB:3WJO and PDB:2B4C.
In recent years, great progress has been made in defining atomic level structures of Env. The crystal structure of the gp120 core monomer was first resolved in complex with d1d2 domains of CD4 [39]. These studies revealed the organization of Env into three areas defined as inner domain, outer domain, and bridging sheet [39]. Of interest, the inner domain is the target of some CD4-inducible (CD4i) Abs with broad and potent ADCC activity, represented by prototypic mAbs A32 and C11 [24,33]. More complete molecular models of virion-associated envelope trimmers have been recently resolved by cryo-electron tomography (Cryo-EM) [40-42]. These studies have shed new light onto the functional structures of the envelope required for virus infectivity. The trimer model indicates that the CD4 and co-receptor binding sites could be potentially masked by V1 and V2 loops [43,44]. The CD4 binding site, the co-receptor binding site, and the V1V2 regions are putative targets for both human ADCC and neutralizing Abs (Table 1). At this time it is unclear whether more than one envelope structural configuration is present on virions and/or on the cell surface, as initially suggested by Moore et al. [45], or if only envelope trimers are present, as recently suggested by Haim et al. [46]. If the latter is the case, we can hypothesize that envelope trimers are not in a static configuration and may exist in two or more different configurations depending on whether they are unliganded (Fig. 2A) or CD4-bound (Fig. 2B) [42,46]. Both structural configurations could be present on virions at the time of entry, or during the HIV-1 budding process on the surface of infected cells. For the purpose of vaccine design, it is fundamental for us to understand the accessibility of these structures and other Env conformers to ADCC-mediating Abs, similar to our current understanding of the binding of neutralizing Abs (NAbs).
HIV-1 GP120 CD4-INDUCED (CD4i) EPITOPES
The ability of CD4i antibodies to bind to Env is linked to the conformational changes that follow the binding of CD4 to gp 120. 17b and 48d were the first mAbs described that recognize CD4i epitopes and were capable of neutralizing HIV-1 infection [37]. Subsequent studies revealed that these mAbs were not very effective at recognizing Env epitopes exposed during the interaction of CD4 and Env present on the surface of infected cells during the formation of syncytia [47]. In contrast, CD4i mAb A32 that binds to a conformational epitope involving the C1 region does not bind Env expressed on infectious virions and is therefore incapable of virus neutralization [37]. However, A32 can rapidly bind to Env-target cell interfaces following the start of syncytium formation [47] and can mediate ADCC with significantly higher potency than mAb 17b [24]. These data indicated that the accessibility of CD4i epitopes for Ab recognition differs when Env is expressed on the surface of infected cells in the presence of CD4 molecules, thus impacting the antiviral functions of antibodies recognizing these epitopes.
The presence of CD4i ADCC-mediating Abs has been demonstrated in both acute and chronic HIV-1 infection as well as following vaccination. An analysis of the frequency of B cells producing anti-Env Ab in HIV-1 acutely infected subjects receiving anti-retroviral therapy revealed that >47% of cells produced CD4i Abs [48]. This study suggested that CD4i epitopes might be more immunogenic than other Env epitopes during acute infection.
In the context of chronic infection, pre-incubation of target cells with the A32 or 17b Ab Fab fragments resulted in blocking more than 50% of ADCC activity in 7 of 14 HIV-1 chronically infected individuals [24]. The data suggest that CD4i are indeed relevant in the context of ADCC responses induced by natural HIV-1 infection. Guan et al. finely characterized ADCC mediated by CD4i Abs by studying a panel of 41 CD4i mAbs generated from infected individuals and classified them in three clusters (Fig. 2C) depending on cross-blocking by mAbs A32 and/or C11 [Cluster A], E51-M9 (Cluster B), and 17b and/or 19e (Cluster C) in ELISA [33]. These mAbs were then evaluated for their ability to eliminate CD4 T cells at time of virus entry by ADCC. The ADCC half-maximal effective concentrations of the Cluster A and B mAbs were always 0.5-1 Log lower than those of the Cluster C mAbs with the only exception being mAb N10-i3.1. In addition, none of the Cluster A or B mAbs had the ability to neutralize HIV-1. These findings suggest that there is a hierarchy in the accessibility of epitopic regions to Ab recognition on the surface of infected cells, during HIV-1 entry, or during the time of Env expression on cell membranes preceding the release of mature virions. In the same study, Guan and his collaborators suggested that the A32- and C11-blockable mAbs most likely recognize conformational epitopes within the inner domain of gp120 that involve the C1 region [33].
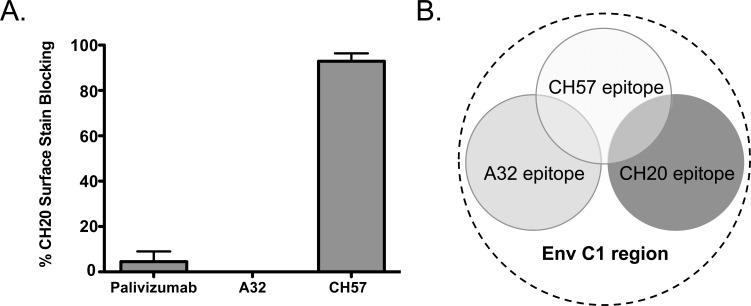
In characterizing ADCC responses of vaccine recipients in the RV144 trial, Bonsignori et al. demonstrated that the vaccine regimen induced binding and ADCC Ab responses that could be blocked by competition with the A32 Fab in >90% of the vaccine recipients [32]. These data supported the hypothesis that CD4i epitopes might play a relevant role in the vaccine-induced ADCC response. Moreover, the same investigators generated 21 ADCC-mediating mAbs from vaccine recipients. The ADCC activity of each mAb was blocked by A32 Fab competition. Interestingly, each vaccine-induced mAb differed in its ability to cross-compete A32 mAb for binding to recombinant gp120, and each of the vaccine-induced mAbs displayed a unique pattern of cross-clade ADCC activity [32]. Of note, one of the A32-blockable mAbs, CH57, was able to block binding of the CH20 mAb to the surface of infected cells (Fig. 3A). CH20 was originally described as directed to a conformational epitope that could not be blocked by competition with the A32 Fab. The data suggest that these vaccine-induced A32-blockable Ab responses may recognize several overlapping binding sites (Fig. 3B) within the conformational epitope recognized by the A32 mAb [32].
Fig. (3).
Vaccine-induced ADCC Abs recognize distinct overlapping epitopes. (A) Unlabeled mAb Palivizumab (negative control), mAb A32, and vaccine-induced anti-C1 region mAb CH57 were used to compete the binding of fluorescently labeled CH20 mAb to the surface of HIV-1-infected CD4+ T-cells and the % blocking was determined by flow cytometry. The A32 mAb was unable to block CH20 surface binding while competition with CH57 resulted in near total blocking. The A32 Fab fragment has been previously shown to inhibit ADCC activity of mAb CH57 but not CH20 [32]. Collectively these data suggest the epitope model proposed in (B). The conformational epitope of mAb A32 is located predominantly within the C1 region (represented as dashed circle) and overlaps with the conformational epitope recognized by mAb CH57. The conformational epitope recognized by mAb CH20 overlaps with the epitope of CH57, but not that of A32.
Taken together, these results indicate that CD4i epitopes are commonly recognized throughout the course of HIV-1 infection and also represent an immuno-dominant region of Env for ADCC responses induced by the RV144 vaccine regimen.
EPITOPES WITHIN THE HIV-1 GP120 V2 REGION
The β2-β3 strands constitute the V1/V2 stem and are located in the vicinity of the inner domain of gp120. Upon CD4 binding, the inner domain undergoes conformational changes that result in a shift of the V1/V2 stem giving these variable loops a new spatial disposition [43,49]. These observations, once again, underscore the importance of changes in the conformation of gp120 that affect the accessibility of the V1/V2 region to ADCC-mediating Ab responses, whether it is in a trimeric unbound state or in a CD4-bound conformation.
The results of the immune correlates of risk analysis of the RV144 trial demonstrate that high levels of anti-V1/V2 antibody responses detected in the plasma of vaccine recipients using the clad B gp70-V1/V2 CaseA2 recombinant protein scaffold inversely correlate with rate of infection [14]; and analysis of breakthrough infections found evidence of immune pressure on the V2 amino acid residue K169 [50]. The secondary analysis of correlates of risk also revealed an inverse correlation between the risk of infection and the level of ADCC responses in vaccines with low anti-Env IgA responses [14]. To study the function of vaccine-induced V2-specific Ab responses, Liao et al. generated recombinant versions of V2 mAbs (CH58, CH59, HG107 and HG120] isolated from memory B cells of multiple vaccine recipients [6]. None of these mAbs were capable of capturing free Tier 2 virions or neutralizing Tier 2 HIV-1 isolates [6]. Of particular interest, mAb CH58 mediated ADCC of HIV-infected target cells, bound the clade B gp70-V1/V2 Case-A2 scaffold used for interrogation of the correlates of risk, and requires a lysine in position 169 of the V2 for Env binding [6] that is also the residue identified as site of immune pressure by sieve analysis [50]. These findings suggest that CH58-like mAbs represented the type of Ab response that correlate with lower risk of infection and are compatible with the hypothesis that such V2 Ab responses might be protective because of their ability to mediate ADCC.
Liao et al. demonstrated that the V2 loop can assume different conformations which are specifically recognized by different V2 Abs [6]. RV144 vaccine-induced V2 mAbs CH58 and CH59 bind to the same region recognized by V2 BNAb PG9 generated from an HIV-1 infected individual, and all three mAbs are able to mediate ADCC against HIV-1 infected cells [6,51,52]. However, differently from PG9, CH58 and CH59 do not display preferential binding to trimeric gp120 and do not bind directly to the glycans in positions 156 and 160 [6,9,53]. Moreover, mAbs CH58 and CH59 recognize linear epitopes within two distinct V2 loop conformations: an α-helix conformation followed by a coil structure for CH58, and a coil or turn sequence followed by a short 310 helix for CH59 [6], both of which differ from the conformation recognized by PG9. These findings indicated that different conformations of the V2 loop could be recognized by ADCC-mediating Ab and BNAbs. How these differences in fine specificities of Abs that recognizes the V2 region relates to their ability to contribute to protection from infection will need to be further evaluated in passive protection trials conducted in non-human primates.
EPITOPES WITHIN THE CD4 BINDING SITE (CD4bs)
CD4 binds to gp120 within a cavity formed at the interface of the outer and inner domains, and the bridging sheet [54]. The gp120 residues involved in the binding of the CD4 molecule have been identified and are located exclusively within the outer domain [54]. The first CD4bs mAb described to mediate ADCC against HIV-1 infected target cells is mAb 15e [55]. 15e mAb was initially reported as a neutralizing antibody with broad specificity that blocked the binding of gp120 to CD4 and recognized a conformational epitope [56]. The recognition of conformational epitopes is a common feature of other mAbs directed against the CD4bs, and such mAbs were initially reported as broadly neutralizing and, subsequently, shown to be capable of also mediating ADCC (Table 1). Each CD4bs mAb displays unique features in its ability to bind and neutralize HIV-1. As an example, though both BNAbs b12 [57] and VRC01 [58] primarily recognize a region in the outer domain of gp120 Env involved in binding with CD4, mAb b12 recognizes gp120 using only the Ab heavy chain [59] whereas both the heavy and light chains of VRC01 contribute to binding [60]. These differences have been shown to be crucial for their neutralization breadth [60].
Zhou et al. designed molecules where the antigenic structure of the neutralizing surface of the CD4bs was preserved but the exposed surface residues were substituted by simian immunodeficiency virus (SIV) and other non-HIV-1 residues [60]. This class of molecules were named Resurfaced Stabilized Core [RSC] proteins and were used to isolate the potent VRC01 BNAb [60]. In addition, Lynch et al. utilized RSC protein to determine the ontogeny of VRC01-like Ab responses following HIV-1 infection in 18 subjects [61]. They observed that Abs directed to the CD4bs were detectable within 4-16 weeks from infection, but that RSC-binding Abs were only detectable in two patients at 10 and 152 weeks post-infection [61]. Moreover, the cross-sectional analysis of sera from 113 patients collected at 99 to 147 weeks post-infection within the CHAVI and CAPRISA cohorts demonstrated the presence of RSC-binding Abs in 21% of the samples [61]. Taken together, these data suggest that while CD4bs specific Ab responses may be generated early after infection, RSC-binding Abs, suggestive of broadly neutralizing activity, may require years to mature, as had been suggested for general NAb responses [61,62], and therefore may also be difficult to induce by vaccine candidates. The relative contribution of early CD4bs Abs to the overall ADCC response during acute HIV-1 infection, their potential to develop into BNAbs as somatic mutations are accumulated, and their ability to induce selective pressure on the autologous virus are still unclear.
EPITOPES WITHIN THE HIV-1 GP120 V3 REGION
Five monoclonal antibodies directed against the V3 region of gp120 Env have been reported as capable of mediating ADCC. Three were isolated from HIV-1 infected individuals [694/98D, 41117C, and 41148D] [34,35], and two were generated by a vaccine recipient enrolled in the phase II RV135 clinical trial (CH22 and CH23) [32,63]. All were mapped to linear sequences of the V3 loop.
Anti-V3 Ab responses are generally detectable in chronically HIV-1 infected individuals, but the biological relevance of anti-V3 Ab responses contribution to total ADCC responses is still unresolved. In addition, the sequences of the V3 region are by definition variable [64], therefore ADCC mediated by the V3 mAbs can be isolate-specific as demonstrated by the analysis of their ADCC profile [32,34,35].
ADCC EPITOPES WITHIN GP41
The structure of the gp41 Env glycoprotein includes an ectodomain portion that is responsible for trimerization and a membrane-spanning anchor followed by the cytoplasmic tail. Non-covalent interactions between the gp41 ectodomain and the discontinuous structure of gp120 at its NH2- and COOH-terminal ends contribute to the assembled structure of un-triggered envelope trimers [65,66]. In this conformation the most immunogenic epitopes on gp41, which are located at the surface directly interacting with gp120 [65,66], are buried within a compressed trimeric coiled-coil and inaccessible to neutralizing and non-neutralizing Ab that can mediate ADCC prior to gp120 binding to CD4.
Tomaras et al. have demonstrated that anti-gp41 Ab responses are the first detectable anti-HIV-1 plasma antibodies appearing approximately 13 days after the detection of plasma virus and represent the early immuno-dominant antibody response to HIV-1 [67]. However, these responses did not significantly impact the early dynamics of virus load in plasma nor were they able to select early viral escape mutations [67].
Among the first reports on ADCC responses to HIV-1, Tyler et al. reported that five non-neutralizing anti-gp41 mAbs (120-16, 126-50, 98-6, 50-69, and 98-43) isolated from HIV-1 infected individuals mediated ADCC [22]. The least potent mAb, 98-43, mapped to amino acids 579-604, whereas mAb 120-16 was the most potent and mapped to amino acids 644-663 [22]. Interestingly, the latter mAb mapped to the same Membrane Proximal External Region (MPER) recognized by the potent and broadly neutralizing cluster II mAbs 2F5 and 4E10, which are also capable of mediating ADCC [36]. Shen et al. have reported that cluster II 2F5-blockable neutralizing Ab responses are not detectable earlier than 12-27 months after acute HIV-1 infection [68] and it has been proposed that non-neutralizing responses such as those represented by the 120-6 and 98-43 mAbs may inhibit the development of potent NAbs to gp41 by masking these epitopes from recognition by B cell clones with potential to mature into neutralizing Nabs [67]. Furthermore, it has been hypothesized that events related to immune dysregulation that allow for the development of autoantibody responses may contribute to the delayed appearance of 2F5-like antibody responses [69]. In addition to the non-neutralizing mAbs reported by Tyler et al., two more non-neutralizing mAbs, 246-D and 4B3 [34], directed against the Principal Immune Domain [PID] of gp41 have been shown to mediate ADCC [36]. Take together, these studies indicate that gp41 is a target for ADCC responses and are compatible with the hypothesis that gp41 ADCC mAbs and NAbs may require different levels of maturation to exert their function, with ADCC antibodies being easier to elicit than NAbs. The importance of ADCC antibodies that recognize gp41 in preventing onset of disease or altering its progression needs further evaluation.
ESCAPE FROM EPITOPE - SPECIFIC ADCC RESPONSES
As noted throughout the review, many ADCC epitopes coincide with epitopes recognized by neutralizing antibody responses. HIV-1 has devised several mechanisms linked to sequence diversity and inherent structure of the envelope to avoid recognition by neutralizing Ab responses reviewed in [70,71], and these mechanisms also impact ADCC responses. The first obstacle Ab responses must overcome in order to broadly and effectively recognize the circulating HIV-1 is to account for the large diversity in the sequence of the HIV-1 envelope that has been reported to be at least 20%, and greater than any other virus [64]. The inherent structure of the envelope provides a shield whereby key regions of the envelope such as the gp41 and the vulnerable CD4bs are protected by highly variable and often poorly immunogenic structures [65]. In addition, the high level of glycosylation of gp120, defined as a “glycan shield”, protects relevant epitopes from recognition by neutralizing Abs [72-75]. Some Abs are however able to recognize the glycosylated structures of Env. One classic example that recognizes a conformational glycan structure is BNAb 2G12 [76]; it has been successfully used in passive protections studies, and can mediate ADCC, but escape mutants have been described [77]. Moore et al. have recently reported how these different mechanisms can account for the ability of gp120 to escape recognition of regions targeted by Ab responses that can mediate both neutralizing and ADCC functions [78].
A fundamental question is whether ADCC-mediating Abs that lack neutralizing activity are also able to exert selective pressure. If so, this evidence would give a strong indication of ADCC protective function. Chung et al. demonstrated that at least two epitopes within the C1 region are under immune pressure by ADCC-mediating Abs [31]. Of note, the sequence of both epitopes escaping the ADCC response is within the region of the gp120 recognized by the Cluster A mAbs reported by Guan et al. [33]. Most recently, Rolland et al. identified the amino acid position 169 in the V2 region as a site of immune pressure exerted on the transmitted/founder HIV-1 isolated from the breakthrough infections in RV144 vaccines [50]. As previously discussed, this amino acid residue is one of those necessary for the recognition of the envelope by the V2 mAbs isolated from RV144 vaccine recipients [6], suggesting that these vaccine-induced Ab responses might also exert immune pressure and generate escape mutants [6]. Overall, it is still unclear whether ADCC responses targeted to the C1 and V2 regions contribute to control of virus replication and studies specifically designed to answer this question are needed.
It is interesting to note that common epitopes targeted by Abs that mediate ADCC but not neutralization are situated in regions of the gp120 and gp41 exposed only following CD4 binding, with CD4i Abs and the coiled-coil structure of the gp41 being a clear example, and this may represent an ADCC-specific mechanism of viral escape. In this regard, the ability of the viral regulatory Nef and Vpu gene products to down-regulate the CD4 molecule on the surface of infected cells might be another mechanism by which HIV-1 avoids recognition by these relatively potent group of ADCC Ab responses [79,80]. Similarly, the coiled-coil structure of the gp41, which is underneath gp120 in the intact trimer, likely becomes fully accessible to Ab responses only during the fusion process [65], limiting the time that most gp41-specific ADCC can be effective.
CONTRIBUTION OF EPITOPE-SPECIFIC ADCC RES-PONSES TO PROTECTION FROM OR CONTROL OF HIV-1 INFECTION
In the context of the protection induced by vaccines, it has been proposed that ADCC might have mediated the lower risk of infection observed in the RV144 vaccine clinical trial. The observed protection correlated with Ab responses against the V1/V2 scaffold with properties that are characteristic of the CH58 mAb generated by Liao et al. [6]. The fact that mAb CH58 could only neutralize Tier 1 HIV-1 isolates but was capable of mediating ADCC against Tier 2 isolates suggests that protection mediated by Ab responses with this type of antigen specificity could be related to their ADCC function. Passive protection studies in the non-human primates model have been planned to address this issue.
Passive immunization experiments conducted with b12 mAb and its LALA variant (which lacks the ability to bind the Fc receptor of effector cells populations capable of mediating ADCVI/ADCC) suggested that the b12 protective function in the SHIV model could have been related to its ability to mediate ADCVI/ADCC [81]. However, subsequent studies testing the non-fucosilated variants of b12 mAb revealed that in vivo protection was not enhanced despite the observed higher ability to mediate ADCC and ADCVI in vitro [82]. These studies raised the question of which Fc-related Ab function could be the most relevant to confer protection, if these functions can be singled out [83].
Recently, Moog and collaborators studied two non-neutralizing ADCC-mediating mAb directed against the PID of gp41 for their ability to prevent mucosal infection when delivered directly at the mucosal site and observed that despite the fact that SHIV infection was not prevented, the combination of the two mAbs was capable of lowering the peak of virus load [36]. The contribution of vaccine-induced gp41 Abs to protection from challenge and control of infection was evaluated in a study conducted by Bomsel and collaborators [84]. The investigators identified associations between transcytosis inhibition and gp41-specific mucosal ADCC activity with protection from mucosal SHIV challenge. Additionally, they demonstrated a significant inverse correlation between MPER-specific ADCC activity of Cervico-Vaginal Secretions and peak viral load. No significant correlations were identified for neutralization activity. These findings further implicate ADCC and other non-neutralizing antiviral Ab functions in protection and control of HIV/SHIV infection.
CONCLUSION
Several HIV-1 epitopes have been identified as targets for ADCC responses. Thus far, only Abs directed against epitopes within the HIV-1 Env have been demonstrated to target ADCC responses to HIV-infected cells. There is overlap between epitopes recognized by ADCC and neutralizing Ab responses, although mAbs directed against the same epitope can mediate mutually exclusive activities. ADCC antibody responses arise earlier than neutralizing responses, but many of their specificities remain to be determined. HIV-1 has devised several mechanisms to limit recognition by ADCC responses that in part overlap with those identified for escape from neutralizing Abs. The studies conducted by Hessel [81], Moldt [82], and Moog [36] using the SHIV model suggest that Fc-related Ab functions that includes ADCC play a role in either preventing infection or reducing peak plasma viremia. However, there is still no definitive understanding of the relative contribution of each of the numerous ADCC Ab specificities to an overall protective response. Ultimately, given the overlap between the neutralizing and ADCC functions for many of the finely characterized mAb combinations used in these studies, the relevance to protection of ADCC as well as other Fc-related functions have yet to be fully defined.
ACKNOWLEDGEMENTS
This study was supported in part by a Collaboration for AIDS Vaccine Discovery (CAVD) grant from the Bill & Melinda Gates Foundation (Grants ID: 1032144, OPP1032325 and OPP1033098), and by grant from NIH/NIAID the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery (Grant U01 AI067854; National Institutes of Health, National Institute of Allergy and Infectious Diseases Division of Acquired Immunodeficiency Syndrome (NIH/NIAID/DAIDS). We thank Dr. George Lewis for the helpful discussion of this review.
ABBREVIATIONS
- HIV-1
= Human Immunodeficiency Virus-1
- ADCC
= Antibody Dependent Cellular Cytotoxicity
- Ab
= Antibody
- mAb
= Monoclonal Antibody
- NAb
= Neutralizing Antibody
- CD4i
= CD4 Inducible
- CD4bs
= CD4 Binding Site
- ADCVI
= Antibody Dependent Cellular Viral Inhibition
- gp120
= Glycoprotein 120
- gp41
= Glycoprotein 41
CONFLICT OF INTEREST
None of the authors has any conflict of interest to declare for the content of this manuscript.
PATIENT CONSENT
Declared none.
HUMAN/ANIMAL RIGHTS
Declared none.
REFERENCES
- 1.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: Understanding Nature's Pathways. Immunol Rev. 2013;254(1):225–44. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements Mann ML, Weinhold K, Matthews TJ. Immune Responses to Human Immunodeficiency Virus (HIV) Type 1 Induced by Canarypox Expressing HIV-1 Mngp120 HIV-1 SF2Recombinant gp120 or Both Vaccines in Seronegative Adults. J Infect Dis. 1998;177(5):1230–46. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 3.Karnasuta C, Paris RM, Cox JH. Antibody-Dependent Cell-Mediated Cytotoxic Responses In Participants Enrolled In A Phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 Vaccine Trial In Thailand. Vaccine. 2005;23(19):2522–9. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Goepfert PA, Tomaras GD, Horton H. Durable hiv-1 antibody and t-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25(3):510–8. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Currier JR, Ngauy V, de Souza MS. Phase I safety and immunogenicity evaluation of MVA-CMDR. a multignic.recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS ONE. 2010;5(11):e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao H-X, Bonsignori M, Alam SM. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity. 2013;38(1):176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS. 2009;4(5):373–9. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum LL, Cassutt KJ, Knigge K. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–73. [PubMed] [Google Scholar]
- 9.Forthal DN, Landucci G, Haubrich R. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180(4):1338–41. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 10.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75(15):6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nag P, Kim J, Sapiega V. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis. 2004;190(11):1970–8. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambotte O, Ferrari G, Moog C. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23(8):897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLoS Pathog. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BF, Gilbert PB, McElrath MJ. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies Curr Opin HIV AIDS. NIH Public Access. 2009;4(5):388–93. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 18.Lyerly HK, Reed DL, Matthews TJ. Anti-GP 120 Antibodies from HIV Seropositive Individuals Mediate Broadly Reactive Anti-HIV ADCC. AIDS Res Hum Retroviruses. 1987;3(4):409–22. doi: 10.1089/aid.1987.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Tyler DS, Stanley SD, Nastala CA. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection.Humoral and cellular defects. J Immunol. 1990;144(9):3375–84. [PubMed] [Google Scholar]
- 20.Koup RA, Robinson JE, Nguyen QV. Antibody-dependent cell-mediated cytotoxicity directed by a human monoclonal antibody reactive with gp120 of HIV-1. AIDS 1991 . ov 1;5(11):1309–14. doi: 10.1097/00002030-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Chung A, Rollman E, Center R, Kent S, Stratov I. Rapid Degranulation of NK Cells following Activation by HIV-Specific Antibodies. J Immunol. 2009;182(2):1202–10. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 22.Tyler DS, Stanley SD, Zolla-Pazner S. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990;145(10):3276–82. [PubMed] [Google Scholar]
- 23.Pollara J, Hart L, Brewer F. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A. 2011;79A(8):603–12. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari G, Pollara J, Kozink D. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol. 2011;85(14):7029–36. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr. HIV Res. 2008;6(6):515–9. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- 26.Alpert MD, Heyer LN, Williams DEJ. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol. 2012;86(22):12039–52. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology. 2011;412(1):110–6. doi: 10.1016/j.virol.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada T, Watanabe N, Nakamura T, Iwamoto A. Antibody-Dependent Cellular Cytotoxicity via Humoral Immune Epitope of Nef Protein Expressed on Cell Surface. J. Immunol. 2004;172(4):2401–6. doi: 10.4049/jimmunol.172.4.2401. [DOI] [PubMed] [Google Scholar]
- 29.Tiemessen CT, Shalekoff S, Meddows-Taylor S. Cutting Edge: Unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182(10):5914–8. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wren LH, Chung AW, Isitman G. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138(2):116–23. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung AW, Isitman G, Navis M. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci USA. 2011;108(18):7505–10. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonsignori M, Pollara J, Moody MA. Antibody-Dependent Cellular Cytotoxicity-Mediating Antibodies from an HIV-1 Vaccine Efficacy Trial Target Multiple Epitopes and Preferentially Use the VH1 Gene Family. J Virol. 2012;86(21):11521–32. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan Y, Pazgier M, Sajadi MM. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci USA. 2013;110(1):E69–78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forthal DN, Landucci G, Gorny MK, Zolla-Pazner S, Robinson WE. Functional activities of 20 human immunodeficiency virus type 1 (HIV-1)-specific human monoclonal antibodies. AIDS Res Hum Retroviruses. 1995;11(9):1095–9. doi: 10.1089/aid.1995.11.1095. [DOI] [PubMed] [Google Scholar]
- 35.Alsmadi O, Tilley SA. Antibody-Dependent Cellular Cytotoxicity Directed against Cells Expressing Human Immunodeficiency Virus Type 1 Envelope of Primary or Laboratory-Adapted Strains by Human and Chimpanzee Monoclonal Antibodies of Different Epitope Specificities. J Virol. 1998;72(1):286–93. doi: 10.1128/jvi.72.1.286-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moog C, Dereuddre-Bosquet N, Teillaud J-L. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2013;17:1–11. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69(9):5723–33. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moldt B, Schultz N, Dunlop DC. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fc? receptors to define the role of effector functions in protection against HIV. J Virol. 2011;85(20):10572–81. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt R, Kwong PD, Desjardins E. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(18):705–11. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455(7209):109–13. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran E, Borgnia MJ, Kuybeda O, Schauder DM. Structural Mechanism of Trimeric HIV-1 Envelope Glycoprotein Activation. PLoS Pathog. 2012;8(7):e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao Y, Wang L, Gu C. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat Struct Mol Biol. 2012;19(9):893–9. doi: 10.1038/nsmb.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433(7028):834–41. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, Kwon YD, Zhou T. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009;326(5956):1123–7. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore PL, Crooks ET, Porter L. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80(5):2515–28. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haim H, Salas I, Sodroski J. Proteolytic Processing of the Human Immunodeficiency Virus Envelope Glycoprotein Precursor Decreases Conformational Flexibility. J Virol. 2013;87(3):1884–9. doi: 10.1128/JVI.02765-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finnegan CM, Berg W, Lewis GK, DeVico AL. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J Virol. 2001;75(22):11096–105. doi: 10.1128/JVI.75.22.11096-11105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson JE, Elliott DH, Martin EA, Micken K, Rosenberg ES. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum Antibodies. 2005;14(3-4):115–21. [PubMed] [Google Scholar]
- 49.Da L-T, Quan J-M, Wu Y-D. Understanding of the bridging sheet formation of HIV-1 glycoprotein gp120. J Phys Chem B. 2009;113(43):14536–43. doi: 10.1021/jp9081239. [DOI] [PubMed] [Google Scholar]
- 50.Rolland M, Edlefsen PT, Larsen BB. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490(7420):417–20. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker LM, Phogat SK, Chan-Hui P-Y. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biedma ME, Lederle A, Decoville T, Schmidt S, Laumond G, Moog C. Lysis of primary infected CD4 T cells by ADCC is virus dependent.HIV Vaccines Keystone Symposia on Molecular and Cellular Biology 2013. Keystone CO. 2013 [Google Scholar]
- 53.Pancera M, McLellan JS, Wu X, Zhu J. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. J Virol. 2010;84(16):8098–110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–59. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koup RA, Pikora CA, Mazzara GP, Panicali D, Sullivan JL. Broadly Reactive Antibody-Dependent Cellular Cytotoxic Response to HIV-1 Envelope Glycoproteins Precedes Broad Neutralizing Response in Human Infection. Viral Immunol. 1991;4(4):215–23. doi: 10.1089/vim.1991.4.215. [DOI] [PubMed] [Google Scholar]
- 56.Robinson JE, Holton D, Pacheco-Morell S, Liu J, McMurdo H. Identification of conserved and variant epitopes of human immunodeficiency virus type 1 (HIV-1) gp120 by human monoclonal antibodies produced by EBV transformed cell lines. AIDS Res Hum Retroviruses. 1990;6(5):567–79. doi: 10.1089/aid.1990.6.567. [DOI] [PubMed] [Google Scholar]
- 57.Roben P, Moore JP, Thali M, Sodroski J, Barbas CF, Burton DR. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68(8):4821–8. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou T, Xu L, Dey B. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou T, Georgiev I, Wu X. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010 A. 2013;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynch RM, Tran L, Louder MK. The Development of CD4 Binding Site Antibodies during HIV-1 Infection. J Virol. 2012;86(14):7588–95. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1. PLoS Pathog. 2011;7(1):e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nitayaphan S, Pitisuttithum P, Karnasuta C. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative.Thai adults. . J Infect Dis. 2004;190(4):702–6. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 64.Gaschen B, Taylor J, Yusim K. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296(5577):2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 65.Wyatt R, Sodroski J. The HIV-1 Envelope Glycoproteins.Fusogens. Antigens and Immunogens. . Science. 1998;280(5371):1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 66.Finzi A, Xiang S-H, Pacheco B. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell. 2010;37(5):656–67. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alam SM, Scearce RM, Parks RJ. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics induction and potential for regulation in acute infection. J Virol. 2008;82(1):115–25. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen R, Drelichman ER, Bimczok D. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 2010;184(7):3648–55. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen X, Parks RJ, Montefiori DC. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J Virol. 2009;83(8):3617–25. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pantophlet R, Wrin T, Cavacini LA, Robinson JE, Burton DR. Neutralizing activity of antibodies to the V3 loop region of HIV-1 gp120 relative to their epitope fine specificity. Virology. 2008;381(2):251–60. doi: 10.1016/j.virol.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. Journal of Experimental Medicine Feb 11. 2013;210(2):209–23. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Losman B, Bolmstedt A, Schonning K. Protection of Neutralization Epitopes in the V3 Loop of Oligomeric Human Immunodeficiency Virus Type 1 Glycoprotein 120 by N-Linked Oligosaccharides in the V1 Region. AIDS Res Hum Retroviruses. 2001;17(11):1067–76. doi: 10.1089/088922201300343753. [DOI] [PubMed] [Google Scholar]
- 73.Wei X, Decker JM, Wang S. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 74.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. Antibody 2G12 Recognizes Di-Mannose Equivalently in Domain- and Nondomain-Exchanged Forms but Only Binds the HIV-1 Glycan Shield if Domain Exchanged. J Virol Sep 20. 2010;84(20):10690–9. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doores KJ, Bonomelli C, Harvey DJ. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107(31):13800–5. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trkola A, Purtscher M, Muster T. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70(2):1100–8. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore PL, Gray ES, Wibmer CK. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012;18(11):1688–92. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore PL, Sheward D, Nonyane M. Multiple pathways of escape from HIV broadly cross-neutralizing V2-dependent antibodies. J Virol. 2013;87(9):4882–94. doi: 10.1128/JVI.03424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oldridge J, Marsh M. Nef—an adaptor adaptor?. Trends Cell Biol. 1998;8(8):302–5. doi: 10.1016/s0962-8924(98)01318-x. [DOI] [PubMed] [Google Scholar]
- 80.Magadan JG, Bonifacino JS. Transmembrane Domain Determinants of CD4 Downregulation by HIV-1 Vpu. J Virol. 2011;86(2):757–72. doi: 10.1128/JVI.05933-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hessell AJ, Hangartner L, Hunter M. Fc receptor but not complement binding is important in antibody protection against HIV. Nature Sep 5. 2007;449(7158):101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 82.Moldt B, Shibata-Koyama M, Rakasz EG. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced Fc?RIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–96. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson HL. Non-neutralizing antibodies in prevention of HIV infection Expert opinion on biological therapy Informa. UK Ltd London. 2013;13(2):197–207. doi: 10.1517/14712598.2012.743527. [DOI] [PubMed] [Google Scholar]
- 84.Bomsel M, Tudor D, Drillet A-S. Immunization with HIV-1 gp41 Subunit Virosomes Induces Mucosal Antibodies Protecting Nonhuman Primates against Vaginal SHIV Challenges. Immunity. 2011;34(2):269–80. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Pettersen EF, Goddard TD, Huang CC. UCSF Chimera?.A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 86.Tudor D, Bomsel M. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a Fc?RI-dependent manner. AIDS. 2011;25(6):751–9. doi: 10.1097/QAD.0b013e32834507bd. [DOI] [PubMed] [Google Scholar]
- 87.Friedman J, Alam SM, Shen X. Isolation of HIV-1-Neutralizing Mucosal Monoclonal Antibodies from Human Colostrum. PLoS ONE. 2012;7(5):e37648. doi: 10.1371/journal.pone.0037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alsmadi O, Herz R, Murphy E, Pinter A, Tilley SA. A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection. J Virol. 1997;71(2):925–33. doi: 10.1128/jvi.71.2.925-933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Posner MR, Elboim HS, Cannon T, Cavacini L, Hideshima T. Functional Activity of an HIV-1 Neutralizing IgG Human Monoclonal Antibody: ADCC and Complement-Mediated Lysis. AIDS Res Hum Retroviruses. 1992;8(5):553–8. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- 90.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JGJ, Parren PWHI. Effector Function Activities of a Panel of Mutants of a Broadly Neutralizing Antibody against Human Immunodeficiency. Virus Type 1 J Virol. 2001;75(24):12161–8. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]