Abstract
Ingestion of vegetables rich in inorganic nitrate has emerged as an effective method, via the formation of a nitrite intermediate, for acutely elevating vascular NO levels. As such a number of beneficial effects of dietary nitrate ingestion have been demonstrated including the suggestion that platelet reactivity is reduced. In this study we investigated whether inorganic nitrate supplementation might also reduce platelet reactivity in healthy volunteers and have determined the mechanisms involved in the effects seen. We conducted two randomised crossover studies each in 24 (12 of each sex) healthy subjects assessing the acute effects of dietary nitrate (250 ml beetroot juice) or potassium nitrate capsules (KNO3, 8 mmol) vs placebo control on platelet reactivity. Inorganic nitrate ingested either from a dietary source or via supplementation raised circulating nitrate and nitrite levels in both sexes and attenuated ex vivo platelet aggregation responses to ADP and, albeit to a lesser extent, collagen but not epinephrine in male but not female volunteers. These inhibitory effects were associated with a reduced platelet P-selectin expression and elevated platelet cGMP levels. In addition, we show that nitrite reduction to NO occurs at the level of the erythrocyte and not the platelet. In summary, our results demonstrate that inorganic nitrate ingestion, whether via the diet or through supplementation, causes a modest decrease in platelet reactivity in healthy males but not females. Our studies provide strong support for further clinical trials investigating the potential of dietary nitrate as an adjunct to current antiplatelet therapies to prevent atherothrombotic complications. Moreover, our observations highlight a previously unknown sexual dimorphism in platelet reactivity to NO and intimate a greater dependence of males on the NO-soluble guanylate cyclase pathway in limiting thrombotic potential.
Abbreviations: BP, blood pressure; IBMX, 3-isobutyl-1-methylxanthine; LTA, light transmission aggregometry; PBS, phosphate-buffered saline; PPP, platelet-poor plasma; PRP, platelet-rich plasma; Sper-NO, spermine-NONOate.
Keywords: Inorganic nitrate, Platelet, cGMP, Nitrite
Highlights
-
•
Dietary nitrate or nitrate salt supplementation attenuates platelet activation in healthy male but not female volunteers.
-
•
These effects of nitrate relate to its bioactivation via the enterosalivary circuit to nitrite and then NO.
-
•
Nitrite attenuates platelet activation following reduction to NO at least in part at the level of the erythrocyte.
-
•
The antiplatelet effects of inorganic nitrate or nitrite in males are due to elevation of cGMP: an effect not evident in females.
-
•
Our findings intimate moderate antiplatelet effects of inorganic nitrate that might prove useful in therapeutics.
Introduction
Cardiovascular disease (CVD) remains a global killer affecting every ethnic group and accounts for approximately one-third of all deaths (www.who.int, www.heartstats.org). Over the past few decades there have been major advances in the treatment of patients presenting with a cardiovascular event, including primary percutaneous coronary intervention (PPCI) with stent insertion in acute myocardial infarction (AMI) [21] and rapid thrombolysis with tissue plasminogen activator (tPA) poststroke [49]. This first line treatment coupled with effective secondary prevention using aspirin and other antiplatelet drugs, such as prasugrel [54], have made substantial reductions in both mortality and morbidity. However, progress with respect to primary prevention of atherothrombotic events, despite considerable reductions already achieved through changes in lifestyle factors, remains an issue. Strong support for population approaches to prevention exist as articulated in the recent “European Guidelines on Cardiovascular Disease Prevention” [47]. Until recently it was thought that aspirin offered an effective option, and its use was routinely recommended in the primary prevention of coronary heart disease and perhaps to a lesser extent in stroke [1,2,33,34]. However, recent meta-analyses [6] and population studies [13] indicate serious bleeding risks with aspirin, that in many cohorts outweigh the potential benefits, suggesting that alternatives with a more moderate antiplatelet profile might be of some value. The use of “statins” in primary prevention has received substantial support as also has the concept, first mooted by Wald and Law in 2003 [48], of a cardiovascular polypill. However, the medicalisation of “healthy” individuals, noncompliance, and cost-effectiveness remain significant hurdles and uncertainties. Such issues have fuelled desires to better harness the beneficial and potentially antithrombotic effects of diets rich in fruit and vegetables [16,17]. This focus in part stems from the view that dietary interventions may be more acceptable and achievable for many individuals.
In this regard there has been growing interest in the possibility that dietary nitrate may underlie some of the beneficial effects of healthy diets. Inorganic nitrate is found in vegetables and is especially abundant in green-leafy vegetables and beetroot (Beta vulgaris). Small-scale clinical studies demonstrate that orally ingested inorganic nitrate is sequentially bioactivated within the enterosalivary circuit to inorganic nitrite (NO2-) [20,22,52]. Dietary nitrate is rapidly absorbed across the upper intestine to enter the circulation. While the majority of this nitrate is eventually excreted in the urine, ∼25% is actively extracted by the salivary glands and then secreted into the saliva where it comes into close contact with facultative bacteria expressing nitrate reductases [46] that have colonized the dorsal surface of the tongue. These bacteria reduce nitrate to nitrite that is then swallowed. Evidence suggests that at least some of this swallowed nitrite then appears within the circulation where it is converted to NO: a reaction thought to be facilitated by a number of enzyme-dependent and independent nitrite reductase pathways [50]. This nitrite-derived NO, in turn, exerts a number of beneficial effects within the circulation, most notably that administration of dietary nitrate or nitrate supplementation, via reduction to nitrite, results in NO-mediated vasodilatation and decreases in blood pressure (BP) [19,22,52]. Interestingly, post hoc analysis in one BP study also suggested that while females appeared to express a superior capacity to generate bioactive nitrite through this pathway that the activity of the nitrite generated was greater in males than in females [19].
There is also some suggestion that dietary nitrate, via nitrite as an intermediate, may repress platelet reactivity. We have previously shown that consumption of dietary nitrate (as beetroot juice) attenuates ex vivo stimulated platelet aggregation; an effect that was lost if the oral conversion of nitrate to nitrite was prevented [52], thereby preventing elevations of systemic nitrite levels. However, whether inorganic nitrate was responsible for this effect, whether NO was the underlying mediator of this response, and whether sex differences occurred in the effects of nitrate on platelet reactivity and the mammalian nitrite reductase pathways that might be involved in these effects remain uncertain. We have conducted clinical studies in healthy volunteers to interrogate these unknowns.
Methods
Volunteers
The studies were peer-reviewed by the institutional review board and were granted full ethics approval by The London Stanmore Research Ethics Committee (11/LO/0715). Informed, written consent was taken after satisfying the inclusion criteria. Volunteers were included if they fulfilled the following Inclusion criteria: 18–45 years of age, body mass index (BMI) of 18–40 kg/m2, and no systemic medication (other than the oral contraceptive pill). Volunteers adhered to a low nitrate diet and refrained from caffeine consumption and strenuous exercise the day before and the day of the respective study, and were fasted overnight before all study visits.
Investigation of the effect of dietary nitrate ingestion (beetroot juice) on platelet aggregation assessed ex vivo using light transmission aggregometry (LTA)
In a single-blind (investigator blind), randomised, placebo-controlled crossover study, male (n=12) and female (n=12) volunteers received dietary nitrate (as beetroot juice giving a nitrate dose: 3.1±0.35 mmol equivalent to approximately 190 mg) or matched-volume of low nitrate-containing water (Zepbrook Ltd., London, UK) placebo control, on two separate occasions, at least 7 to 28 days apart. For LTA, blood was collected into a 60 ml syringe preprepared with 3.2% sodium citrate through a 19 gauge butterfly needle and then immediately centrifuged (170g, 21 °C, 15 min) to generate platelet rich plasma (PRP) that was stored at 37 °C and used within 30 min. Blood was sequentially centrifuged (170g, 21 °C, 15 min, and then 15,000g, 21 °C, 2 min) to generate PRP and platelet-poor plasma (PPP), respectively. PRP was taken as equivalent to 0% aggregation and PPP was taken as equivalent to 100% aggregation, respectively. Platelet aggregation in response to adenosine diphosphate (ADP) (0.1–30 µmol/L), collagen (0.1–3 µg/ml), and epinephrine (0.001–100 µmol/L) was measured using a 96-well plate light transmission assay over 16 min, as previously described [3]. For comparisons between groups and treatments the AUC of percentage aggregation response over 16 min was used. All measures were performed at baseline and at 3 h post intervention. This 3 h time point was selected in view of our previous findings demonstrating that circulating levels of nitrite, and the associated bioactivity, peak 3 h post nitrate ingestion [52].
Assessment of the in vitro effects of nitrite on platelet aggregation
Blood collected from healthy male volunteers (n=6–8) was incubated (10–30 min, 37 °C) with potassium nitrite (KNO2, 0.1–3 μmol/L), spermine-NONOate (Sper-NO; 1–10 μmol/L), or phosphate-buffered saline (PBS, as control). Platelet aggregation was assessed in response to ADP (0.1–10 µmol/L) and collagen (0.1–30 µg/ml) in PRP by LTA.
To assess the effect of nitrite on platelet aggregation in whole blood, blood was collected and incubated with nitrite for 10 min prior to assessment of aggregation in response to ADP or collagen using impedance aggregometry (n=12–15 males). In a separate series of experiments the effects of Sper-NO (1–10 μmol/L) on ADP-induced aggregation of whole blood were determined (n=5 males, n=5 females). An aliquot of blood was collected from each volunteer for in vitro incubation with nitrite (1 µmol/L, 10 min, 37 °C) followed by isolation of a platelet pellet for determination of cyclic guanosine monophosphate (cGMP) levels using a commercially available ELISA. In addition, a separate aliquot of blood was collected for erythrocyte isolation (n=7 males; n=5 females) and assessment of nitrite reductase activity using gas phase ozone chemiluminescence as previously described [51].
Investigation of the effects of inorganic nitrate supplementation (KNO3 capsules) on whole blood platelet aggregation assessed ex vivo
In a double blind, randomised, placebo controlled crossover study male (n=12) and female (n=13) volunteers received either 8 mmol potassium nitrate (KNO3, Martindale Pharmaceuticals, Ipswich, UK, equivalent to 496 mg of nitrate) or matched potassium chloride (KCl, Martindale Pharmaceuticals, Ipswich, UK) placebo control, and returned for the cross-over limb between 7 and 28 days later. Capsules were consumed with 2 slices of dry wholemeal toast and 250 ml low-nitrate-containing water (Zepbrook Ltd., London, UK). Blood, urine, and saliva were collected for analysis of [nitrate] and [nitrite] and blood was separately collected for determination of P-selectin expression under unstimulated conditions. Platelet aggregation was assessed in whole blood in response to ADP (10 μmol/L), collagen (3 μg/ml), epinephrine (10 µmol/L), and PBS (as control) using an impedance aggregometer (MultiplateRanalyzer, Dyabyte Medical, Germany) measured over a 6 min period. Aggregation is quantified as AUC giving a measure of total resistance Ω*time. Briefly 300 µL of citrated whole blood was added to 300 µL of normal saline with 3 mmol/L CaCl2 (Sigma, UK) and equilibrated with constant magnetic stirring for 3 min prior to addition of agonist and platelet aggregation measurement. For the incubation of whole blood with nitrite following a 3 min equilibration period, the relevant concentration of potassium nitrite was added and a further 10 min incubation period ensued prior to addition of aggregating agonist and measurement of platelet aggregation. All measures were performed at baseline and at 3 h.
Blood pressure measurement
Blood pressure was measured at baseline to confirm healthy volunteer status and effective randomisation. An Omron 705IT was used for all BP measurements while participants were seated and readings were performed in triplicate according to established guidelines [53]. Laminated coverings were used for the machine and the printer so that both investigators and participants were blinded to the readings. The means of the 2nd and 3rd readings were used to calculate the final BP measurement.
Blood sampling
Blood was collected into either 1.8 mg EDTA per ml of blood (for NOx), 3.2% buffered sodium citrate (for aggregation assays), or 0.1 mmol/L 3-isobutyl-1-methylxanthine (IBMX) (for cGMP assay) containing vacutainers (BD) through a 21-gauge butterfly needle inserted into an antecubital vein. For the generation of platelet-rich plasma (PRP), citrated blood was immediately centrifuged (170g at 21 °C for 15 min) and PRP separated, stored at 37 °C, and used within 30 min. For measurement of NOx levels, blood samples were centrifuged immediately (1300g, 4 °C, 10 min) and the supernatant was collected and stored at -80 °C pending analysis by ozone chemiluminescence.
Urine and saliva sampling
Mid-stream urine samples were collected into sterile pots and an aliquot was stored at -80 °C pending analysis by ozone chemiluminescence. Unstimulated saliva was collected into sterile Eppendorfs and centrifuged (14,000g, 4 °C, 10 min) and the supernatant was stored at −80 °C pending analysis by ozone chemiluminescence.
Ozone chemiluminescence
Briefly, to determine total nitrate and nitrite levels (collectively termed [NOx]), samples were added to 0.1 mmol/L vanadium (III) chloride in 1 mmol/L hydrochloric acid refluxing at 95 °C under nitrogen. Nitrite concentrations were determined by addition of samples to 0.09 mmol/L potassium iodide in glacial acetic acid under nitrogen at room temperature. [Nitrate] were calculated by subtraction of [nitrite] from [NOx] as previously described. [14]
Measurement of platelet cGMP
For assessment of platelet cGMP following in vivo interventions blood (12 ml) was collected into 3 citrated tubes via the same puncture and 0.1 mmol/L IBMX (Sigma, UK) added. This blood was immediately centrifuged (170g, 15 min, 21 °C) to generate PRP that was then further centrifuged (1800g, 10 min, 4 °C) to obtain a platelet pellet and PPP. Both were then stored at −80 °C pending cGMP measurement. For assessment of the effects of the in vitro effects of nitrite on platelet cGMP, blood was collected as above and immediately incubated with nitrite at 37 °C for 10 min. Following this PRP and then a platelet pellet were generated and then stored at −80 °C pending cGMP measurement. Platelet cGMP was measured using an ELISA (GE Healthcare, Amersham) according to the manufacturer's instructions.
P-selectin expression
Two-colour whole blood flow cytometry was used to measure platelet P-selectin using a modification of previously published protocols and recommendations [41]. Whole blood was collected from individuals prior to and following ingestion of nitrate or placebo capsules. Samples were immediately incubated with selective antibodies, at room temperature for 20 min, and then fixed using 1% paraformaldehyde (Sigma, UK), stored at 4 °C and analysed 2 h after completion of each study visit using a Becton Dickinson FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The platelet population was identified preliminarily based on forward and side scatter properties, then further delineated via labeling with CD42b monoclonal antibody conjugated to allophycocyanin (APC). Gates were used to isolate this population, and CD62 (P-selectin) monoclonal antibody conjugated to (fluoroscenisothiocyanate) FITC was used to determine P-selectin expression. Populations were further confirmed by use of antibody negative isotypes to P-selectin and CD42b. All samples were run in duplicate. 10,000 platelets were acquired in the CD42b region, and results were expressed as the percentage of platelets positive for P-selectin.
Erythrocyte nitrite reductase assay
For the measurement of the nitrite reductase activity of tissue and erythrocyte samples gas phase chemiluminescence was used. Experiments were performed in a sealed 10-ml glass reaction chamber containing citric acid/Na₂HPO₄ buffer at pH 7.4 (physiological levels) or pH 6.8 (representing acidosis), and KNO2 (10–300 µmol/L) in a total volume of 1 ml. This solution was bubbled with nitrogen gas (100%) by means of an NO scrubbing air filter (Sievers, Boulder, CO, USA). The headspace NO concentration was measured in parts per billion by continuous sampling for ozone chemiluminescence (Sievers 280 A nitric oxide analyzer). The impact of biological tissue samples on NO production from nitrite, was determined by the addition of washed human RBCs from healthy human volunteers and measurement of NO over 2 min, calculating the rate of NO production (pmol per g per s) from the area under the curve.
Statistical analysis
The data were analysed using Graphpad Prism software version 5. All data are expressed as mean±SEM. Data for LTA aggregometry represent the area under the curve (over 16 min)*% platelet aggregation and are reported in arbitrary units, presented in concentration–response curves and were analysed using repeated-measures two-way ANOVA and Bonferroni post hoc tests. Erythrocytic nitrite reductase activity was analysed by two-way repeated-measures ANOVA. Nitrite incubation whole blood aggregometry data were analysed using one-way ANOVA and Dunnett's post hoc test compared to control (PBS). Systemic NOx levels were analysed using one-way ANOVA and Bonferroni post hoc tests. For comparison of all other data paired Student's t tests were used. Values of P<0.05 were considered significant.
Results
All interventions were tolerated without adverse effects.
Dietary nitrate treatment attenuates ex vivo sensitivity of platelets to ADP
There were no significant differences in the demographics of healthy volunteers between visits (Table 1).
Table 1.
Demographics, hemodynamic and plasma NOx values for both limbs of the dietary nitrate (beetroot juice) vs low-nitrate-containing water supplementation study.
| Characteristic | Placebo | Dietary Nitrate | P value | |
|---|---|---|---|---|
| Male (n) | 12 | |||
| Age (years) | 26.1±0.8 | |||
| BMI (kg/m2) | 23.8±1.2 | |||
| Baseline SBP (mm Hg) | 126.4±3.9 | 126.5±3.8 | 0.98 | |
| Baseline DBP (mm Hg) | 71.8±2.8 | 72.1±1.9 | 0.87 | |
| Female (n) | 12 | |||
| Age (years) | 24.1±1.9 | |||
| BMI (kg/m2) | 22.9±1.1 | |||
| Baseline SBP (mm Hg) | 106.8±2.3 | 105.1±1.9 | 0.26 | |
| Baseline DBP (mm Hg) | 64.0±1.6 | 65.0±1.4 | 0.39 |
Statistical analysis conducted using paired t test.
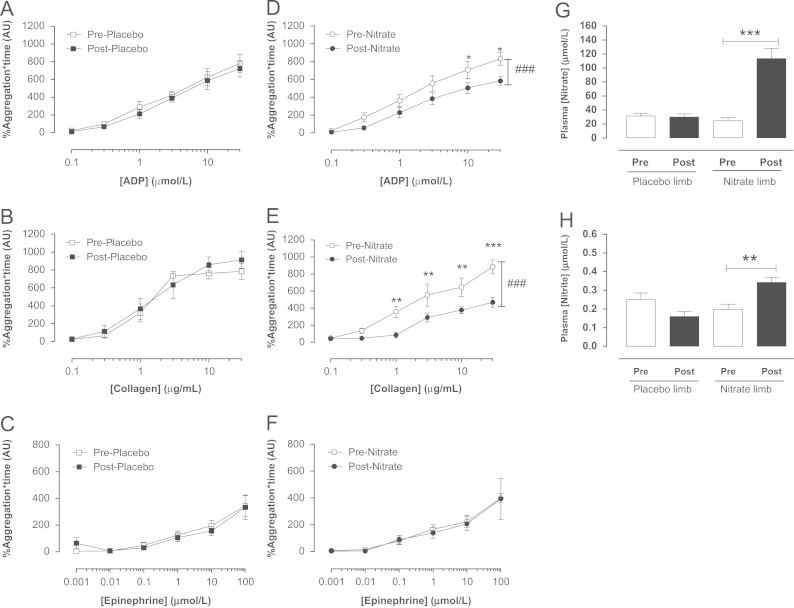
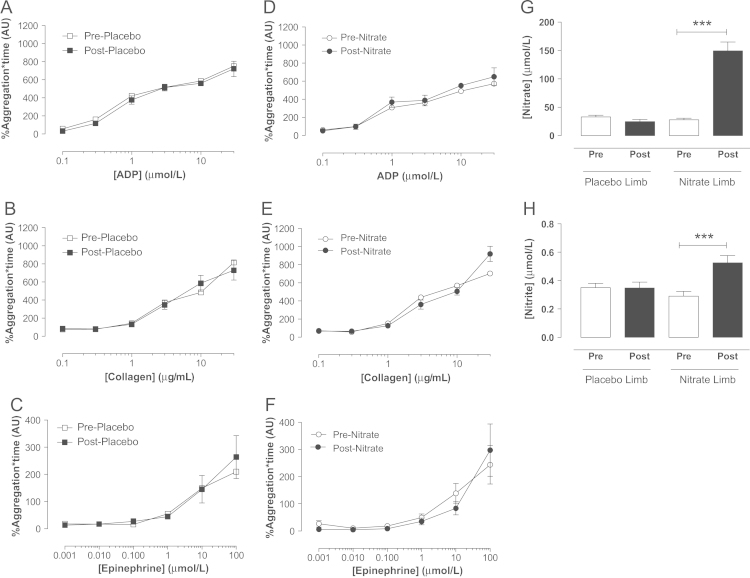
In both male (Fig. 1) and female (Fig. 2) volunteers, dietary nitrate administration, in the form of beetroot juice, caused a significant rise in plasma [nitrite] and [nitrate]. Ex vivo assessment of platelet reactivity using LTA demonstrated concentration-dependent aggregation of platelets isolated from either male or female volunteers in response to collagen, ADP, and epinephrine (Figs. 1 and 2). Importantly, the concentration–response curves to both ADP and collagen, but not epinephrine, were significantly suppressed following dietary nitrate treatment in males (Fig. 1) but not females (Fig. 2). The placebo had no effect on platelet responses to any aggregating stimuli in either sex (Figs. 1 and 2).
Fig. 1.
Dietary nitrate supplementation elevates plasma nitrite levels and attenuates platelet reactivity in healthy male volunteers. Ex vivo platelet (PRP) aggregation assessed by light transmission aggregometry in response to ADP (0.1–0.3 µmol/L), collagen (0.1–30 µg/ml), and epinephrine (0.001–100 µmol/L), before and 3 h post placebo (A–C) or dietary nitrate (beetroot juice, ∼3.1 mmol nitrate, D–F) (n=12). Effect of dietary nitrate on plasma nitrate (G) and nitrite (H) concentrations. Data are expressed as mean±SEM. Significance shown as ###P<0.001 for two-way repeated-measures ANOVA, and ⁎P<0.05,⁎⁎P<0.01, and ⁎⁎⁎P<0.001 for Bonferroni post hoc tests following one-way or two-way repeated-measures ANOVA as appropriate. (ADP, adenosine diphosphate; PRP, platelet-rich plasma).
Fig. 2.
Dietary nitrate supplementation elevates plasma nitrite levels but does affect platelet reactivity in healthy female volunteers. Ex vivo platelet (PRP) aggregation assessed by light transmission aggregometry in response to (A, B) ADP (0.1–0.3 µmol/L), (C, D) collagen (0.1–30 µg/ml), and (E, F) epinephrine (0.001–100 µmol/L), and (G) plasma nitrate and (H) nitrite levels before and 3 h post placebo or dietary nitrate (beetroot juice, ∼3.1 mmol nitrate) consumption in females (n=12). Data are expressed as mean±SEM. Significance shown as ⁎⁎⁎P<0.001 for Bonferroni post hoc tests following one-way ANOVA. (ADP, adenosine diphosphate; PRP, platelet-rich plasma).
In vitro incubation of platelets with nitrite does not alter platelet reactivity
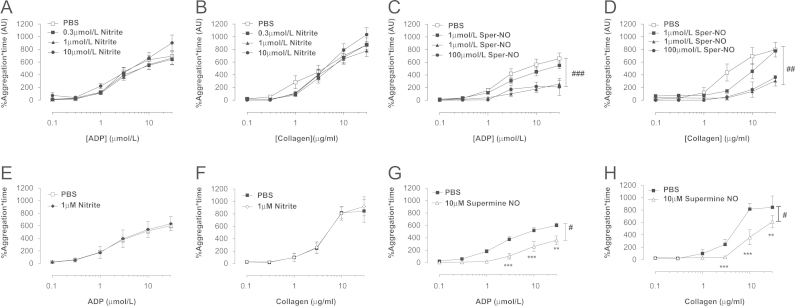
We next investigated whether the effects of dietary nitrate in male volunteers were related to a direct effect of nitrite on circulating platelets. Nitrite treatment did not alter the aggregating response to either ADP or collagen of PRP collected from untreated healthy male volunteers following a 30 min (Fig. 3A and B) or a 10 min pretreatment period (Fig. 3E and F). In contrast, prior incubation of platelets with Sper-NO caused a concentration-dependent attenuation of platelet aggregation (Fig. 3C, D, G, and H).
Fig. 3.
Spermine-NONOate but not KNO2 inhibits aggregation of platelet-rich plasma (PRP). Platelet aggregation induced by ADP (0.1–0.3 µmol/L) or collagen (0.1–30 µg/ml) assessed by light transmission aggregometry in PRP from healthy untreated male volunteers incubated ex vivo for 30 min with either KNO2 (A, B) or spermine-NONOate (C, D) or for 10 min with KNO2 (1 µmol/L, E, F) or spermine-NONOate (10 µmol/L G, H). Data are expressed as mean±SEM of n=5–13. Significance shown as #P<0.05, ##P<0.01, and ###P<0.001 for two-way repeated-measures ANOVA followed by Bonferroni post tests shown as ⁎⁎P<0.01 and ⁎⁎⁎ for P<0.001. (ADP, adenosine diphosphate; PRP, platelet-rich plasma; Sper-NO, Spermine-NONOate).
In vitro incubation of whole blood with nitrite attenuates platelet aggregation responses to ADP
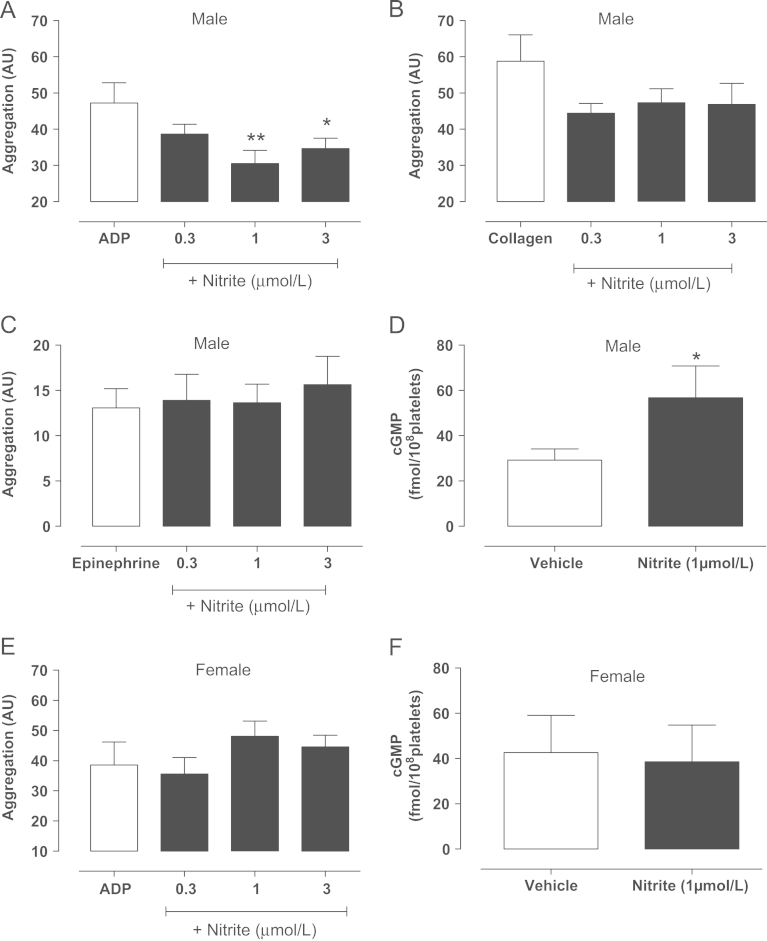
To investigate the possibility that nitrite bioactivity might be dependent on the presence of erythrocytes (a previously identified important site for nitrite conversion to NO within the circulation [11]) we investigated the effect of incubation in vitro of whole blood, collected from male volunteers, with nitrite and subsequent sensitivity to platelet aggregating stimuli using whole blood impedance aggregometry. Nitrite incubation (KNO2, 10 min) caused a modest (maximum effect 27±8% inhibition) concentration-dependent reduction in platelet aggregation in response to ADP (Fig. 4A) and to a lesser extent collagen (Fig. 4B), although the latter did not reach statistical significance. Nitrite treatment had no effect on epinephrine-induced aggregation responses (Fig. 4C). Incubation with an equivalent salt solution control of KCL had no significant effect on responses to ADP (PBS 42±5.1 AU, 0.3 µmol/L 42.1±3.9 AU, 1 µmol/L 35±4.9 AU, 3 µmol/L 35.4±7.1 AU, n=5 for each). This effect of nitrite was associated with a near doubling of platelet cGMP levels (Fig. 4D). In contrast, nitrite did not alter responses of whole blood collected from female healthy volunteers to ADP and nitrite treatment was not associated with any elevation of platelet cGMP levels (Fig. 4E and F). In support of a reduced sensitivity of females to NO, Sper-NO caused concentration-dependent attenuation of platelet aggregation in response to ADP that was significantly reduced in females compared to males (Table 2)
Fig. 4.
KNO2 reduces platelet aggregation in whole blood with an associated rise in platelet cGMP in males. Platelet aggregation assessed by impedance aggregometry of whole blood of healthy male volunteers incubated ex vivo with KNO2 (0.3—3 µmol/L) in response to (A) ADP (10 µmol/L, n=13), (B) collagen (3 µg/ml, n=11), and (C) epinephrine (10 µmol/L, n=11). Platelet cGMP levels in whole blood of males incubated with KNO2 (D, 1 µmol/L, n=12). Effect of ex vivo incubation of whole blood with KNO2 (0.3–3 µmol/L) on (E) ADP-induced (10 µmol/L, n=7) aggregation and (F) platelet cGMP levels (n=6) collected from untreated healthy female volunteers. Data are expressed as mean±SEM. Significance shown as ⁎P<0.05, ⁎⁎P<0.01 for Dunnett's post hoc test compared to control (PBS) following one-way ANOVA. For cGMP data, significance shown as ⁎P<0.05 following paired t test. White bars represent PBS and black bars nitrite (ADP, adenosine diphosphate).
Table 2.
Effect of the NO donor Spermine NONOate on ADP (10 μmol/L)-induced platelet aggregation in whole blood of male (n=5) and female (n=5) healthy volunteers.
| Spermine NONOate (μmol/L) | Male (% control ADP response) | Female (% control ADP response) |
|---|---|---|
| 0.1 | 117.9±2.6 | 107.9±4.6 |
| 1.0 | 106.9±4.7 | 99.9±4.2 |
| 3.0 | 46.9±17.1⁎⁎ | 75.6±14.6 |
| 10.0 | 3.5±2.1⁎⁎⁎ | 10.0±2.2⁎⁎⁎ |
Data shown as mean±SEM. Statistical significance determined using one-way ANOVA followed by Dunnett's comparison to the control response to ADP in each <respective sex (male aggregation response= 37.0±2.9 and female aggregation= 50.5±1.6).
Inorganic nitrate supplementation attenuates sensitivity of platelets to ADP and collagen in males only
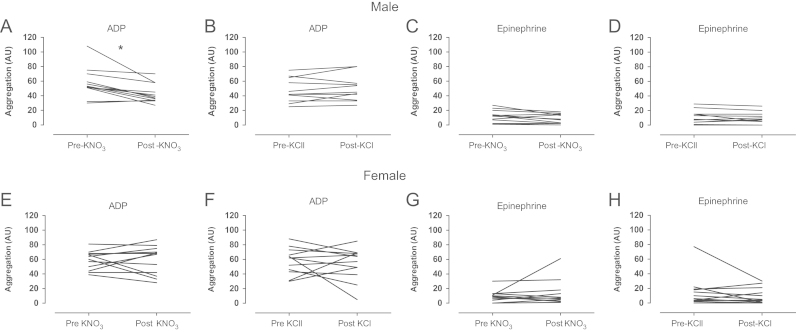
There were no significant differences in the demographics of healthy volunteers between visits (Table 3). Of the females recruited for this study 6 were taking the contraceptive pill and 7 were not. Following KNO3 supplementation there was significant elevation in plasma, salivary, and urinary [nitrate] and [nitrite] in both male (Fig. 5) and female (Fig. 6) healthy volunteers, an effect not seen following KCl ingestion. In male volunteers these elevations were associated with a significant suppression of ADP (Fig. 7A and B) but not epinephrine-induced aggregatory response (Fig. 7C and D). In contrast, despite elevations in systemic [nitrite] and [nitrate] following KNO3 ingestion in female volunteers, no change in ex vivo platelet reactivity was demonstrated (Fig. 7E–H).
Table 3.
Baseline demographic, hemodynamic, and analytical parameters for both limbs of the potassium nitrate (8 mmmol) versus potassium chloride (8 mmol) capsule study.
| Characteristic | Placebo | Inorganic nitrate | P value | |
|---|---|---|---|---|
| Male (n) | 12 | |||
| Age (years) | 26.7±1.4 | |||
| BMI (kg/m2) | 22.8±2.0 | |||
| Baseline SBP (mm Hg) | 120.3±3.2 | 121.0±3.7 | 0.80 | |
| Baseline DBP (mm Hg) | 67.2±1.8 | 66.8±2.0 | 0.77 | |
| Female (n) | 12 | |||
| Age (years) | 29.3±1.8 | |||
| BMI (kg/m2) | 22.9±0.8 | |||
| Baseline SBP (mm Hg) | 107.8±2.6 | 106.1±2.3 | 0.44 | |
| Baseline DBP (mm Hg) | 68.8±2.0 | 67.3±2.2 | 0.35 |
Statistical analysis conducted using paired t test.
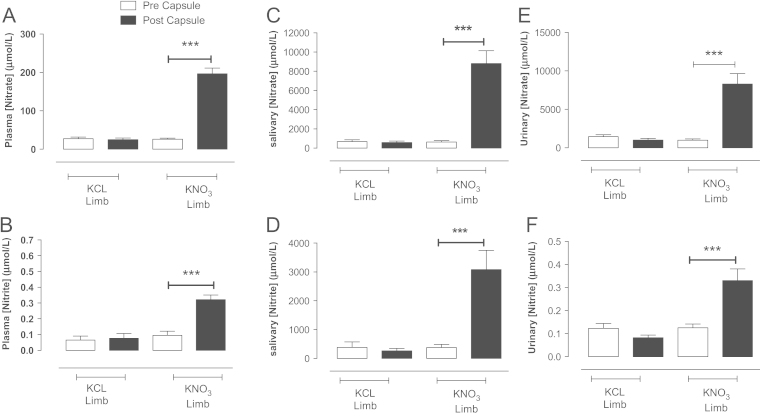
Fig. 5.
KNO3 but not KCl (8 mmol) supplementation elevates systemic nitrate and nitrite levels in healthy male volunteers. The effects of KNO3 or KCl ingestion 3 h prior on circulating plasma (A,B), salivary (C,D), and urinary (E,F) nitrate and nitrite levels. Data are expressed as mean±SEM of n=12. Significance shown for Bonferroni post hoc tests between groups as ⁎⁎P<0.01 and ⁎⁎⁎P<0.001 following one-way ANOVA.
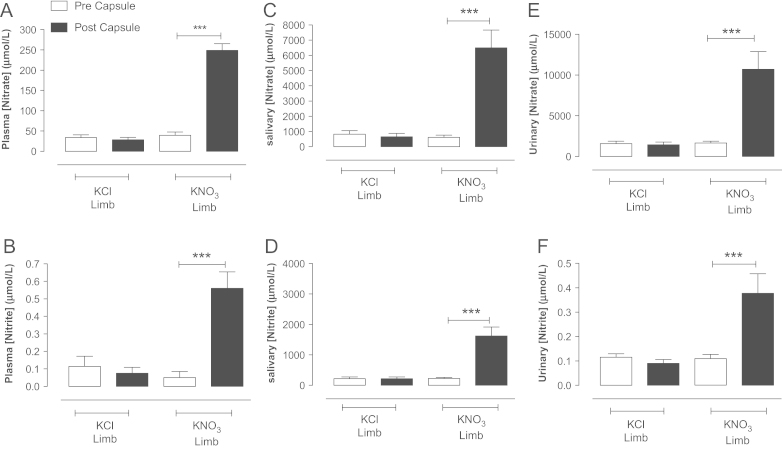
Fig. 6.
KNO3 but not KCl (8 mmol) supplementation elevates systemic nitrate and nitrite levels in healthy female volunteers. The effects of KNO3 or KCl ingestion 3 h prior on circulating plasma (A, B), salivary (C, D), and urinary (E, F) nitrate and nitrite levels. Data are expressed as mean ±SEM of n=12. Significance shown for Bonferroni post hoc tests between groups as ⁎⁎P<0.01 and ⁎⁎⁎P<0.001 following one-way ANOVA.
Fig. 7.
KNO3 (8 mmol) supplementation attenuates platelet aggregation in healthy male but not female volunteers. Platelet aggregation assessed by impedance aggregometry of whole blood collected from healthy male volunteers in response to ADP (10 µmol/L) and epinephrine (10 µmol/L) before and 3h post KNO3 (A, C) and KCl (B, D) supplementation and female volunteers (E, G and F, H respectively). Data are expressed as mean±SEM of n=12. Significance shown for Bonferroni post hoc tests between groups as ⁎P<0.05 following one-way ANOVA (ADP, adenosine diphosphate).
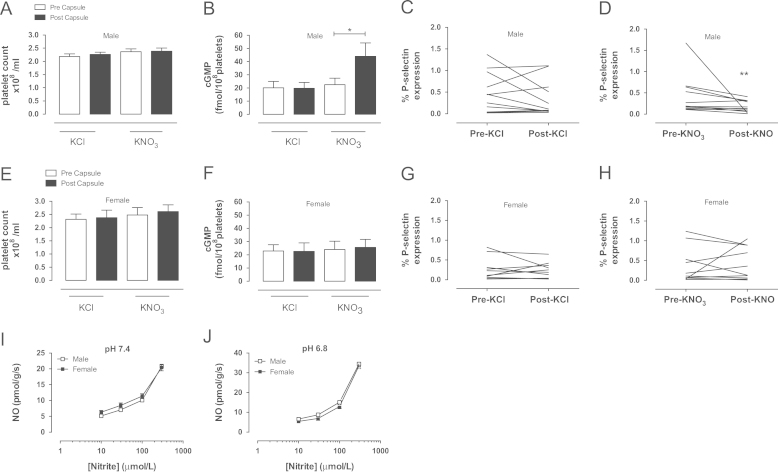
Suppression of platelet aggregation with dietary nitrate is associated with significant increase in platelet cGMP and suppression of P-selectin exposure
In males, attenuation of ex vivo platelet reactivity following inorganic nitrate ingestion was associated with a doubling of platelet cGMP levels and a significant suppression of P-selectin expression (Fig. 8). Neither KNO3 or KCl ingestion affected platelet number in either males or females (Fig. 8A and E). In contrast, the lack of effect of inorganic nitrate consumption in females was associated with an absence of any rise in platelet cGMP levels and no changes in P-selectin expression (Fig. 8F–H). There were no differences in P-selectin mean fluorescence intensity between the groups (data not shown). The differences in the functional effects of oral nitrate ingestion between the sexes is unlikely to be due to differences in the nitrite reductase potential of erythrocytes since activity, assessed in vitro, at both pH 7.4 and 6.8 (optimal conditions for nitrite reduction) was identical (Fig. 8I and J).
Fig. 8.
KNO3 supplementation elevates platelet cGMP levels and simultaneously suppresses unstimulated platelet P-selectin expression in healthy male but not female volunteers. Platelet count (A, n=12), platelet cGMP levels (B, n=9) and platelet P-selectin expression (C and D, n=11–12) in healthy males following KNO3 or KCl (8 mmol) supplementation and in healthy females (E–H, respectively). Erythrocytic nitrite reductase activity at pH 7.4 (I) and pH 6.8 (J), in males (n=7) and females (n=5). All data are expressed as mean±SEM. Significance shown as ⁎P<0.05 and ⁎⁎P<0.01 following paired t test for two groups. No significant differences in nitrite reductase activity assessed using two-way repeated-measures ANOVA.
Discussion
Dietary (inorganic) nitrate may underpin the cardioprotective effects offered by fruit- and vegetable-rich diets [16,17,20]. In support of this possibility, recent studies in healthy volunteers have shown a range of beneficial effects of acute administration of a dietary nitrate load [52] or inorganic nitrate supplementation [19,22,23] on the cardiovascular system including some suggestion of reduced platelet reactivity [38,52]. In the present study we demonstrate a modest antiplatelet effect of both dietary and inorganic nitrate supplementation in male but not female healthy volunteers. This effect in males is dependent on elevation of circulating nitrite followed by reduction of nitrite to NO, in part, at the level of the erythrocyte. In turn this NO suppresses platelet reactivity by elevation of cGMP. Our results also suggest that while the enterosalivary circuit and nitrite reductive pathways are intact in females, the antiplatelet effect is absent due to an absence of platelet cGMP increase. These findings suggest that dietary nitrate or inorganic nitrate supplementation may prove useful in providing a modest reduction of platelet activity either in primary prevention or as an adjunct to antiplatelet therapies in secondary prevention of atherothrombotic events. Moreover, our observations describe a novel sexual dimorphism that may help to better understand the sex differences evident in thrombotic potential in humans.
Inorganic nitrate administration caused a significant rise in both circulating nitrate, and consequently nitrite levels, whether administered in a dietary form or via inorganic nitrate supplementation. These changes in NOx are similar to those demonstrated in healthy volunteers previously [5,19,52]. It is accepted that, following its ingestion, inorganic nitrate is rapidly absorbed across the intestine and then either excreted by the kidneys or concentrated in the salivary glands, where in the case of the latter, it is secreted into the saliva [46]. Our results demonstrate clear evidence of this pathway occurring in the healthy volunteers recruited into this clinical study. Within the oral cavity approximately two-thirds of inorganic nitrate is thought to be converted to nitrite by bacterial nitrate reductases [18,43,46,52]: a view likewise supported by our findings herein. This nitrite, once swallowed, enters the circulation with levels peaking at approximately 3 h following nitrate ingestion [19,52]. Importantly, the biological effects of inorganic nitrate ingestion correlate directly with the levels of nitrite in the circulation, peak simultaneously with the peak in circulating nitrite levels, and are thought to be due to the conversion of this nitrite to NO within the circulation [19]. It was on this basis that all assessments of platelet function in the studies described herein were conducted 3 h following nitrate ingestion in order to observe the maximum possible effects.
We have demonstrated that the rise in circulating nitrite levels following either dietary nitrate ingestion or following inorganic nitrate supplementation was associated with a reduction in ex vivo platelet aggregation induced by either ADP or collagen using two distinct methods of assessment of platelet aggregation. Importantly, however, we found no effect on responses to the weak platelet activator epinephrine. Both ADP and collagen, while activating distinct receptors (P2Y1/P2Y12 and GPVI) and molecular pathways within the platelet, trigger a number of common phenomena implicated in platelet activation, including platelet granule secretion, thromboxane A2 release, and platelet aggregation events also commonly associated with increases in phosholipase C and phosphoinositide-3-kinase activity (see review [25]). In contrast, epinephrine alters platelet reactivity by binding to α2-receptors coupled to Gi resulting in a reduction in intracellular cAMP levels. It has been suggested that per se epinephrine exerts little change in platelet response; however, when present in combination with other stronger activating stimuli such as ADP or histamine, α2-receptor activation enhances the stimulatory effect of the agonist [7,40]. Since epinephrine was not used in combination with other agonists this may underlie the absence in effect of inorganic nitrate on responses seen but also suggests that nitrate acts to suppress pathways implicated in platelet activation commonly associated with stronger stimuli.
It is likely that the repressive effects of inorganic nitrate on platelet reactivity are due to the formation and activity of NO in vivo. NO is a potent inhibitor of platelet function and basal NO generation is thought to play a crucial role in suppressing platelet reactivity in physiology [26]. This activity of NO has been attributed, primarily, to activation of sGC and consequent elevation of platelet cGMP levels [12,30,35,39]. Although, there is also some suggestion that there may be sGC-independent effects of NO and NO donor drugs on platelets [27,32,42,55] and also controversially prostimulatory effects of the NO-sGC-cGMP pathway in platelets [28,45,55]. More recently both of these possibilities have been challenged by demonstration of an absence of any anti- or indeed prostimulatory platelet effects of NO in mice lacking the β1 subunit of sGC [12] or in mice with selective deletion of platelet sGC [39]. Although, in contrast using the same platelet-specific mice evidence for a stimulatory effect of NO-induced cGMP has also been proffered [55]. Nevertheless, irrespective of this controversy, our studies support an inhibitory role for the NO-sGC-cGMP pathway in platelets. Indeed, we show that inorganic nitrate ingestion in males elevates platelet cGMP levels implicating NO in the antiaggregatory effects. This effect likely underlies the beneficial effects since in females where no change in platelet reactivity was evident there was likewise no change in platelet cGMP levels.
The exact mechanisms involved in cGMP-induced suppression of platelet reactivity in the present study are uncertain. It has been suggested that elevation of cGMP in platelets results in PKG activation and consequent phosphorylation of a range of proteins that influence platelet function including inositol triphosphate receptors and phosphodiesterase 5 to name just two (see review [15]). Irrespective of the exact molecular pathways involved, NO has been shown to cause a number of phenomena in platelets that would ultimately result in decreased activity including reduced granule secretion [8,29] and platelet adhesion [36]: phenomena shown to be both cGMP dependent [29,37] and independent [27,31]. As an index of both of these characteristics we measured P-selectin expression and demonstrated that, in males treated with inorganic nitrate, unstimulated platelet P-selectin expression was suppressed and accordingly in females where cGMP was not changed no difference in P-selectin expression was evident.
The conversion of nitrite to NO within the circulation is thought to be facilitated by a number of distinct nitrite reductases that have been localised to either the erythrocyte or the blood vessel wall. To determine whether the platelet itself might be the site of nitrite reduction we investigated the effects of incubation of purified platelets with nitrite, at similar concentrations to those achieved following oral inorganic nitrate ingestion (i.e., 0.1–3 µmol/L), prior to assessment of aggregation using LTA. Our data show no effect of nitrite when incubated with platelets for 10 or 30 min despite clear evidence of sensitivity to NO, demonstrated using the NO donor Sper-NO. These observations are in agreement with recent studies testing similar concentrations of nitrite with human PRP demonstrating no effect of nitrite on ADP or collagen-induced aggregation [44] and suggest that nitrite has no direct effect on platelets. In contrast, there are some suggestions that nitrite exerts direct inhibitory effects on platelets; however, the in vivo relevance of these observations are uncertain since the effects of nitrite were achieved with concentrations 10–100 times above that found circulating in vivo [4,24]. Thus, in agreement with recently published observations [44], our findings intimate that while physiological concentrations of nitrite in vivo exert antiplatelet effects this is not due to its conversion to NO at the platelet itself.
To determine whether the erythrocyte might be the site of nitrite conversion within the circulation we assessed the effects of nitrite incubation on platelet aggregation in whole blood. Our data demonstrate that in vitro nitrite causes moderate concentration-dependent inhibition of platelet aggregation in response to ADP when incubated with whole blood collected from healthy male volunteers. This effect of nitrite was likely due to its conversion to NO since it was associated with elevations in platelet cGMP levels. It is therefore probable that in vivo nitrite reduction occurs, at least in part, at the erythrocyte. Indeed, there are several reports demonstrating erythrocytic nitrite reduction [11,51]. However, whether the erythrocyte accounts for all of the nitrite reduction occurring in vivo that underlies the reduced platelet sensitivity is uncertain. The exact nitrite reductase that might be involved in this effect is not certain. Recent in vitro studies suggest that deoxyhemoglobin might be the nitrite reductase on erythrocytes influencing platelet function since reductions in oxygen tension associated with increases in deoxyhemoglobin levels resulted in improved nitrite bioactivity [44]. Whether this might be the pathway involved in our study is uncertain and warrants investigation.
Our studies also suggest that the pathways for NO bioactivity within the circulation are different between the sexes. More specifically, our data imply that the NO-sGC-cGMP pathway in platelets is impaired in females compared to males, resulting in a reduction of the antiplatelet effect of nitrite-derived NO in females. This difference in activity was not due to differences in the capacity to reduce nitrite to NO since the nitrite reductase activity of erythrocytes assessed ex vivo was identical between the sexes under varying pH conditions and nitrite concentrations. We suggest that the differences in bioactivity relate to a reduced sGC activity in females compared to males. Such a proposal is supported by our data demonstrating a reduced sensitivity of female blood to NO donors as well as an absence of effect of both dietary nitrate and in vitro nitrite administration on platelet function in women. Accordingly, a number of recent preclinical studies indicate a greater role for the NO-sGC-cGMP pathway in vascular reactivity in males compared to females. Indeed, while male sGCα1 knockout mice are hypertensive, female knockouts are not [9]. In addition, our own recent investigations suggest that while sGC activators cause potent vasodilatation and decreases in BP in male mice, the activity is much reduced in females, an effect reflecting reduced sGC expression [10]. Our observations shown herein suggest that this phenomenon translates to humans, although further molecular investigations are required to confirm this possibility.
Conclusions
Thus, inorganic nitrate ingestion, whether taken via the diet or through supplementation, causes a modest decrease in platelet reactivity in healthy males but not females. This effect in males is associated with elevations of circulating nitrite resulting in NO-induced elevations of platelet cGMP, an effect that is absent in females and likely underlying the lack of responsiveness in terms of platelet activity in females. The effects of inorganic nitrate on platelet activity may offer potential in the therapeutics of cardiovascular disease in secondary prevention, as an adjunct to current antiplatelet therapies to prevent atherothrombotic complications. However, it is possible that the greatest potential of dietary nitrate may lie in primary prevention. The modest antiplatelet activity of such a dietary intervention may provide a superior option to aspirin possibly via providing an important but less dramatic antiplatelet effect versus aspirin, in addition to a reduced bleeding complications profile. We suggest that our studies support the case for large-scale and long-term clinical studies assessing the therapeutic potential of a dietary nitrate intervention on platelet reactivity in patients at risk of atherothrombotic events.
Acknowledgments
This work and S.V. are supported by a British Heart Foundation Clinical Fellowship. V.K. was supported by a British Heart Foundation Special Project Grant and R.S.K. by a British Heart Foundation Project Grant. S.M.G. is supported by an MRC MRes/PhD studentship. A.A. is a director of Heartbeet Ltd.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.The International HapMap Project Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 2.Aspirin for the prevention of cardiovascular disease: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong P.C., Dhanji A.R., Truss N.J., Zain Z.N., Tucker A.T., Mitchell J.A., Warner T.D. Utility of 96-well plate aggregometry and measurement of thrombi adhesion to determine aspirin and clopidogrel effectiveness. Thromb. Haemost. 2009;102:772–778. doi: 10.1160/TH09-04-0215. [DOI] [PubMed] [Google Scholar]
- 4.Arora S., Tyagi Y.K., Kumar A., Majumder S., Saluja D., Raj H.G., Chatterjee S., Saso L., Prasad A.K., Parmar V.S. The role of calreticulin transacetylase in the activation of human platelet nitrite reductase by polyphenolic acetates. Biol. Pharm. Bull. 2009;32:161–165. doi: 10.1248/bpb.32.161. [DOI] [PubMed] [Google Scholar]
- 5.Bahra M., Kapil V., Pearl V., Ghosh S., Ahluwalia A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide. 2012;26:197–202. doi: 10.1016/j.niox.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baigent C., Blackwell L., Collins R., Emberson J., Godwin J., Peto R., Buring J., Hennekens C., Kearney P., Meade T., Patrono C., Roncaglioni M.C., Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevan S., Hothi S., Hughes G., James I.F., rang H.P., Shah K., Walpole C.S., Yeats J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broekman M.J., Eiroa A.M., Marcus A.J. Inhibition of human platelet reactivity by endothelium-derived relaxing factor from human umbilical vein endothelial cells in suspension: blockade of aggregation and secretion by an aspirin-insensitive mechanism. Blood. 1991;78:1033–1040. [PubMed] [Google Scholar]
- 9.Buys E.S., Sips P., Vermeersch P., Raher M.J., Rogge E., Ichinose F., Dewerchin M., Bloch K.D., Janssens S., Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCα1 knockout mice. Cardiovasc. Res. 2008;79:179–186. doi: 10.1093/cvr/cvn068. [DOI] [PubMed] [Google Scholar]
- 10.Chan M.V., Bubb K.J., Noyce A., Villar I.C., Duchene J., Hobbs A.J., Scotland R.S., Ahluwalia A. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br. J. Pharmacol. 2012;167:805–817. doi: 10.1111/j.1476-5381.2012.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., III, Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 12.Dangel O., Mergia E., Karlisch K., Groneberg D., Koesling D., Friebe A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J. Thromb. Haemost. 2010;8:1343–1352. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 13.De Berardis G. ASsociation of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro L.J., Fukuto J.M., Griscavage J.M., Rogers N.E., Byrns R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C., Barrett N., Moraes L., Gibbins J., Jackson D. Endogenous inhibitory mechanisms and the regulation of platelet function. In: Gibbins J.M., Mahaut-Smith M.P., editors. Platelets and megakaryocytes. Springer; New York: 2012. pp. 341–366. [DOI] [PubMed] [Google Scholar]
- 16.Joshipura K.J., Ascherio A., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Hennekens C.H., Spiegelman D., Willett W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 17.Joshipura K.J., Hu F.B., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Colditz G., Ascherio A., Rosner B., Spiegelman D., Willett W.C. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kapil V., Haydar S.M., Pearl V., Lundberg J.O., Weitzberg E., Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapil V., Milsom A.B., Okorie M., Maleki-Toyserkani S., Akram F., Rehman F., Arghandawi S., Pearl V., Benjamin N., Loukogeorgakis S., MacAllister R., Hobbs A.J., Webb A.J., Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 20.Kapil V., Webb A.J., Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96:1703–1709. doi: 10.1136/hrt.2009.180372. [DOI] [PubMed] [Google Scholar]
- 21.Keeley E.C., Boura J.A., Grines C.L. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet. 2006;367:579–588. doi: 10.1016/S0140-6736(06)68148-8. [DOI] [PubMed] [Google Scholar]
- 22.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 23.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Laustiola K.E., Vuorinen P., Porsti I., Metsa-Ketela T., Manninen V., Vapaatalo H. Exogenous GTP enhances the effects of sodium nitrite on cyclic GMP accumulation, vascular smooth muscle relaxation and platelet aggregation. Pharmacol. Toxicol. 1991;68:60–63. doi: 10.1111/j.1600-0773.1991.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Delaney M.K., O'Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 27.Marcondes S., Cardoso M.H.M., Morganti R.P., Thomazzi S.M., Lilla S., Murad F., De Nucci G., Antunes E. Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: A role for α-actinin nitration. Proc. Natl. Acad. Sci. USA. 2006;103:3434–3439. doi: 10.1073/pnas.0509397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marjanovic J.A., Li Z., Stojanovic A., Du X. Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation. J. Biol. Chem. 2005;280:37430–37438. doi: 10.1074/jbc.M506518200. [DOI] [PubMed] [Google Scholar]
- 29.Michelson A.D., Benoit S.E., Furman M.I., Breckwoldt W.L., Rohrer M.J., Barnard M.R., Loscalzo J. Effects of nitric oxide/EDRF on platelet surface glycoproteins. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H1640–H1648. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 30.Moro M.A., Russell R.J., Cellek S., Lizasoain I., Su Y., Darley-Usmar V.M., Radomski M.W., Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell C.N., Matsushita K., Chiles K., Scharpf R.B., Yamakuchi M., Mason R.J.A., Bergmeier W., Mankowski J.L., Baldwin W.M., Faraday N., Lowenstein C.J. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawloski J.R., Swaminathan R.V., Stamler J.S. Cell-free and erythrocytic S-nitrosohemoglobin inhibits human platelet aggregation. Circulation. 1998;97:263–267. doi: 10.1161/01.cir.97.3.263. [DOI] [PubMed] [Google Scholar]
- 33.Pearson T.A., Blair S.N., Daniels S.R., Eckel R.H., Fair J.M., Fortmann S.P., Franklin B.A., Goldstein L.B., Greenland P., Grundy S.M., Hong Y., Houston Miller N., Lauer R.M., Ockene I.S., Sacco R.L., Sallis J.F., Smith S.C., Stone N.J., Taubert K.A. AHA Guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 34.British Cardiac Society JBS 2 Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91:v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radomski M.W., Palmer R.M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radomski M.W., Palmer R.M.J., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;330:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 37.Radomski M.W., Palmer R.M.J., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 38.Richardson G., Hicks S.L., O'Byrne S., Frost M.T., Moore K., Benjamin N., McKnight G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 39.Rukoyatkina N., Walter U., Friebe A., Gambaryan S. Differentiation of cGMP-dependent and -independent nitric oxide effects on platelet apoptosis and reactive oxygen species production using platelets lacking soluble guanylyl cyclase. Thromb. Haemost. 2011;106:922–933. doi: 10.1160/TH11-05-0319. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh M., Salzman E.W., Smith M., Ware J.A. Activation of protein kinase C in platelets by epinephrine and A23187: correlation with fibrinogen binding. Blood. 1989;74:2001–2006. [PubMed] [Google Scholar]
- 41.Sidhu J.S., Cowan D., Tooze J.A., Kaski J.C. Peroxisome proliferator-activated receptor-+¦ agonist rosiglitazone reduces circulating platelet activity in patients without diabetes mellitus who have coronary artery disease. Am. Heart J. 2004;147:1032–1037. doi: 10.1016/j.ahj.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 42.Sogo N., Magid K.S., Shaw C.A., Webb D.J., Megson I.L. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem. Biophys. Res. Commun. 2000;279:412–419. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- 43.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 44.Srihirun S., Sriwantana T., Unchern S., Kittikool D., Noulsri E., Pattanapanyasat K., Fucharoen S., Piknova B., Schechter A.N., Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojanovic A., Marjanovic J.A., Brovkovych V.M., Peng X., Hay N., Skidgel R.A., Du X. A Phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J. Biol. Chem. 2006;281:16333–16339. doi: 10.1074/jbc.M512378200. [DOI] [PubMed] [Google Scholar]
- 46.Tannenbaum S.R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet. Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 47.Task, F. M.; Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, +. e.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; Deaton, C.; Ebrahim, S.; Fisher, M.; Germano, G.; Hobbs, R.; Hoes, A.; Karadeniz, S.; Mezzani, A.; Prescott, E.; Ryden, L.; Scherer, M.; Syv+ñnne, M.; Scholte Op Reimer, W. J.M.; Vrints, C.; Wood, D.; Zamorano, J.L.; Zannad, F.; Other experts who contributed to parts of the guidelines:; Cooney, M. T.; ESC Committee for Practice Guidelines (CPG); Bax, J.; Baumgartner, H.; Ceconi, C.; Dean, V.; Deaton, C.; Fagard, R.; Funck-Brentano, C.; Hasdai, D.; Hoes, A.; Kirchhof, P.; Knuuti, J.; Kolh, P.; McDonagh, T.; Moulin, C.; Popescu, B.A.; Reiner, +. e.; Sechtem, U.; Sirnes, P.A.; Tendera, M.; Torbicki, A.; Vahanian, A.; Windecker, S.; Document Review; Funck-Brentano, C.; Sirnes, P.A.; Aboyans, V.; Ezquerra, E.A.; Baigent, C.; Brotons, C.; Burell, G.; Ceriello, A.; De Sutter, J.; Deckers, J.; Del Prato, S.; Diener, H.C.; Fitzsimons, D.; Fras, Z.; Hambrecht, R.; Jankowski, P.; Keil, U.; Kirby, M.; Larsen, M.L.; Mancia, G.; Manolis, A.J.; McMurray, J.; Paj-àk, A.; Parkhomenko, A.; Rallidis, L.; Rigo, F.; Rocha, E.; Ruilope, L.M.; van der Velde, E.; Vanuzzo, D.; Viigimaa, M.; Volpe, M.; Wiklund, O.; Wolpert, C.European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J.33:1635-1701; 2012. [DOI] [PubMed]
- 48.Wald N.J., Law M.R. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326 doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wardlaw J.M., Murray V., Berge E., del Zoppo G., Sandercock P., Lindley R.L., Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 1923;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb A.J., Ahluwalia A. Mechanisms of nitrite reduction in ischemia in the cardiovascular system. In: Ignarro L.J., editor. Nitric oxide: biology and pathobiology. Elsevier; Los Angeles: 2010. pp. 555–586. [Google Scholar]
- 51.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M., Perrett D., Raimondo C., Khoshbin E., Ahmed Z., Uppal R., Benjamin N., Hobbs A.J., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;103:957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S., Rashid R., Miall P., Deanfield J., Benjamin N., MacAllister R., Hobbs A.J., Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams B., Poulter N.R., Brown M.J., Davis M., McInnes G.T., Potter J.F., Sever P.S., Thom S.M. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiviott S.D., Braunwald E., McCabe C.H., Montalescot G., Ruzyllo W., Gottlieb S., Neumann F.J., Ardissino D., De Servi S., Murphy S.A., Riesmeyer J., Weerakkody G., Gibson C.M., Antman E.M. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 55.Zhang G., Xiang B., Dong A., Skoda R.C., Daugherty A., Smyth S.S., Du X., Li Z. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood. 2011;118:3670–3679. doi: 10.1182/blood-2011-03-341107. [DOI] [PMC free article] [PubMed] [Google Scholar]