Abstract
The apical membrane antigen 1 (AMA1), merozoite surface antigen 2 (MSA2), and merozoite surface protein 1 (MSP1) are asexual-stage proteins currently being evaluated for inclusion in a vaccine for Plasmodium falciparum. Accordingly, it is important to understand factors that control antibody responses to these antigens. Antibody levels in plasma from residents of Etoa, Cameroon, between the ages of 5 and 70 years, were determined using recombinant AMA1, MSA2, and the N-terminal region of MSP1 (MSP1-190L). In addition, antibody responses to four variants of the C-terminal region of MSP1 (MSP119) were assessed. Results showed that all individuals produced antibodies to AMA1, MSA2, and MSP1-190L; however, a proportion of individuals never produced antibodies to the MSP119 variants, although the percentage of nonresponders decreased with age. The influence of age and human leukocyte antigen (HLA)-DRB1/DQB1 alleles on antibody levels was evaluated using two-way analysis of variance. Age was correlated with levels of antibodies to AMA1 and MSP119 but not with levels of antibodies to MSA2 and MSP1-190L. No association was found between a single HLA allele and levels of antibodies to MSA2, MSP1-190L, or any of the MSP119 variants. However, individuals positive for DRB1*1201 had higher levels of antibodies to the variant of recombinant AMA1 tested than did individuals of all other HLA types. Since the effect was seen across all age groups, HLA influenced the level but not the rate of antibody acquisition. This association for AMA1, combined with the previously reported association between HLA class II alleles and levels of antibodies to rhoptry-associated protein 1 (RAP1) and RAP2, indicates that HLA influences the levels of antibodies to three of the five vaccine candidate antigens that we have evaluated.
Malaria, caused by Plasmodium falciparum, is a major public health problem in the world, killing greater than 1 million individuals each year (43). The erythrocyte stage of the P. falciparum parasite is the stage that causes clinical disease (7). Immunity to this stage develops following repeated clinical and subclinical infections, suggesting that it may be possible to develop a vaccine for malaria. Several erythrocyte-stage molecules are currently being considered for vaccine development. Among these are the apical membrane antigen 1 (AMA1), the merozoite surface antigen 2 (MSA2), and the merozoite surface protein 1 (MSP1) (35). All of these antigens are expressed on the surface of merozoites (18) and, thus, are accessible to antibody and effector cells. AMA1 is a polymorphic protein that is localized to the apical region in late schizonts and free merozoites (30). Initially, AMA1 is synthesized as an 83-kDa molecule that rapidly undergoes N-terminal processing to a 66-kDa form (19). Around the time of schizont rupture, it is thought that the processed form is transported to the merozoite surface (30). MSA2 is a highly polymorphic merozoite surface protein of 40 to 50 kDa that consists of conserved carboxyl- and amino-terminal regions flanking a central variable region composed of both repetitive and nonrepetitive sequences (37, 42). MSP1 is a polymorphic glycoprotein of approximately 195 kDa that is the major surface antigen of the invasive merozoite stage (18). Posttranslational processing of MSP1 at the time of schizont rupture generates multiple fragments that are displayed on the surface of the mature merozoite (4, 17). One of these proteins is the 19-kDa C-terminal fragment (MSP119). Recombinant protein MSP1-190L, located at the N terminus of MSP1, contains 175 amino acids of blocks 3 and 4 (15).
All three antigens are reported to be targets of parasite invasion-inhibitory or growth-inhibitory antibodies (4, 8, 10, 11, 16, 31, 32, 50). High-titer antibodies to MSA2 and MSP1 have been associated with fewer clinical malaria episodes and lower prevalences of anemia and/or parasite densities (1, 2, 5, 9, 27, 38, 49, 52). Because all three asexual-stage molecules are candidates for vaccine development, it is important to understand the factors that control the antibody response to them. Human leukocyte antigen (HLA) class II alleles are known to influence antibody production (13). In fact, the genes that encode class II alleles were originally identified as immune response genes because of their influence on antibody levels (26). It has been reported that specific HLA-DR and -DQ alleles influence levels of antibodies to rhoptry-associated protein 1 (RAP1) and RAP2 (23). Other investigators have reported an association between an HLA class II allele and the acquisition of antibodies to a B-cell epitope in the ring-erythrocyte-stage antigen (RESA) (38), the subunit vaccine antigen SPf66 (3), and a malaria sporozoite antigen (44). Although field studies showed no influence of HLA on the acquisition of antibodies to the circumsporozoite protein repeat region (6, 14, 39), a strong influence of HLA-DR on responsiveness to circumsporozoite protein was observed in phase I vaccine trials (28).
In the study reported here, we evaluated the influence of HLA-DRB1 and -DQB1 allelic products on the level and rate of acquisition of antibodies to recombinant AMA1 (rAMA1), rMSA2, and rMSP1 (MSP1-190L and four variants of MSP119) using plasma collected in a cross-sectional study of Cameroonian individuals between the ages of 5 and 70 years. Results show that, in addition to the previously reported influence of HLA on levels of antibodies to RAP1 and RAP2 (23), HLA class II allelic products influence the level of antibodies to the variant of rAMA1 tested. No HLA influence was observed for the variant of MSA2 and MSP1-190L tested or for any of the MSP119 variants used in the study.
MATERIALS AND METHODS
Study design.
In 1995, a cross-sectional study was conducted in the rural village of Etoa, Cameroon. Etoa is a village of ∼485 individuals where P. falciparum malaria is holoendemic (36). Malaria transmission is perennial with an estimated 2.4 infectious bites per night during each of the two rainy seasons and 0.4 infectious bites per night during the two dry seasons (36). Previous studies demonstrated that the prevalence of P. falciparum was ∼65% in children 5 to 10 years, 34% in adolescents 11 to 15 years, and 29% in individuals over 15 years of age. Peripheral blood samples were obtained from 200 volunteers representing 146 households. The overall average number of individuals per household in the entire sample was less than 2. The majority of individuals studied (79.6%) were single representatives of 116 different households. Of the remaining volunteers, most (12.2%) came from households represented by two individuals, and those household members who volunteered often were related by marriage only. The age distribution for the total sample was as follows: age 5 to 9 years, n = 31; 10 to 14 years, n = 59; 15 to 29 years, n = 44; 30 to 44 years, n = 23; ≥45 years, n = 43. Children less than 5 years of age were not included in the study design. Plasma samples were assayed for antibodies to rAMA1, MSA2, and MSP1 (MSP1-190L and four MSP119 variants). The project was approved by the Institutional Review Board of Georgetown University and the Ministry of Health, Cameroon.
Determination of HLA alleles.
DNA was isolated from whole blood drawn in EDTA by using the Puregene DNA isolation kit (Gentra Systems Inc., Minneapolis, Minn.). Allele level typing was performed for HLA-DRB1 and -DQB1 (except for DRB1*04 and DQB1*02) with commercially available kits for medium-level and group-specific resolution (Lifecodes Corporation, Stamford, Conn.) according to the Lifecodes protocol. In addition, because of the high occurrence of DR52 group alleles in the Cameroonian population (33), ambiguous combinations of DR52-associated DRB1 alleles were resolved using DR52 group-specific primer sets that employed two reverse primers specific for the Val-Gly polymorphism at amino acid 86 of DRB1 (41). The amplified products were spotted on membranes and probed with the set of DR52 group-specific probes from Lifecodes according to the Lifecodes protocol. The following alleles could be resolved with the sets of primers and probes used: DRB1*0101 to -0104, *1501 to -1506, *1601 to -1608, *03011 to -0309, *04, *0701, *0801 to -0816, *0901, *1001, *1101 to -1126, *1201 to -1205, *1301 to -1315, *1317 to -1327, and *1401 to -1425 and DQB1*02, *0301 to -0305, *0501 to -0504, *0601 to -0608, and *0401 and *0402.
rAMA1, rMSA2, and rMSP1. (i) rAMA1.
The AMA1 protein was derived from the full-length Pf83/AMA1 (including putative signal peptide) of the 7G8 strain (rPF83-7G8-1). The protein was produced in a baculovirus expression system and was purified using high-performance ion-exchange chromatography in which elution conditions were defined by charge and hydrophobicity (29). This recombinant protein was used to induce antibodies that inhibited parasite invasion in vitro, and from these a conformation-dependent monoclonal antibody (MAb) (4G2) was isolated (25).
(ii) rMSA2.
The MSA2 protein used has the near-full-length sequence of the 3D7 parasite clone and a molecular mass of 25 kDa, consisting of 250 amino acids, i.e., 229 of the central domain and 21 of leader and C-terminal sequences [MRGS(H6)GSV and DLQPSLIS, respectively] (40, 47). MSA2 was expressed in Escherichia coli and purified by nickel chelate chromatography with nitrilotriacetic acid resin (Qiagen, Inc., Valencia, Calif.) followed by reverse-phase and ion-exchange chromatography (40, 46).
(iii) rMSP1.
Two regions of the MSP1 protein were used, four variants of the 19-kDa C-terminal MSP119 fragment and the N-terminal MSP1-190L fragment. The four MSP119 variants (Q-KNG [FVO-Q], E-KNG [FVO-E], Q-TSR [3D7-Q], and E-TSR [3D7-E]) were expressed in Saccharomyces cerevisiae and purified as described previously (24). MSP1-190L consists of a 175-amino-acid fragment of blocks 3 and 4 derived from the K1 line, fused to the CST3 T-cell epitope from the P. falciparum circumsporozoite protein (15). The MSP1-190L protein was expressed in E. coli and purified by nickel chelate chromatography with nitrilotriacetic acid resin (Qiagen) followed by ion-exchange chromatography (40).
ELISA.
Plasma samples were tested by enzyme-linked immunosorbent assay (ELISA) for the presence of immunoglobulin G (IgG) antibodies to AMA1, MSA2, MSP1-190L, and MSP119 variants according to previously described procedures (20, 24, 34, 45, 51). ELISAs were performed at three different points in time, and the number of serum samples available for testing differed slightly (AMA1, n = 188; MSA2 and MSP1-190L, n = 136; and MSP119 variants, n = 187). Positive and negative controls were included on every plate. For negative controls, plasma samples collected from 30 nonexposed American Caucasians (ELISAs with MSA2, MSP1-190L, and MSP119 variants) or from 45 nonexposed European Caucasians (ELISAs with AMA1) with no history of malaria were used. A test sample was considered positive if it had an optical density (OD) greater than twice the standard deviation (SD) of the geometric mean OD of the negative-control samples. The positive-control samples consisted of a pool of plasma collected from adult individuals living in an area of holoendemicity of Ivory Coast or Cameroon; rabbit antisera raised against recombinant proteins (for MSA2 and MSP1); rat MAb 28G2dcl, which recognizes the C-terminal region of AMA1 (51); and murine MAbs to rMSA2 (40), and rMSP1 (8, 20). Results are given without background subtracted. During the optimization and standardization of each ELISA, conditions were extensively tested to control for nonspecific binding and to ensure that the measured human antibody responses were specific for each antigen. Endpoint titrations of immune sera with high and low ODs were performed for each antigen to ensure that the single serum dilution selected for measurement would produce an OD that correlated with antibody titer.
For detection of antibodies to AMA1, high protein-binding microtiter ELISA plates (Greiner, Laborchnik, Solingen, Germany) were coated with recombinant Pf83/AMA1 (75 ng/ml in 50 μl of phosphate-buffered saline [PBS], pH 8.0, plus 0.02% azide) overnight at 4°C in a humidified chamber (51). Plates were blocked with 3% bovine serum albumin (BSA) in PBS. Test and control samples were diluted 1:200 in 0.5% BSA in PBS (pH 8.0), and 100 μl was added to duplicate antigen-coated wells. Alkaline phosphatase-conjugated goat anti-human IgG (heavy plus light chains) (Pierce, Rockford, Ill.) was used as the secondary antibody. Bound antibody was detected by adding 50 μl of enzyme substrate (p-nitrophenyl phosphate; Sigma, St. Louis, Mo.)/well at 1 mg/ml in 1 M diethanolamine-0.5 M MgCl2 (pH 9.8), incubating the plates for 30 min at room temperature in the dark, and then reading them at 405 nm. A serum sample was considered positive if the value was >0.2 OD units (i.e., greater than the mean OD + 2 SDs of the negative-control plasma).
For detection of antibodies to MSA2 and MSP1-190L, microtiter ELISA plates (Immunoplate [Maxisorb]; Nunc) were coated with 50 ng of MSA2 or MSP1-190L/well in 50 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) overnight at 4°C (45). Plates were blocked with 1% BSA in PBS, pH 7.4. Test and control samples were diluted 1:200 in 0.05% Tween 20 and 1% BSA in PBS, and 100 μl was added in triplicate to antigen-coated wells. Peroxidase-conjugated goat anti-human IgG (gamma chain specific) (Bio-Rad Laboratories, Hercules, Calif.) was used as a secondary antibody. Bound antibody was detected with the enzyme substrate o-phenylenediamine dihydrochloride (Pharmacia/Amersham, Piscataway, N.J.) and read at 450 nm. A serum sample was considered positive if the value was >0.2 OD units (i.e., greater than the mean OD + 2 SDs of the negative-control plasma).
For detection of antibodies to the four MSP119 variants, microtiter ELISA plates (Immunoplate [Maxisorb]) were coated with 50 ng of MSP119 antigen/well for each of the four alleles in 100 μl of coating buffer overnight at 4°C (20). Plates were blocked with 1% BSA in PBS, pH 7.4. Test and control samples were diluted 1:100 in 1% BSA-0.5% normal yeast extract (Difco)-0.05% Tween 20 in PBS and added to duplicate antigen-coated wells. The secondary antibody was alkaline phosphatase-conjugated goat anti-human IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Bound antibody was detected by adding 50 μl of enzyme substrate (p-nitrophenyl phosphate; Sigma)/well at 1 mg/ml in 1 M diethanolamine-0.5 M MgCl2 (pH 9.8), incubating the plates for 30 min at room temperature in the dark, and then reading them at 405 nm. A serum sample was considered positive if the value was >0.4 OD units (i.e., greater than the mean OD + 2 SDs of the negative-control plasma).
Detection of sickle cell trait.
Hemoglobin polymorphisms were determined by electrophoresis with a commercially available kit (Helena Laboratories, Beaumont, Tex.).
Statistical methods.
The statistical analyses were done using the SAS (SAS Institute Inc., Cary, N.C.) package. First, analyses were done to determine whether age or presence of the sickle cell allele accounted for a significant proportion of the variability in antibody level, i.e., Spearman correlation coefficients between OD to a recombinant antigen and age were calculated as a preliminary analysis. Analysis of variance was done to determine whether the presence of the sickle cell allele (AS) was associated with a change in mean OD to each of the recombinant malaria antigens. Any variable(s) showing a significant association with a change in mean OD was included in the analysis of the OD association with HLA. For those recombinant antigens for which the OD value showed a significant correlation with age, the linear relationship was investigated and determined not to be linear. The variability in OD associated with age was best accounted for by dichotomizing with respect to individuals under age 15 versus those age 15 or greater, with the exception of RAP2. In the case of RAP2, the difference in OD was observed between those less than age 30 and those age 30 or greater.
Multiway (“multiallele”) and two-way (“single-allele”) analyses of variance were used to determine the effect of HLA on antibody levels. In the multiallele analyses, age group (<15 years versus ≥15 years) was one factor and the HLA alleles at a given locus were the other factors. Only those HLA-DRB1 and -DQB1 alleles that were present in at least 20 individuals in the total sample of Cameroonians were considered. As a result, seven DRB1 alleles and five DQB1 alleles were included in the analysis. These alleles are referred to hereafter as “frequent” alleles. To test for a locus effect, the multiallele analysis of variance used all the frequent alleles at a given locus as the factors. In the single-allele analyses, age group was one factor and a single HLA allele was the second factor. In each case, the dependent variable was the antibody level (i.e., OD value), a quantitative value.
To rule out any HLA association possibly due to common household environmental conditions or genetics, associations were reanalyzed using a subsample that consisted of only one individual from each of the 146 distinct households. From the 30 households with more than one member in the study, an individual who was less than 15 years of age and/or was positive for the HLA allele of interest (i.e., the allele associated with high mean OD for the recombinant antigen of interest) was chosen. Data were tested by nonparametric analysis (i.e., Wilcoxon rank sum test comparing age-adjusted values of those individuals positive for the HLA allele with those negative for the allele).
In the first pass, the effect of variance in OD due to frequent HLA alleles at each locus was evaluated. Thus, the general hypothesis of partial association between mean OD and presence of the frequent HLA alleles was tested for each locus separately. The F test of no partial effect of any of the seven frequent DRB1 alleles on mean OD had df = 7 and df = 179 [i.e., 188 (n) − 1 − 1 (for age group) − 7 alleles]. Similarly, the F test of no partial effect of presence of any of the five frequent DQB1 alleles on mean OD had df = 5 and df = 181. If a locus showed a significant finding at the 0.10 level, backward elimination of nonsignificant HLA alleles was performed to determine those alleles that were significantly associated with the increased antibody level.
Analyses also were performed that allowed for interaction between age and HLA allele effects. Only results that were consistently significant at the 0.01 level in the initial screen considering all age-antigen-antibody combinations and that held up at the 0.05 level in a corresponding analysis of age group-adjusted values were considered noteworthy.
A Bayesian analysis was performed to determine the optimal subset of predictors of antibody level for those malaria antigens shown in the “frequentist” analyses, described above, to be associated with an HLA allele, i.e., AMA1. The method involved an extension of a method for choosing the optimal subset of predictors from a set of p candidate independent predictors proposed by George and McCullough (12). These authors proposed that the presence of a variable j in the model is a Bernoulli variable, γj, and that the prior distribution of the coefficients, βj, each in turn depends on γj. Markov chain Monte Carlo sampling methods were used to determine the posterior distribution of the 2p values of the vector (px1) of the γj and to choose, as the final model, that model for which the posterior probability is largest. Suh et al. (48) extended this approach to the problem of choosing a combination of m markers from a larger set of n candidate markers in a linkage analysis of a complex disease. Here, the George and McCullough (12) method is extended to the analysis of a locus with m candidate alleles plus one factor (age) to determine the combination of frequent HLA alleles and age that has the highest posterior probability. Thus, stochastic search variable selection (SSVS) involved considering a regression model that includes all p candidate predictors, Y = α + ΣβkXk + ɛ, ɛ ∼ N(0, σ2), with prior distribution of the β set as βk | γk ∼ (1 − γk) N(0, τ2) + γk N(0, c2 τ2). The prior for the γk is identically Bernoulli with parameter P = 1/2, and we consider σβk/τ, c) = (1, 5), (1, 10), (10, 100), and (10, 500) where σβk denotes the standard error (SE) of βk. For the Etoa data, we applied SSVS to AMA1 by setting Y = AMA1 and considering P = 8 predictors of Xi (i = 1, 2,…7) and X8 = Xage.
RESULTS
HLA-DRB1 and -DQB1 allele and haplotype frequencies in Etoa.
HLA-DRB1 and -DQB1 typing was performed on DNA samples from the total sample of 200 Cameroonians. For the purpose of analysis with antibody levels, the number of individuals positive for each allele was determined. Both the number of individuals positive for the HLA-DRB1 and -DQB1 alleles and the relative frequency of each allele are given in Table 1. Only those alleles present in at least 20 individuals (i.e., frequent alleles) were considered in the subsequent analyses of the effect of HLA on antibody level. Of the ∼130 DRB1 alleles assessed, only 17 were detected. Seven DRB1 alleles, in the order of frequency, were present in at least 20 individuals, i.e., DRB1*1503, *1301, *0102, *03011, *0701, *1101, and *1201. HLA-DRB1*08041, *1102, and *1302 were present at intermediate frequencies (7 to 9%). All other DRB1 alleles, DRB1*0101, *03021, *0901, *1001, *1202, *1303, and *1401, were observed at frequencies of ≤2%. Of the 20 DQB1 alleles assessed, 13 were detected (Table 1). The five DQB1 alleles present in at least 20 individuals, in order of frequency, were DQB1*0602, *02, *0501, *03032, and *0301. DQB1*0605 was present at an intermediate frequency (7%), while all other DQB1 alleles were detected at frequencies of ≤3.5%.
TABLE 1.
HLA-DR/DQ allele frequencies in individuals living in Etoaa
| Allele (2n = 400) | No. of individuals with allele | Relative frequency (no. of individuals/200) |
|---|---|---|
| DRB1b | ||
| *0101 | 2 | 0.01 |
| *0102 | 22 | 0.11 |
| *03011 | 36 | 0.18 |
| *03021 | 3 | 0.015 |
| *0701 | 26 | 0.13 |
| *08041 | 15 | 0.075 |
| *0901 | 3 | 0.015 |
| *1001 | 4 | 0.02 |
| *1101 | 25 | 0.125 |
| *1102 | 13 | 0.065 |
| *1201 | 25 | 0.125 |
| *1202 | 1 | 0.005 |
| *1301 | 68 | 0.34 |
| *1302 | 18 | 0.09 |
| *1303 | 1 | 0.005 |
| *1401 | 2 | 0.01 |
| *1503 | 109 | 0.545 |
| DQB1c | ||
| *02xx | 67 | 0.335 |
| *0301 | 28 | 0.14 |
| *03032 | 49 | 0.245 |
| *0401 | 1 | 0.005 |
| *0402 | 3 | 0.015 |
| *0501 | 56 | 0.28 |
| *0502 | 1 | 0.005 |
| *05031 | 1 | 0.005 |
| *0602 | 128 | 0.64 |
| *0603 | 1 | 0.005 |
| *0604 | 7 | 0.035 |
| *0605 | 14 | 0.07 |
| *0608 | 2 | 0.01 |
Sample represents total number of individuals in sample (n = 200).
DRB: 27 homozygotes, 173 heterozygotes.
DQB: 42 homozygotes, 158 heterozygotes.
HLA-DR/DQ haplotypes were assigned based on HLA-DR and -DQ haplotypes determined in a previous Cameroonian population study that employed a standard maximum likelihood estimation procedure (33). The previous population was composed, in part, of the same ethnic group as the residents of Etoa. The frequently determined haplotypes present in Etoa, in the order of frequency, are listed in Table 2. These were used for antibody level association analyses.
TABLE 2.
Association of frequent HLA-DR/DQ haplotypes in Etoa with antibody levels
| HLA-DR/DQ haplotypea | Result for antigen(s)
|
|||
|---|---|---|---|---|
| AMA1 | RAP1 | RAP2 | MSA2, MSP1-190L, and MSP119 | |
| DRB1*1503, DQB1*0602 | NSb | NS | NS | NS |
| DRB1*1301, DQB1*03032 | NS | NS | NS | NS |
| DRB1*03011, DQB1*02 | NS | NS | 0.11, SE = 0.048, P = 0.020c | NS |
| DRB1*0701, DQB1*02 | NS | NS | NS | NS |
| DRB1*0102, DQB1*0501 | NS | NS | NS | NS |
| DRB1*1201, DQB1*0501 | 0.17, SE = 0.065, P = 0.009c | NS | NS | NS |
| DRB1*1101, DQB1*0602 | NS | NS | NS | NS |
Haplotypes are listed in order of frequency.
NS, nonsignificant (estimated coefficient <0.05, P > 0.15).
Estimated difference in mean OD value between individuals with the indicated haplotype and those without the haplotype, followed by the estimated SE of difference.
Production of antibodies to AMA1, MSA2, and MSP1-190L.
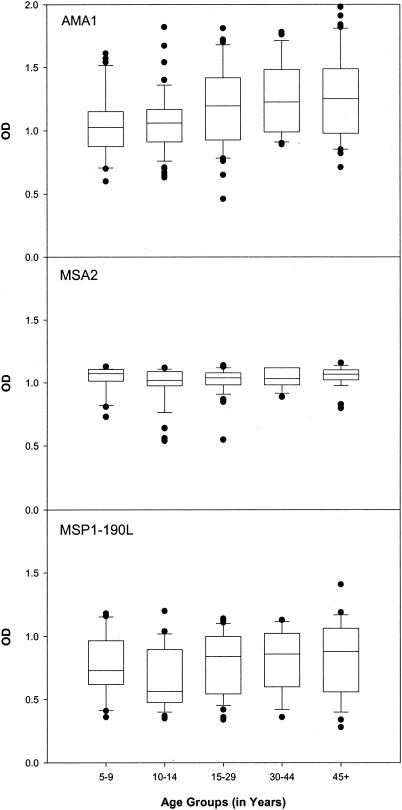
Because of the high rate of malaria transmission in Etoa, children acquire antibodies to many asexual-stage antigens by the time they reach 5 years of age (36). Thus, we determined if the proportion of individuals with antibodies (i.e., percent responders) to AMA1, MSA2, and MSP1-190L increased with age. Results showed that all individuals evaluated had antibodies to AMA1, MSA2, and MSP190L by the age of 5 years and beyond (Fig. 1). That is, all plasma samples had OD values above the cutoff for positivity, which was OD = 0.20. Thus, there was no evidence of major histocompatibility complex (MHC) restriction to these antigens in this population.
FIG. 1.
Relative levels of antibodies to AMA1, MSA2, and MSP1-190L, in each of five age groups (5 to 9, 10 to 14, 15 to 29, 30 to 44, and ≥45 years). Plasma samples from individuals residing in Etoa were tested by ELISA at a 1:200 dilution. Outlying values are shown as individual dots. Values above OD = 0.2 were considered antibody positive (i.e., greater than the mean + 2 SDs of the negative-control plasma sample). The OD values for each malarial antigen are displayed in box plots, with the median values represented by the horizontal line within each box. The boxed regions represent the interquartile ranges, while the upper and lower vertical bars represent the 90th and 10th percentiles, respectively.
Next, the effect of age on antibody level (i.e., OD) was evaluated. Results of Spearman nonparametric correlation analyses showed a correlation between age and OD level for AMA1 but not for MSA2 or MSP1-190L. To determine if the age effect was present across all age groups, median antibody levels were compared among five age groups (ages 5 to 9, 10 to 14, 15 to 29, 30 to 44, and ≥45 years) formed so as to have approximately 20% of the sample in each group. Data are shown in Fig. 1. As expected, median levels of antibodies to MSA2 and MSP1-190L did not change with increasing age (P > 0.1). For AMA1, median antibody levels for age groups 5 to 9 and 10 to 14 years (i.e., <15 years) differed significantly from median antibody levels for age groups 15 to 29, 30 to 44, and ≥45 years (i.e., 15 years and older) (P < 0.05). When the median level of antibodies to AMA1 was compared between children <15 years and individuals 15 years and older, a significant difference was detected (P < 0.03). Thus, levels of antibodies to AMA1 did not reach maximal levels until age 15 years, but thereafter they did not change.
Production of antibodies to four MSP119 variants.
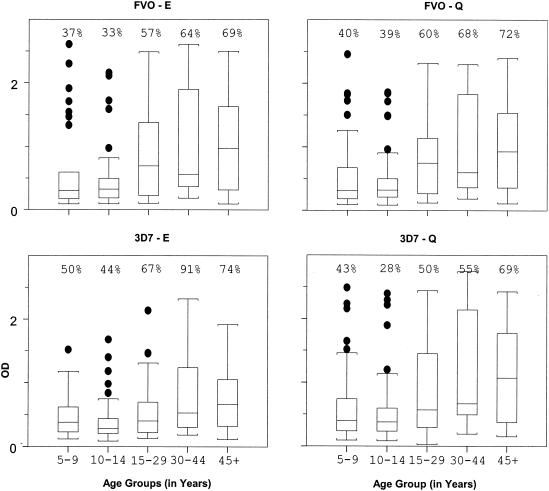
In contrast to the above recombinant antigens, not all individuals produced antibodies to MSP119 C-terminal variants (i.e., a proportion of the population appears to be nonresponders, defined by an OD of ≤0.4) (Fig. 2). The percentage of responders increased with age but never reached greater than 90%. For example, 37% of children aged 5 to 9 years, 33% aged 10 to 14 years, 57% aged 15 to 29 years, 64% aged 30 to 44 years, and 69% 45 years or older had antibodies to the MSP119 variant FVO-E (Fig. 2). The percentage of responders in different age groups was similar for FVO-Q, 3D7-E, and 3D7-Q. The median level of antibodies to each of the four MSP119 variants also increased with age (Fig. 2). Comparison of antibody levels showed that the median OD for age groups less than 15 years differed significantly from those for age groups of 15 years and older (P < 0.001). This implies that levels of antibodies to each of the four MSP119 variants, at least, did not reach maximum until age 15 years, similar to the level of antibodies to AMA1.
FIG. 2.
Relative levels of antibodies to four variants of the C-terminal fragment, MSP119, of the asexual-stage antigen MSP1 (variants FVO-E, FVO-Q, 3D7-E, and 3D7-Q), in each of five age groups (5 to 9, 10 to 14, 15 to 29, 30 to 44, and ≥45 years). Plasma samples from individuals residing in Etoa were tested by ELISA at a 1:100 dilution. Values above OD = 0.4 were considered antibody positive (i.e., greater than the mean + 2 SDs of the negative-control plasma sample). Values above each age group indicate percent responders. The OD values for each malarial antigen are displayed in box plots, with the median values represented by the horizontal line within each box. The boxed regions represent the interquartile ranges, while the upper and lower vertical bars represent the 90th and 10th percentiles, respectively.
Correlation analyses were performed among the antibody responses to the four naturally occurring MSP119 variants tested in the population of endemicity of Etoa. Correlations among the responses to all four variants were highly significant (r = 0.85 to 0.98; P < 0.0001) (Table 3). The highest correlation was between responses to FVO-E and FVO-Q (r = 0.98). The correlation was slightly lower between responses to 3D7 variants, 3D7-E and 3D7-Q (r = 0.90). In fact, the response to 3D7-E showed a higher correlation with those to FVO-E and FVO-Q than with that to 3D7-Q (r = 0.94), the variant with which it shares the greatest amino acid sequence homology. Although antibody levels were also highly correlated between MSP1-190L and the MSP119 variants (P < 0.0001), the correlation coefficient between MSP1-190L (N-terminal blocks 3 and 4) and each of the MSP119 variants (C-terminal block) (r = 0.42 to 0.46) was lower than those among the variants (r = 0.85 to 0.98).
TABLE 3.
Correlation among antibody responses to different antigens
| Antigen | Pearson correlation coefficienta
|
||||||
|---|---|---|---|---|---|---|---|
| MSP119
|
MSP1-190L | MSA2 | AMA1 | ||||
| FVO-E | FVO-Q | 3D7-E | 3D7-Q | ||||
| FVO-E | 0.98* | 0.94* | 0.85* | 0.42* | 0.34* | 0.25*** | |
| FVO-Q | 0.94* | 0.87* | 0.45* | 0.36* | 0.25*** | ||
| 3D7-E | 0.90* | 0.46* | 0.31** | 0.26*** | |||
| 3D7-Q | 0.46* | 0.31** | 0.22*** | ||||
| MSP1-190L | 0.44* | 0.25*** | |||||
| MSA2 | 0.24*** | ||||||
| AMA1 | |||||||
*, P < 0.0001; **, P < 0.001 > 0.0001; ***, P < 0.01 > 0.001.
Hemoglobin S does not influence levels of antibodies to AMA1, MSA2, MSP1-190L, or MSP119.
Since the sickle cell trait (AS) provides a protective effect against malaria, the influence of sickle (AS) versus normal hemoglobin (AA) on the level of antibodies to AMA1, MSA2, MSP1-190L, and the four MSP119 variants was evaluated. No significant difference in levels of antibodies to any of the antigens was observed for individuals who were AS heterozygotes compared to those who were AA homozygotes. Therefore, sickle cell trait was not considered in the analyses of the influence of frequent HLA alleles on antibody level.
HLA influences levels of antibodies to AMA1 but not to MSA2, MSP1-190L, or the MSP119 variants.
The influences of HLA-DRB1 and -DQB1 alleles and haplotypes on levels of antibodies to AMA1, MSA2, MSP1-190L, and the MSP119 variants were analyzed. Since everyone in the population produced detectable amounts of antibodies to the three recombinant antigens, AMA1, MSA2, and MSP1-190L, restriction to these antigens did not appear to be a problem. However, since a proportion of the population appeared to be nonresponders to MSP119, restriction could have influenced the nonresponder status.
Multiallele and single-allele regression analyses were used to evaluate if a given frequent HLA-DR or -DQ allele or frequent HLA-DR/DQ haplotype had an influence on antibody levels independent of age. That is, did a single HLA allele or haplotype correlate with higher or lower mean OD values relative to individuals positive for all other HLA alleles or haplotypes? The multiallele regression analyses of seven DRB1 alleles plus age (<15 years versus ≥15 years) resulted in only one new locus-wide partial association (significant at the 0.1 level) between antibody levels and the HLA alleles. This finding was observed for AMA1 with the DRB1 alleles (F [7, 179] = 1.87, P = 0.07). Age (<15 years versus ≥15 years) was a significant factor as well for the AMA1 antibody level (F [1, 179] = 14.8, P < 0.0001).
Results of the multiallele regression analyses with AMA1 OD values as the dependent variable are shown in Table 4. The coefficient estimates, their SEs, and the P value associated with each coefficient are reported. By the use of forced entry of the seven dummy variable values corresponding to each of the seven frequent DRB1 antigens, the only antigen showing a significant partial association with AMA1 antibody levels is DRB1*1201 (P = 0.05). By the use of backward elimination of the nonsignificant alleles, the final model obtained includes an increase of AMA1 OD associated with an age of <15 years (P < 0.0001) and a significant increase in AMA1 OD associated with DRB1*1201 (P < 0.05). Table 4 also reports the coefficient estimates obtained on doing separate, single-allele regressions, their standard errors, and the P value associated with each coefficient. Single-allele analyses of the frequent DRB1 alleles with age also showed a significant association between levels of antibodies to AMA1 and those to DRB1*1201 (single-allele estimated coefficient = 0.177, SE = 0.062, P = 0.005). The estimated coefficient was greater and P value was smaller with single-allele regressions than with multiallele regressions. The estimated coefficient for age equals 0.17, SE = 0.04. This estimated coefficient for age is, coincidentally, the same as that for DRB1*1201 (i.e., estimated coefficient = 0.18 with SE = 0.06).
TABLE 4.
Results of regression analyses of AMA1 antibody level on DRB1 and DQB1 allele and agea
| HLA allele | No. of individuals | Multiallele regression
|
Single-allele regression
|
||
|---|---|---|---|---|---|
| Coefficient (SE) | P | Coefficient (SE) | P | ||
| DRB1*0102 | 20 | −0.030 | 0.68 | −0.002 | 0.95 |
| DRB1*03011 | 35 | −0.020 | 0.73 | 0.035 | 0.50 |
| DRB1*0701 | 25 | −0.124 (0.067) | 0.065 | −0.088 | 0.15 |
| DRB1*1101 | 23 | −0.073 | 0.27 | −0.075 | 0.2 |
| DRB1*1201 | 23 | 0.141 (0.069) | 0.05 | 0.177 (0.062) | 0.005 |
| DRB1*1301 | 66 | −0.068 | 0.17 | −0.048 | 0.24 |
| DRB1*1503 | 103 | −0.032 | 0.53 | −0.025 | 0.55 |
| DQB1*02 | 64 | −0.032 | 0.5 | −0.003 | 0.9 |
| DQB1*0301 | 25 | −0.050 | 0.45 | −0.007 | 0.9 |
| DQB1*03032 | 47 | −0.077 | 0.15 | −0.057 | 0.2 |
| DQB1*0501 | 52 | 0.051 | 0.40 | 0.102 (0.041) | 0.03 |
| DQB1*0602 | 121 | −0.072 | 0.20 | −0.070 | 0.10 |
All correlations were significant at the level of 0.01 or less. SEs were all approximately 0.06. Exact values are given where P is <0.1 for the allele indicated. The multiallele regression model is as follows. For DRB1, E (AMA1) = α + ΣβiXi + β≥15X≥15. Here Xi = 1 if the individual has DRB1 allele i (i = *0102, *03011, *0701, *1101, *1201, *1301, or *1503) and Xi = 0 otherwise and X≥15 = 1 if the individual's age is 15 or over and X≥15 = 0 otherwise. One multiallele regression model is considered. For DQB1, E (AMA1) = α + ΣβiXi + β≥15X≥15 where Xi = 1 if the individual has allele i (i = *02, *0301, *03032, *0501, or *0602) and Xi = 0 otherwise and X≥15 = 1 if the individual's age is 15 years or more and X≥15 = 0 otherwise. One multiallele regression model is considered. The single-allele regression model is as follows. For DRB1, E (AMA1) = α + βiXi + β≥15X≥15 where Xi = 1 if the individual has DRB1 allele i (i = *0102, *03011, *0701, *1101, *1201, *1301, or *1503) and Xi = 0 otherwise and X≥15 = 1 if the individual's age is 15 years or over and X≥15 = 0 otherwise. Seven separate single-allele regression models are considered. For DQB1, E (AMA1) = α + βiXi + β≥15X≥15 where Xi = 1 if the individual has DQB1 allele i (i = *02, *0301, *03032, *0501, or *0602) and Xi = 0 otherwise and X≥15 = 1 if the individual's age is 15 years or more and X≥15 = 0 otherwise. Five separate single-allele regression models are considered.
Similar multiallele and single-allele regression analysis of AMA1 antibody level with age and the presence of the five frequent DQB1 alleles resulted in a nonsignificant locus-wide finding (F [5, 181] = 1.45, P > 0.2). Additionally, none of the DQB1 alleles indicated a significant partial association with AMA1 antibody levels, all having P values of 0.15 or more. However, single-allele analyses of the DQB1 alleles with age resulted in DQB1*0501 showing a significant positive association (estimated coefficient = 0.102, SE = 0.041, P = 0.03) (Table 4).
Single-allele analysis of the DQB1 alleles with RAP1 antibody level resulted in confirmation of the previously reported association between RAP1 and DQB1*03 (23). Detailed results are not given since these analyses are not a replication on a new or additional sample but rather a replication on an enriched sample from the same group as the original study. The multiallele analysis of RAP1 OD with the frequent DQ locus alleles and age showed, as suspected, significance at the 0.1 level, F (4, 130) = 1.76 (0.05 < P < 0.10). The corresponding statistic for RAP1 antibody level with the seven frequent DRB1 alleles indicated no decrease in variance associated with DRB1, F (7, 127) = 0.70 (P > 0.5). However, as before (23), significant single-allele findings are observed for RAP1 and the “supertype” DQB*03 (P = 0.0247) with an estimated increase in OD for RAP1 of 0.077 (SE = 0.035) associated with DQB1*03. The DQB1 supertype includes the alleles DQB1*0301 and *03032. When regressed separately, multiallele regression results showed an increase in mean OD of 0.107 (P = 0.056) with DQB1*0301 and an increase in mean OD of 0.078 (P = 0.074) with DQB1*03032. Results of single-allele analyses of DQB1*0301 and *03032 were also nonsignificant, P = 0.11 and P = 0.16, respectively. An increase in OD of 0.10 (SE = 0.03, P = 0.0003) is estimated to be associated with being aged 15 years or more.
Similarly, the previously reported finding of an association of RAP2 antibody level with DRB1*03011 (23) was confirmed in the single-allele analysis. As for RAP1, detailed results are not given since these analyses are not a replication on a new sample but rather a replication on an enriched sample from the same group as the original study. No findings are significant at the 0.1 level in the locus-wide multiallele analysis of RAP2 for DRB1, F (7, 127) = 0.94, P > 0.5, and for DQB1, F (5, 129) = 1.6, 0.1 < P < 0.25. Results of single-allele analyses showed that an estimated increase in RAP2 mean OD is 0.114 (SE = 0.05, P = 0.0199). Results of analyses with the five frequent DQB1 alleles showed a significant although weak association with DQB1*02. The estimated increase in OD as a result of being DQB1*02 positive is 0.079 (SE = 0.039, P = 0.047). No change in RAP2 OD value is observed in comparing those individuals under age 30 to those who are 30 years of age or more.
The corresponding statistics for tests for MSA2 indicated no decrease in variance associated with the DRB1 locus alleles (F [7, 127] = 0.85, P > 0.5) or with the DQB1 (F [5, 129] = 0.77, P > 0.5). Similarly, strongly nonsignificant findings were observed for MSP1 (i.e., for example, MSP119 variant FVO-E, F [7, 178] = 0.55, P > 0.5 with DRB1, and F [5, 180] = 0.46, P > 0.5 for DQB1 and for MSP1-190L, F [7, 127] = 0.45, P > 0.5 with DRB1 and F [5 < 129] = 0.3, P > 0.5 with DQB1). Results of multiallele and single-allele regression evaluation of frequent HLA-DRB1 and -DQB1 alleles on OD values to the recombinant antigens, MSA2 and MSP1, are not given since all findings were strongly nonsignificant.
Multiallele and single-allele analyses of levels of antibodies to each of the malarial antigens (AMA1, MSA2, and MSP1) with the seven most frequently observed HLA-DR/DQ haplotypes revealed no additional significant associations. Data are summarized in Table 2. The haplotype DRB1*1201/DQB1*0501 was positively associated with an estimated increase in mean OD for AMA1 of 0.17 (SE = 0.065, P = 0.009), and the DRB1*03011, DQB1*02 haplotype was associated with an increase in mean OD for RAP2 of 0.11 (SE = 0.05, P = 0.019). Only one frequent haplotype contained one of the DQB1*03 supertype alleles, DRB1*1301/DQB1*03032. This haplotype showed no significant association with antibody level (estimated coefficient = 0.05, SE = 0.04, P = 0.16).
Bayesian optimal subset analysis does not identify additional HLA associations for AMA1.
The Bayesian optimal subset analyses with the SSVS method were done for AMA1 OD with the seven most frequent DRB1 alleles plus age group to determine the optimal subset of predictors of antibody level. This analysis was done with the objective of obtaining additional rather than confirmatory information on associations between the HLA alleles and antibody level. By using SSVS, DRB1*1201 and age were in the optimal subset with the highest posterior probability under three very different sets of prior distributions for the model parameters.
HLA does not influence the age of acquisition of antibodies to AMA1.
Since children less than 15 years of age have lower levels of antibodies to AMA1 than do individuals over the age of 15 years (Fig. 1), we evaluated whether HLA influenced this difference. Such interactions would occur if an HLA allele had an effect on individuals less than age 15 but not on individuals aged 15 or over or, equivalently, if an age effect was dependent on the HLA allele of the individual. We did two-way analyses of variance that included tests for interaction between age group and each of the frequent DRB1 and DQB1 allele on level of antibodies to any of the recombinant antigens. No interaction was found between any frequent DRB1 or DQB1 allele and age group and level of antibodies to any of the recombinant antigens. These results show that the increase in level of AMA1-specific antibodies observed with age is not related directly to the HLA alleles possessed by the individual. Thus, HLA does not appear to influence the rate of acquisition of antibodies to AMA1. However, with a sample of this size, the number of individuals in each age group was of sufficient power to detect only large differences in rates of acquisition associated with a given HLA allele.
DISCUSSION
Results reported herein indicate that age influences antibody responses to AMA1 and MSP119 and that HLA has a direct influence on production of antibodies to AMA1 from the 7G8 strain. In the Cameroonian village of Etoa, where malaria transmission is intense and perennial, all individuals produced antibodies to AMA1, MSA2, and MSP1-190L by 5 years of age (Fig. 1). Levels of antibodies to MSA2 and MSP1-190L remained constant throughout life, suggesting a maximal response to these antigens very early in life. Levels of antibodies to AMA1 and the MSP119 variants, however, increased until the age of 15 years and then remained constant thereafter (Fig. 1 and 2). Thus, the age at which maximal IgG antibody levels were acquired differed among the antigens, varying from 5 to 15 years of age.
Antibody responses to AMA1, MSA2, and MSP1-190L molecules were not restricted by HLA, since all individuals, even children 5 to 9 years of age, tested positive for antibody. No association with any HLA-DRB1 or -DQB1 allele or HLA-DR/DQ haplotype, positive or negative, was observed for levels of antibodies to MSA2 or MSP1-190L. However, HLA has a moderate but significant effect, regardless of age, on levels of antibodies to the AMA1 polypeptide molecule from the 7G8 strain. Individuals positive for DRB1*1201 have higher levels of antibodies (i.e., mean OD) to AMA1 than do individuals with all other HLA-DRB1 alleles. Since the estimated coefficient for age and AMA1 mean OD is essentially equal to the estimated coefficient for DRB1*1201 and AMA1 mean OD, one can conclude that DRB1*1201-positive individuals who are under age 15 have the same mean AMA1 OD as do DRB1*1201-negative individuals who are age 15 or over. However, those individuals over age 15 and DRB1*1201 positive have an even greater mean level of antibodies to AMA1.
Individuals positive for DQB1*0501 also have higher levels of antibodies to AMA1 than do individuals with all other HLA-DQB1 alleles. A significant association is observed between levels of antibodies to AMA1 and those to DQB1*0501 in the single-allele analysis but not in the multiallele analysis (Table 4). The association is not as strong between mean AMA1 OD and DQB1*0501 (estimated coefficient = 0.102, SE = 0.041, P = 0.03) as observed with DRB1*1201 (estimated coefficient = 0.177, SE = 0.062, P = 0.005). In Cameroon, HLA-DRB1*1201 is observed most frequently (9 of 13) on the same haplotype with DQB1*0501 (32). On the other hand, in Cameroon, DQB1*0501 is frequently present on haplotypes with other DRB1 alleles, i.e., HLA-DRB1*0102/DQB1*0501, DRB1*1101/DQB1*0501, DRB1*1302/DQB1*0501, DRB1*1401/DQB1*0501, and DRB1*1001/DQB1*0501 (32). Thus, the observed increase in levels of antibodies to AMA1 is either with DRB1*1201 or another gene located closer to the DRB1*1201 gene than to the DQB1*0501 gene. Because most DRB1*1201-positive individuals are also DQB1*0501 positive, as expected, a significant association was observed between levels of antibodies to AMA1 and the DRB1*1201/DQB1*0501 haplotype. The estimated coefficient for DRB1*1201 alone (0.177, SE = 0.062) is essentially identical to that for the DRB1*1201/DQB1*0501 haplotype (0.17, SE = 0.065).
The multiallele regression model resulted in higher P values and slightly higher estimates of the SE of the coefficient associated with DRB1*1201 than did the single-allele analysis, in which only DRB1*1201 was considered. This is as expected, in this case, because DRB1*1201 is the only DRB1 allele that is associated with higher AMA1 antibody levels. Inclusion of the six other frequent DRB1 alleles, all of which are not associated with AMA1 antibody levels and whose presence, because they are allelic, is negatively associated with DRB1*1201, would result in an overspecified model. Thus, the multiallele regression model is a less precise model than the simple single-allele model with only DRB1*1201 and age. If more than one HLA allele was associated with an increase in AMA1 antibody level, this would not be the case.
Additional analyses are supportive of DRB1*1201 being the only DRB1 allele associated with higher levels of antibodies to AMA1 in this African population. These analyses included a multiallele regression approach and a Bayesian optimal subset analysis done with SSVS with different priors that considered all subsets (28) of the seven frequent HLA-DRB1 alleles and age. This analysis was done with the objective of obtaining additional rather than confirmatory information on associations between the HLA alleles and antibody level. By the use of the SSVS method, the subset with the highest posterior probability contained no variables other than age and DRB1*1201. Thus, we have several indications that DRB1*1201 and age (<15 years versus ≥15 years) are the two predictors of the higher AMA1 antibody level. Also, we can be reasonably sure that no other HLA association was observed for AMA1 antibody level.
No interactions are observed in this study between age and the HLA alleles for AMA1. However, we point out that this does not mean, necessarily, that the HLA allele effects are all uniform for all ages. It may simply be a result of not having sufficient power to detect any age-dependent HLA effects. However, we might conjecture that any existing interactions between age and HLA allele on OD value are probably quite small.
Since we were not able to collect longitudinal data for the number of clinical malaria episodes in the study reported here, the clinical significance of the higher levels of antibodies to rAMA1 associated with DRB1*1201, if any, is not known. It should be noted that AMA1 is highly polymorphic, and only one variant has been analyzed in this study, i.e., rAMA1 from the 7G8 Brazilian strain. AMA1 sequences of Cameroonian isolates have not been determined. Thus, we do not know how much of the measured ELISA response may be strain specific and how much may be directed against conserved epitopes. In the assay employed, conserved epitopes are seen early during exposure, while with time, more exposure gives a wider repertoire of antibody specificity including strain-specific 7G8 epitopes. Thus, while serum from adults might be expected to react with most, if not all, rAMA1 from other isolates (3D7, D10, and HB3), the magnitude of the OD might vary among AMA1 variants. We cannot conclude, without testing, that the observed effect of HLA-DRB1*1201 on levels of antibodies to rAMA1 from the 7G8 isolate would extrapolate to the antibody response to rAMA1 molecules from other isolates. Unfortunately, serum is not available to test additional isolates to resolve this question.
A few individuals in all age groups tested negative for MSP119-specific antibodies, thus suggesting that restriction might exist for these moieties. No HLA influence was identified, however, for response to MSP119. Also, no HLA influence was identified for levels of antibodies to MSA2. Since our analyses tested only for the effect of individual frequent HLA alleles and haplotypes that were found frequently in the population (i.e., observed in 10% or more of the population), an association could have been missed if antibody levels are associated with one of the low-frequency HLA alleles not analyzed or if a combination of HLA class II alleles influences antibody levels. Another possibility is that the inability to detect an influence of HLA on antibody level might reflect a strain-specific response to the recombinant protein tested. The response to another strain might show an influence. Alternatively, HLA class II alleles may not affect the level of antibody to these molecules. A study of twins in The Gambia supports a lack of influence of HLA on antibody response to MSP1 and MSA2 (22). Although the study reported a strong genetic component for antibody responses to MSP142 (MSP119 is included in MSP142) and MSA2, the genetic influence appeared to be primarily due to non-HLA genes (22).
In an earlier report, high levels of antibodies to RAP1 and RAP2 were associated with an HLA class II allele (23). Because the previously analyzed data set for RAP1 and RAP2 included individuals living in the same household, we have subsequently reanalyzed the data using only one individual per household. The results of the reanalysis confirmed the previously reported associations. Individuals positive for HLA-DQB1*03 have higher levels of antibodies to RAP1 than do individuals of all other HLA types (estimated coefficient = 0.077, SE = 0.03, P = 0.02), and individuals positive for HLA-DRB1*03011 have higher levels of antibodies to RAP2 than do individuals of all other HLA types (estimated coefficient = 0.11, SE = 0.05, P = 0.05). The significant decrease in variance in RAP1 attributable to the DQB1 alleles is observed only on combining the two DQB*03 alleles into a supertype as was done in a previous paper. Therefore, the previously reported results for RAP1 and RAP2 remain solid following additional analyses of a sample that includes the original analyzed subjects plus additional individuals from the same population. Combining the results of the previous and present studies, higher levels of antibodies to three of five asexual-stage antigens studied are associated with an HLA class II allele. In each case, the associated HLA allele is different.
Published studies correlate high levels of antibodies to MSA2 (1, 49), MSP1 (2, 5, 9, 38, 52), RAP1 and RAP2 (21, 45), and RESA (39) with low parasite densities or lower prevalences of anemia and/or fewer clinical episodes of malaria. Definitive conclusions about the role of high levels of antibodies to AMA1 are not available. The extensive polymorphism of HLA alleles is generally accepted to have evolved as a result of selective pressure caused by pathogens with certain HLA types affording more protection against a given pathogen. If the above associations between HLA and higher levels of antibodies to AMA1, RAP1, RAP2, and RESA have clinical relevance, then protection from malarial disease would be associated with several HLA alleles. Thus, not all “immune” individuals would necessarily share the same HLA alleles or haplotypes. This situation would provide the population with some protection against malaria while still allowing population fitness against other pathogens.
Results of this study have several implications for vaccine development. First, no evidence of HLA restriction was found at the population level for the antibody response to any of the five recombinant antigens that we have studied. Thus, HLA alleles commonly found in African populations do not appear to pose a problem for use of these antigens in a vaccine. Second, everyone produced antibody at an early age (5 years and older) to all of the polypeptide antigens. Thus, the polypeptide antigens are broadly immunogenic at the population level. For MSA2 and MSP1-190L, individuals had already acquired the maximal level of antibodies by 5 years of age. Finally, antibody levels continued to increase until 15 years of age for AMA1 and the four MSP119 variants but remained constant thereafter. Although HLA restriction does not appear to be a problem, HLA alleles do influence levels of antibodies to three of the five malarial antigens that we have studied. Individuals with DRB1*1201, DQB1*03, and DRB1*0301 alleles produced higher levels of antibodies to AMA1, RAP1, and RAP2, respectively.
Acknowledgments
This work was supported in part by Public Health Service grant UO1-AI-35839 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health; MH49487 from the National Institute of Mental Health, National Institutes of Health; grant BK21 from the Korea Research Foundation; the STD-3 program of the European Commission contract TS3*0147; the Queensland Institute of Medical Research and University of Queensland, Brisbane, Australia; and a Travel Fellowship Award from the University of Queensland (I.A.Q.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Al-Yaman, F., B. Genton, R. F. Anders, M. Falk, T. Triglia, D. Lewis, J. Hii, H.-P. Beck, and M. P. Alpers. 1994. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am. J. Trop. Med. Hyg. 51:593-602. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yaman, F., B. Genton, K. J. Krammer, S. P. Chang, G. S. Hui, M. Baisor, and M. P. Alpers. 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am. J. Trop. Med. Hyg. 54:443-448. [DOI] [PubMed] [Google Scholar]
- 3.Beck, H.-P., I. Felger, M. Barker, T. Bugawan, B. Genton, N. Alexander, E. Jazwinska, H. Erlich, and M. Alpers. 1995. Evidence of HLA class II association with antibody response against the malaria vaccine SPf66 in a naturally exposed population. Am. J. Trop. Med. Hyg. 53:284-288. [PubMed] [Google Scholar]
- 4.Blackman, J. J., H.-G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite surface during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58:211-219. [DOI] [PubMed] [Google Scholar]
- 6.Brown, A. E., D. Chandanayingyong, S. V. Fuggle, and H. K. Webster. 1989. Immune responses to the circumsporozoite protein of Plasmodium falciparum in relation to HLA-DR type. Tissue Antigens 34:200-204. [DOI] [PubMed] [Google Scholar]
- 7.Bruce-Chwatt, L. J. Essential malariology, p. 35−52. William Heinemann Medical Books, Ltd., London, United Kingdom.
- 8.Chang, S. P., H. L. Gibson, C. T. Lee-Ng, P. J. Barr, and G. S. N. Hui. 1992. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 149:548-555. [PubMed] [Google Scholar]
- 9.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 10.Egan, A. F., P. Burghaus, P. Druilhe, A. A. Holder, and E. M. Riley. 1999. Human antibodies to the 19 kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. [DOI] [PubMed] [Google Scholar]
- 11.Epping, R. J., S. D. Goldstone, L. T. Ingram, J. A. Upcroft, R. Ramasamy, J. A. Cooper, G. R. Bushell, and H. M. Geysen. 1988. An epitope recognized by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol. Biochem. Parasitol. 28:1-10. [DOI] [PubMed] [Google Scholar]
- 12.George, E. I., and R. E. McCullough. 1993. Variable selection via Gibbs sampling. J. Am. Stat. Assoc. 88:881-889. [Google Scholar]
- 13.Germain, R. N. 1999. Antigen processing and presentation, p. 287-340. In W. E. Paul (ed.), Fundamental immunology. Lippincott-Raven, Philadelphia, Pa.
- 14.Graves, P. M., K. Bhatia, T. R. Burkot, M. Prasad, R. A. Wirtz, and P. Beckers. 1989. Association between HLA type and antibody response to malaria sporozoite and gametocyte epitopes is not evident in immune Papua New Guineans. Clin. Exp. Immunol. 78:418-423. [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera, M. A., F. Rosero, S. Herrera, P. Caspers, D. Rotmann, F. Sinigaglia, and U. Certa. 1992. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect. Immun. 60:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holder, A. A., J. S. Sandhu, Y. Hillman, L. S. Davey, S. C. Nicholls, H. Cooper, and M. J. Lockyer. 1987. Processing of the precursor to the major merozoite antigens of Plasmodium falciparum. Parasitology 94:199-208. [DOI] [PubMed] [Google Scholar]
- 18.Holder, A. A. 1994. Proteins on the surface of the malaria parasite and cell invasion. Parasitology 108:S5-S18. [DOI] [PubMed] [Google Scholar]
- 19.Howell, S. A., C. Withers-Martinez, C. H. Kicken, A. W. Thomas, and M. J. Blackman. 2001. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J. Biol. Chem. 276:31311-31320. [DOI] [PubMed] [Google Scholar]
- 20.Hui, G. S. N., A. Hashimoto, and S. P. Chang. 1992. Roles of conserved and allelic regions of the major merozoite surface protein (gp195) in immunity against Plasmodium falciparum. Infect. Immun. 60:1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobsen, P. H., M. M. Lemnge, Y. A. Abu-Zeid, H. A. Msangeni, F. M. Salum, J. I. K. Mhina, J. A. Akida, A. S. Ruta, A. M. Ronn, P. M. H. Heegaard, R. G. Ridley, and I. C. Bygbjerg. 1996. Immunoglobulin G reactivities to rhoptry-associated protein-1 associated with decreased levels of Plasmodium falciparum parasitemia in Tanzanian children. Am. J. Trop. Med. Hyg. 55:642-646. [DOI] [PubMed] [Google Scholar]
- 22.Jepson, A., W. Banya, F. Sisay-Joof, M. Hassan-King, C. Nunes, S. Bennett, and H. Whittle. 1997. Quantification of the relative contribution of major histocompatibility complex (MHC) and non-MHC genes to human immune responses to foreign antigens. Infect. Immun. 65:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, A. H., R. Leke, L. Harun, C. Ginsberg, J. Ngogang, A. Stowers, A. Saul, and I. A. Quakyi. 2000. Interaction of HLA and age on levels of antibody to Plasmodium falciparum rhoptry-associated proteins 1 and 2. Infect. Immun. 68:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaslow, D. C., G. Hui, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP119) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 63:283-289. [DOI] [PubMed] [Google Scholar]
- 25.Kocken, C. H., A. M. van der Wel, M. A. Dubbeld, D. L. Narum, F. M. van de Rijke, G. J. van Gemert, X. van der Linde, L. H. Bannister, C. Janse, A. P. Waters, and A. W. Thomas. 1998. Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization. J. Biol. Chem. 273:15119-15124. [DOI] [PubMed] [Google Scholar]
- 26.McDevitt, H., and M. Sella. 1967. Genetic control of the antibody response. II. Further analysis of the specificity of determinant specific control and genetic analysis of the response to (H, G)-A-L in CBD and C57 mice. J. Exp. Med. 126:969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migot-Nabias, F., A. J. F. Luty, P. Ringwald, M. Vaillant, B. Dubois, A. Renault, R. J. Mayombo, T. N. Minh, N. Fievet, J. R. Mbessi, P. Millet, and P. Deloron. 1999. Immune responses against Plasmodium falciparum asexual blood-stage antigens and disease susceptibility in Gabonese and Cameroonian children. Am. J. Trop. Med. Hyg. 61:488-494. [DOI] [PubMed] [Google Scholar]
- 28.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, Z. R. Castro, R. S. Nussenzweig, B. Schmeckpeper, B. F. Hall, C. Diggs, S. Bodison, and R. Edelman. 2000. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J. Infect. Dis. 182:1486-1496. [DOI] [PubMed] [Google Scholar]
- 29.Narum, D. L., G. W. Welling, and A. W. Thomas. 1993. Ion-exchange-immunoaffinity purification of a recombinant baculovirus Plasmodium falciparum apical membrane antigen, PF83/AMA-1. J. Chromatogr. 657:357-363. [DOI] [PubMed] [Google Scholar]
- 30.Narum, D. L., and A. W. Thomas. 1994. Differential localization of full-length and processed forms of PF83/AMA-1, an apical membrane antigen of Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 67:59-68. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crab. 2001. Antibodies against merozoite surface protein (MSP)-119 are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrin, L. H., E. Ramirez, P. H. Lambert, and P. A. Miescher. 1981. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature 289:301-303. [DOI] [PubMed] [Google Scholar]
- 33.Pimtanothai, N., C. K. Hurley, R. Leke, W. Klitz, and A. H. Johnson. 2001. HLA-DR and -DQ polymorphism in Cameroon. Tissue Antigens 58:1-8. [DOI] [PubMed] [Google Scholar]
- 34.Quakyi, I. A. 1980. The development and validation of an enzyme-linked immunosorbent assay for malaria. Trop. Med. Parasitol. 42:325-333. [PubMed] [Google Scholar]
- 35.Quakyi, I. A., D. W. Taylor, A. H. Johnson, J. B. Allotey, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1992. Development of a malaria T-cell vaccine for blood stage immunity. Scand. J. Immunol. Suppl. 11:9-16. [DOI] [PubMed] [Google Scholar]
- 36.Quakyi, I. A., R. G. Leke, R. Befidi-Mengue, M. Tsafack, D. Bomba-Nkolo, L. Manga, V. Tchinda, E. Njeungue, S. Kouontchou, J. Fogako, P. Nyonglema, L. Harun, R. Djokam, G. Sama, A. Eno, R. Megnekou, S. Metenou, L. Ndountse, A. Same-Ekobo, G. Alake, J. Meli, L. Ngu, F. Tietche, J. Lohoue, J. Mvondo, E. Wansi, R. Leke, A. Folefack, J. Bigoga, C. Bomba-Nkolo, V. Titanji, A. Walker-Abbey, M. A. Hickey, A. H. Johnson, and D. W. Taylor. 2000. The epidemiology of Plasmodium falciparum malaria in two Cameroonian villages: Simbok and Etoa. Am. J. Trop. Med. Hyg. 63:222-230. [PubMed] [Google Scholar]
- 37.Ramasamy, R. 1987. Studies on glycoproteins in the human malaria parasite Plasmodium falciparum. Identification of a myristilated 45 kDa merozoite membrane glycoprotein. Immunol. Cell Biol. 65:419-424. [DOI] [PubMed] [Google Scholar]
- 38.Riley, E. M., S. J. Allen, J. Wheeler, M. J. Blackman, S. Bennett, B. Takacs, H. J. Schonfeld, A. A. Holder, and B. M. Greenwood. 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14:321-337. [DOI] [PubMed] [Google Scholar]
- 39.Riley, E. M., O. Olerup, S. Bennett, P Rowe, S. J. Allen, M. J. Blackman, M. Troye-Blomberg, A. A. Holder, and B. M. Greenwood. 1992. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciparum. Int. Immunol. 4:1055-1063. [DOI] [PubMed] [Google Scholar]
- 40.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. S. Briggs, R. Reber, and D. Sturchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145-3159. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer, A. L., J. A. Falk-Wade, V. Tortorelli, A. Cigan, C. Carter, K. Hassan, and C. K. Hurley. 1992. HLA-DRw52-associated DRB1 alleles: identification using polymerase chain reaction amplified DNA, sequence specific oligonucleotide probes, and a chemiluminescent detection system. Tissue Antigens 39:84-91. [DOI] [PubMed] [Google Scholar]
- 42.Smythe, J. A., R. L. Coppell, G. V. Brown, R. Ramasamy, D. J. Kemp, and R. F. Anders. 1988. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:5195-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snow, R. W., M. Craig, U. Deichmann, and K. Marsh. 1999. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull. W. H. O. 77:624-640. [PMC free article] [PubMed] [Google Scholar]
- 44.Stephens, H. A. F., A. E. Brown, D. Chandanayingyong, H. K. Webster, M. Sirikong, P. Longta, R. Vangseratthana, D. M. Gordon, S. Lekmak, and E. Rungruang. 1995. The presence of the HLA class II allele DPB1*0501 in ethnic Thais correlates with an enhanced vaccine-induced antibody response to a malaria sporozoite antigen. Eur. J. Immunol. 25:3142-3147. [DOI] [PubMed] [Google Scholar]
- 45.Stowers, A., D. Taylor, N. Prescott, Q. Cheng, J. Cooper, and A. Saul. 1997. Assessment of the humoral immune response against Plasmodium falciparum rhoptry-associated proteins 1 and 2. Infect. Immun. 65:2329-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stowers, A., K. J. Spring, and A. Saul. 1995. Preparative scale purification of recombinant proteins to clinical grade by isotachophoresis. Bio/Technology 13:1498-1503. [DOI] [PubMed] [Google Scholar]
- 47.Sturchler, D., R. Berger, C. Rudin, M. Just, A. Saul, C. Rzepczyk, G. Brown, R. Anders, R. Coppel, G. Woodrow, D. Pye, F. Sorenson, D. Gillessen, H. Matile, and R. Reber-Liske. 1995. Safety, immunogenicity, and pilot efficacy of Plasmodium falciparum sporozoite and asexual blood-stage combination vaccine in Swiss adults. Am. J. Trop. Med. Hyg. 53:423-431. [DOI] [PubMed] [Google Scholar]
- 48.Suh, Y. J., S. J. Finch, and N. R. Mendell. 2001. Application of a Bayesian method for optimal subset regression to linkage analysis of Q1 and Q2. Genetic Epidemiol. 21(Suppl. 1):S706-S711. [DOI] [PubMed] [Google Scholar]
- 49.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, A. W., J. A. Jeans, G. H. Mitchell, T. Alderson, and S. Cohen. 1984. The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol. Biochem. Parasitol. 13:187-199. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, A. W., J.-F. Trape, C. Rogier, A. Goncalves, V. E. Rosario, and D. L. Narum. 1994. High prevalence of natural antibodies against Plasmodium falciparum 83-kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am. J. Trop. Med. Hyg. 51:730-740. [DOI] [PubMed] [Google Scholar]
- 52.Tolle, R., K. Fruh, O. Doumbo, O. Koita, M. M'Diaye, A. Fischer, K. Dietz, and H. Bujard. 1993. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect. Immun. 61:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]