Abstract
This study investigated the effects of controlled reinfection on fertility of cattle naturally preexposed to Chlamydophila abortus. All animals had high prechallenge levels of immunoglobulin M (IgM), IgG, IgG1, and IgG2 serum antibodies against ruminant C. abortus in a chemiluminescent enzyme-linked immunosorbent assay. Twenty virgin heifers were estrus synchronized with prostaglandin F2, artificially inseminated 2 to 3 days later, and challenged immediately by intrauterine administration of 0, 104, 105, 106, or 108 inclusion-forming units (IFU) of C. abortus. Ten heifers were estrus synchronized, inseminated, and uterine challenged 2 weeks later. These animals were also indirectly exposed to C. abortus infection (cohort challenged) by contact with their previously challenged cohorts. Pregnancy was determined by rectal palpation 42 days after insemination. All anti-C. abortus antibody isotypes increased in heifers following uterine challenge with 108 IFU. A total of 11, 83, 50, 66, and 0% of heifers were pregnant after uterine challenge with 0, 104, 105, 106, and 108 IFU of C. abortus, respectively. A total of 50 and 65% of heifers were pregnant with and without cohort challenge, respectively. Uterine inoculum dose and cohort challenge (or, alternatively, a negative pregnancy outcome [infertility]) correlated highly significantly with a rise in postchallenge anti-C. abortus IgM levels over prechallenge levels. Logistic regression modeled fertility, with uterine challenge dose and cohort challenge or prechallenge IgM as predictors (P < 0.05). The models predict that the uterine C. abortus inoculum causing infertility is 8.5-fold higher for heifers without cohort exposure and 17-fold higher for heifers with high IgM levels than for heifers with cohort exposure or with low IgM levels.
The intracellular bacterium Chlamydophila abortus is well recognized as the infectious agent of epizootic bovine abortion, and its role in this disease has been reviewed previously (33). Abortion probably follows systemic infection subsequent to inhalation or ingestion of elementary bodies (EB), the infectious form of bacteria of the order Chlamydiales, but may also result from direct inoculation of the reproductive tract with EB during sexual contact. Although cattle may experience clinical disease subsequent to inoculation with C. abortus, the more typical response is a balance between host and parasite that results in chronic inapparent infection (33). Although economic losses caused by epizootic bovine abortion are readily apparent, infection by the routes described above also occurs during breeding and may result in unrecognized economic losses as a result of subclinical infertility (3, 22).
The ruminant abortion species, C. abortus (previously termed Chlamydia psittaci serovar 1, biovar 1, omp1 strain B577) (9), is not host species or tissue specific and is also a leading cause of abortion in sheep (20-22, 33). Human beings are also susceptible to infection with this strain of C. abortus (contracted predominantly during unprotected aid in delivery of aborted lambs) (10, 17, 33). Infected human beings may exhibit flulike symptoms, and pregnant women have a high probability of abortion when clinical signs of disease are apparent (10, 17, 33).
Cattle raised under normal animal husbandry conditions invariably have high-level antibody titers against Chlamydophila spp., presumably due to the ubiquitous presence of infections such as genital infection with the ruminant abortion strain of C. abortus (6, 7, 32, 42). Bovine infertility associated with C. abortus infection has been addressed but has not been extensively characterized (6, 7, 15, 18, 23, 30-34). The role of the C. abortus challenge dose in fertility and the relative importance of the route of inoculation are unknown even though it is recognized that bulls can shed C. abortus in semen, that breeding bulls that shed C. abortus in semen have decreased fertility, that semen spiked with C. abortus is associated with reduced fertility, and that uterine challenge with C. abortus can cause metritis (3, 16, 38, 39).
Cattle typically experience a first infection with C. abortus and other Chlamydophila strains at an early age and respond by establishing immune responses (6, 7). This presents the following interesting question: if cattle with established immunity have the ability to effectively control infection, what are the consequences when these animals eventually and almost invariably become reinfected?
In this study we analyzed (by uterine and cohort challenge) the consequences of reinfection with C. abortus for the fertility of heifers preexposed to this agent. We report that preexposed heifers are fully susceptible to depression of fertility, with fertility being dependent on the uterine and cohort challenge dose and anti-Chlamydophila immune status. The results suggest that subclinical infection with C. abortus may profoundly affect bovine herd health and production.
MATERIALS AND METHODS
Cultivation of Chlamydophila spp.
C. abortus strain B577 (VR-656; American Type Culture Collection, Manassas, Va.) isolated from ovine abortion (37) was grown in cultures in Buffalo green monkey kidney cells and NCI-H292 cells (BioWhittaker, Walkersville, Md.), as described previously (13). Briefly, cells were maintained in minimal essential medium (Life Technologies, Gaithersburg, Md.) containing 5% fetal bovine serum, 25 μg of gentamicin/ml, and 2 μg of amphotericin B/ml. Confluent monolayers were inoculated with C. abortus by centrifugation, and the medium was changed to Iscove's modified Dulbecco's medium (Life Technologies) supplemented with gentamicin, amphotericin B, and 10% fetal bovine serum. At 1 week later, 0.25% glucose was added; released EB were harvested in culture medium 3 to 4 days later. Crudely purified EB were prepared by sonication and centrifugation, as described previously (13). Briefly, cell culture medium containing released EB was sonicated on ice and cell nuclei were removed by centrifugation. The EB in supernatant were sedimented by high-speed centrifugation on a step gradient composed of 50% sucrose and 30% renografin-76 in 30 mM Tris-HCl (Squibb, Princeton, N.J.) (pH 7.3). Sedimented EB were washed twice in sucrose-phosphate-glutamate buffer and frozen at −85°C and served as a concentrated C. abortus stock preparation. Inclusion-forming units (IFU) of the C. abortus stock were determined in shell vial cultures by staining 30-h-infected Buffalo green monkey kidney cells with monoclonal antibody against chlamydial lipopolysaccharide (Bio-Rad, Woodinville, Wash.). Five different doses of C. abortus strain B577 (0, 104, 105, 106, and 108 IFU) were produced by diluting stock C. abortus preparation in sucrose-phosphate-glutamate buffer to a final volume of 400 μl and were placed in 0.5-ml wick and powder polyvinyl chloride embryo transfer straws (Professional Embryo Transfer Supply, Inc., Canton, Tex.). Straws were sealed with polyvinyl powder (Professional Embryo Transfer Supply, Inc.), cooled slowly to −85°C, and stored in liquid nitrogen.
C. abortus serology.
Single serum samples from each animal were collected at the start of the experiments (before heifers were challenged with C. abortus) and again at the conclusion of the experiments. Sera were tested (using a previously reported method that has been modified as a chemiluminescent assay) (12, 18) in duplicates by enzyme-linked immunosorbent assays (ELISA) for antibodies against C. abortus strain B577 EB lysate. Results are reported as relative light units (rlu) per second. The background signal for negative control sera from gnotobiotic calves (50 to 200 rlu/s in all assays) was subtracted from the data, and results were normalized between assay plates by use of linear least-square fit equations derived from positive-control sera.
Holstein heifer model.
Virgin Holstein heifers (n = 32) were fed Bermuda grass hay supplemented twice a day with a grain-based concentrate and housed on Bermuda grass pasture. The age (mean ± standard deviation) of heifers was 15.2 ± 1.16 months. All animal procedures were approved by Auburn University's Institutional Animal Care and Use Committee.
Heifers were observed for standing estrus twice daily over 30-min observation periods. Standing estrus was defined as the condition in which a heifer would stand to permit another heifer to mount. In addition, heifers were fitted with pressure-sensitive radio transmitters (HeatWatch; DDx Inc., Denver, Colo.) applied immediately cranial to the tailhead. Mounting data were continually collected by a radio receiver that downloaded to a desktop computer. Commercial software (HeatWatch; DDx Inc.) was used to analyze mounting data to determine the onset of estrus. Estrus in all heifers (n = 32) was synchronized with a single injection of dinoprost tromethamine (Lutalyse; Pharmacia & Upjohn Company, Kalamazoo, Mich.) (25 mg; intramuscular injection; prostaglandin F2). At 2 weeks later, heifers that were not bred after the first injection (group 2; n = 10) received an additional injection of dinoprost tromethamine.
Heifers in standing estrus were bred by artificial insemination using semen from a bull of proven fertility (Rubytom; Southeast Select Sires, Franklin, Tenn.). Semen was stored in liquid nitrogen, thawed in a water bath at 35°C for 45 s, and placed immediately into the uterine body (approximately 1 cm from the cervix).
Heifers were challenged with uterine C. abortus 5 min after breeding. The inoculum was thawed in a water bath at 35°C for 45 s and placed approximately 1 cm into the uterine body. Heifers were randomly assigned to five groups, and each group was challenged with a different dose of C. abortus (0, 104, 105, 106, and 108 IFU). There were 6 heifers per treatment group for a total of 30 heifers. Two additional heifers (reserved as spares) were not used in the experiment.
Heifers that received intrauterine challenge with 0 to 108 IFU of C. abortus were randomly assigned to one of two groups. Heifers assigned to group 1 were bred and challenged at the onset of the experiment and served as a group with no cohort challenge with C. abortus. Breeding and challenge of group 2 heifers were delayed for 2 weeks (so that group 2 heifers would be exposed to maximum genital-tract shedding of C. abortus from the heifers in group 1) and served as a group that was exposed to cohort challenge with C. abortus at the time of breeding (as indicated by a significantly increased vaginal C. abortus load) (8). The goal of this approach was to ensure that maximum vaginal shedding of group 1 heifers coincided with the implantation of embryos into the uterine wall of group 2 heifers (at which time the group 2 heifers were at the highest level of susceptibility to infection with Chlamydophila). Pregnancy status was determined by manual rectal palpation at 42 days postbreeding. At 2 months after the completion of this experiment, all heifers that were not diagnosed pregnant were synchronized again with dinoprost tromethamine and bred a second time.
Validation of the cohort challenge model.
Two additional experiments were conducted to validate the cohort challenge model. The first validation experiment was designed to provide a cohort challenge that was similar to the challenge used in the original experiment. The estimated relative cohort challenge of the initial experiment was duplicated with a uniform first-round uterine inoculum of 3 × 107 IFU of C. abortus strain B577 in 15 out of 32 heifers. Relative cohort challenge was estimated as the sum of the log inoculum IFU values for first-round uterine-inoculated heifers multiplied by the number of first-round uterine-inoculated heifers divided by the total number of heifers: relative cohort challenge = (15 × 7.5) × (15/32) = 52.7; relative cohort challenge (initial experiment) = [(5 × 8) + (5 × 6) + (3 × 5) + (3 × 4)] × (16/32) = 48.5. The experiment was also modified (by using a uterine challenge dose [3 × 107 IFU of Chlamydophila] that was 33% of the high-level dose [108 IFU] used in the initial experiment) to reduce the effect of direct uterine challenge on fertility. The goal of this approach was to retain the overall amount of vaginal shedding (compared to the results seen with initial experiment) while allowing some of the uterus-challenged heifers to become pregnant.
The second validation experiment was designed to reduce the effect on fertility of both the overall cohort challenge and direct uterine challenge with C. abortus. This was achieved by (i) reducing the number of heifers that received uterine C. abortus in the first period to 7 heifers (compared to 15 heifers in the first validation experiment) and (ii) reducing the challenge dose to 6.67 × 106 IFU (compared to the 3 × 107 IFU dose that was used in the first validation experiment). This resulted in a relative cohort challenge of (7 × 6.82) × (7/32) = 10.4. At 2 months after the completion of the first and second validation experiments, heifers that did not become pregnant were again synchronized with dinoprost tromethamine and bred a second time.
Statistical analysis.
Paired sera (collected before and after challenge with C. abortus and tested by ELISA) from heifers of each challenge group were evaluated for changes in immunoglobulin (Ig) isotype concentration with the Wilcoxon signed rank test (35).
Changes in concentrations of anti-C. abortus antibodies over time were evaluated by using the ratio of postchallenge to prechallenge antibody concentrations, with values above or below 1 indicating increases or decreases in antibody levels over time, respectively. This ratio was termed the “antibody trend.” Antibody trends (determined by Ig isotype) for all pregnant versus nonpregnant (infertile) and all cohort- versus non-cohort-challenged heifers were analyzed by Student's t test (41).
Pregnancy outcome versus logarithm uterine C. abortus IFU dose and cohort challenge with C. abortus or antibody concentration against C. abortus were statistically modeled with logistic regression (4, 5, 29, 36). IFU dose data were logarithmically transformed, and antibody data were transformed to dichotomous data as either above or below median values before modeling. Log values (odds of infertility) were modeled as log (odds) = b0 + b1x1 + b2x2, where b0 represents the intercept, b1 and b2 represent regression coefficients, and x1 and x2 represent the independent-variable log IFU challenge dose (0, 4, 5, 6, and 8) and cohort challenge (0 and 1) and antibody level (0 and 1) values, respectively. Probability of infertility was calculated as probability = odds/(1 + odds) (4, 5, 28, 36). Values of P of <0.05 were considered significant.
RESULTS
Holstein heifers have high prechallenge anti-C. abortus antibody levels.
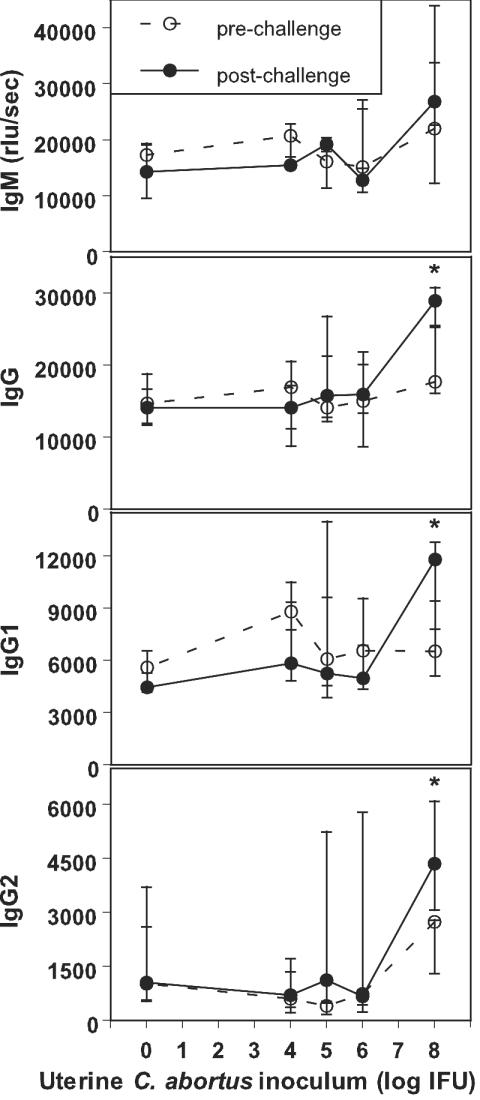
The levels of prechallenge Ig isotypes were high in all heifers, with median (range) values for the entire group of heifers of 17,298 (5,248 to 40,376), 15,761 (3,960 to 28,321), 7,059 (3,168 to 14,838), and 837 (36 to 9,605) for IgM, IgG, IgG1, and IgG2, respectively (Fig. 1). These data indicate that all of these virgin heifers had been exposed to C. abortus prior to challenge infection and that the infection had occurred by nonsexual transmission (6, 7). Antibody levels of this magnitude are consistent with recent or ongoing infection with Chlamydophila spp.
FIG. 1.
Uterine inoculation of heifers with C. abortus increases preexisting anti-C. abortus serum antibody levels in a dose-dependent manner. Estrus was induced in a total of 32 Holstein heifers by injection with dinoprost tromethamine. A total of 30 heifers (6 heifers each per challenge dose of 0, 104, 105, 106, or 108 IFU) responded with estrus and were given a uterine challenge of C. abortus strain B577 via the cervix. Single serum samples were collected 3 to 5 weeks before and 7 to 9 weeks after inoculation. Median and quartile rlu values per second are shown for bovine Ig isotypes IgM, IgG, IgG1, and IgG2 in a chemiluminescent ELISA using C. abortus strain B577 EB lysate antigen. Asterisks indicate significantly different pre- and postchallenge serum antibody levels (P < 0.028 [Wilcoxon signed rank test]). The increase in antibody levels at high levels of challenge inocula suggests that these inocula sufficiently breached anti-C. abortus immunity to establish infections that resulted in substantial stimulation of the immune response to C. abortus.
For each uterine C. abortus inoculation dose, levels of the antibody isotypes in the single pre- and postchallenge paired serum samples were compared using the Wilcoxon signed rank test. IgG, IgG1, and IgG2 values for heifers that received the highest uterine challenge dose of 108 IFU of C. abortus increased significantly (P = 0.028) after challenge with C. abortus. IgM antibody levels also increased in heifers challenged with 108 IFU, but this increase was not significant. Anti-C. abortus IgM, IgG, IgG1, and IgG2 levels did not significantly increase in heifers challenged with 0, 104, 105, or 106 IFU of C. abortus (Fig. 1). The pattern of significant changes suggests that the three significant increases in postchallenge antibody concentrations are not related to type I error in the 20 tests performed (4 antibody isotypes × 5 C. abortus inocula).
The increased postchallenge serum anti-C. abortus antibody concentrations seen after the highest uterine challenge suggest that this inoculum sufficiently breached anti-C. abortus immunity to establish an infection that substantially stimulated the immune response to C. abortus. Despite this clear antibody reaction to C. abortus infection, none of these heifers (and none of the heifers challenged with lower inoculum doses) showed any signs of overt systemic or genital disease (e.g., anorexia or clear signs of vaginal inflammation) throughout the course of the experiment. The only clinical deviation observed after uterine inoculation with C. abortus was that none of the heifers that failed to conceive had a normal estrus cycle of 21 days. These nonpregnant heifers returned to standing estrus after 30 (±10) days.
Cohort and high-level uterine challenge with C. abortus predicts rises of anti-C. abortus IgM.
To gain further insight into the factors affecting the antibody responses, we used the ratios of postchallenge to prechallenge serum anti-C. abortus antibody concentrations as indicators of the change in antibody levels over the course of the experiment, i.e., as markers for the stimulation of the antibody response. These ratios (subsequently termed antibody trends) indicate a rise in antibody levels when they are larger than 1, while they indicate a decline when they are lower than 1 (Fig. 2). Cohort challenge correlated with an increased IgM trend versus no cohort challenge (mean of all intrauterine challenge groups: 1.30 ± 0.46 versus 0.95 ± 0.38; P = 0.04), but IgG isotype trends did not differ significantly between cohort- and non-cohort-challenged animals. The antibody trends for all Ig isotypes were higher in infertile (nonpregnant) animals than in fertile ones (mean values for all intrauterine challenge groups: IgM, 1.39 ± 0.46 versus 0.85 ± 0.24 [P = 0.00]; IgG, 1.58 ± 0.74 versus 0.99 ± 0.24 [P = 0.01]; IgG1, 1.53 ± 0.75 versus 0.98 ± 0.34 [P = 0.01]; IgG2, 3.69 ± 5.04 versus 1.88 ± 2.41 [P = 0.20]). Antibody trend data for IgM, the isotype with the strongest association with experimental parameters and outcomes, are shown with respect to dependence on the uterine inoculum in Fig. 2. Overall, the antibody trend data suggest that IgM anti-C. abortus antibodies (more than IgG antibody isotypes) might be closely associated with parameters affecting the stimulation of the immune response by the C. abortus infection and potentially the functional outcome of the infection.
FIG. 2.
Infertility and cohort challenge are associated with an increased antibody trend for IgM. Median and quartile antibody trends for IgM for all uterine C. abortus inoculum doses are shown. (A) The antibody trend values for IgM of infertile heifers (nonpregnant; n = 12) are consistently higher than those seen with fertile heifers (pregnant; n = 18). (B) IgM trend values for cohort-challenged heifers (n = 10) are higher than those of herd mates that were not cohort challenged (n = 20). Cohort challenge indicates the exposure of inseminated heifers to C. abortus infection by shedding from heifers that had received uterine C. abortus inocula 2 weeks earlier. Animals that were inseminated during the first round of estrus were scored as not cohort challenged by herd mates that received uterine C. abortus inocula during the same time span. Animals inseminated 2 weeks later (during the second round of induced estrus) were scored as having been cohort challenged by their herd mates that were inoculated in the first round. Increased antibody trends for IgM were significantly associated with infertility (P = 0.001) and cohort challenge (P = 0.04) with C. abortus (Student's t test; mean of the results obtained with all intrauterine challenge groups).
The high levels of postchallenge IgM of heifers that were infertile in the challenge experiment predicted increased resistance against Chlamydophila-mediated infertility and likely high-level fertility in a second breeding (provided the challenge inoculation had not caused irreparable uterine injury). At 60 days after initial challenge with C. abortus, 10 nonpregnant heifers were bred a second time. Fertility levels were high for all previous-uterine-challenge groups, with 80% of the heifers becoming pregnant after the second breeding.
High uterine C. abortus challenge levels, cohort challenge with C. abortus, and low prechallenge anti-C. abortus IgM levels reduce fertility.
Uterine challenge and cohort challenge with C. abortus and low-level (below median) prechallenge anti-C. abortus IgM levels were associated with decreased fertility in heifers, as shown in Fig. 3. When modeled with logistic regression, uterine C. abortus inoculum levels, cohort challenge with C. abortus, and IgM levels against C. abortus predicted fertility levels, as shown in Fig. 4. Uterine C. abortus inoculum levels, cohort challenge with C. abortus, and IgM antibody levels against C. abortus significantly affected the models at P values of <0.05. The odds ratios (Wald 95% confidence intervals) per log C. abortus IFU for infertility were 5.4 (1.4 to 21; Fig. 4A) and 3.3 (1.2 to 9.5; Fig. 4B); i.e., the models predicted that infertility would increase by a factor of 5.4 or 3.3 for each log increase in IFU of uterine C. abortus inoculum. For cohort challenge with C. abortus, the odds ratio for infertility was 4.6 (1.01 to 21.15); thus, the model predicted that infertility would increase by a factor of 4.6 with cohort challenge with C. abortus. For low prechallenge IgM levels against C. abortus EB, the odds ratio for infertility was 4.4 (1.05 to 18.50); thus, the model predicted that infertility would increase by a factor of 4.4 for heifers with lower-than-median values of IgM. Overall, the logistic regression models demonstrate that the uterine C. abortus inoculum required to cause infertility is 8.5-fold higher for heifers without Chlamydophila cohort challenge (and 17-fold higher for heifers with high IgM antibody levels against Chlamydophila) compared to the results seen with heifers with cohort challenge or with low IgM antibody levels against Chlamydophila spp.
FIG. 3.
High uterine inoculum and cohort challenge with C. abortus and low prechallenge anti-C. abortus serum IgM levels are associated with reduced fertility in heifers previously exposed to C. abortus. Pregnancy as an indicator of fertility was evaluated 6 weeks after uterine challenge with C. abortus. (A) Overall pregnancy data for Holstein heifers (n = 30) inoculated in the uterus with 0, 104, 105, 106, or 108 IFU (with or without cohort challenge). (B) Pregnancy data for the same group of 30 heifers, with high (above-median) or low (below-median) prechallenge anti-C. abortus IgM levels. Taken together, these data suggest that reduction of bovine fertility by reinfection with C. abortus depends on the epidemiological parameters of herd infection with C. abortus (specifically, on the total challenge) via direct uterine inoculation and via cohort exposure and the immune response to previous infection.
FIG. 4.
Cohort challenge and prechallenge anti-C. abortus serum IgM levels strongly modulate fertility after uterine challenge with C. abortus. Fertility of challenged heifers (i.e., the percentage of pregnant animals) is significantly predicted in logistic regression models by the uterine C. abortus inoculum dose and cohort challenge by C. abortus (A) or by the uterine inoculum dose and concentration of preformed IgM against C. abortus (B). The solid line represents fertility dependence on the uterine inoculum under conditions of cohort challenge to C. abortus (A) or under conditions of below-median (low) levels of anti-C. abortus IgM (B). The dashed line represents fertility without cohort challenge (A) or with above-median (high) levels of anti-C. abortus IgM (B). The graphs represent logistic regression equations: % pregnant heifers (probability of pregnancy) = 100 [e10.56 − 1.68(log IFU) − 1.53(C)/ (1 + e10.56 − 1.68(log IFU) − 1.53(C))] (A) or % pregnant = 100 [e8.73 − 1.21(log IFU) − 1.48(IgM)/(1 + e8.73 − 1.21(log IFU) − 1.48(IgM))] (B), where log IFU represents the log10 of the uterine inoculum IFU dose of C. abortus, C represents either no (0) or cohort (1) challenge by C. abortus, and IgM represents an either below-median (0) or above-median (1) concentration of preformed anti-C. abortus IgM. The Wald P values are as follows: for inoculum dose, 0.016 (A) and 0.024 (B); for cohort challenge, 0.048; and for IgM levels, 0.043. These logistic regression models of fertility of heifers with established immunity against C. abortus indicate that (i) with cohort challenge a uterine infection of 105.38 IFU of C. abortus is necessary to reduce fertility of heifers from 100 to 50% compared to the 8.5-fold-higher dose of 106.31 IFU required for the same reduction without cohort challenge (A) and (ii) at low prechallenge anti-C. abortus IgM levels 106.01 intrauterine IFU of C. abortus reduce fertility of heifers from 100 to 50% compared to the 17-fold-higher dose of 107.24 IFU required for the same reduction at high prechallenge anti-C. abortus IgM levels (B).
Validation experiments corroborate reduction of fertility by cohort challenge.
Results for the validation experiments are shown in Fig. 5. The first validation experiment approximated the total cohort challenge of the initial experiment using a uniform challenge dose. A total of 3 of 14 (21%) or 4 of 5 (80%) heifers without cohort challenge became pregnant when challenged or not challenged by uterine inoculation with Chlamydophila spp., respectively. In contrast, 0 of 7 (0%) or 0 of 3 (0%) heifers with cohort challenge became pregnant with or without uterine challenge with Chlamydophila spp., respectively. These results suggested that cohort challenge with C. abortus induces infertility in cattle.
FIG. 5.
Uterine C. abortus inoculation experiments at high and low C. abortus cohort challenge levels corroborate the influence of cohort challenge on bovine fertility. Validation experiments for the effect of cohort challenge on fertility were performed with two groups of heifers. Group A received a cohort challenge estimated to be similar to that of the first experiment. Group B received 20% of the estimated original cohort challenge. (A) Pregnancy data for Holstein heifers (n = 29) with or without cohort challenge to C. abortus. The uterine inoculum dose was either 3 × 107 IFU of C. abortus strain B577 or a placebo challenge. In this experiment, the estimated relative cohort challenge of the initial experiment was duplicated with a first-round uterine inoculum of 3 × 107 IFU C. abortus strain B577 administered to 15 heifers. The relative cohort challenge value was estimated as 52.7 (compared to that of the initial experiment [48.5]). (B) Pregnancy data for Holstein heifers (n = 32) with or without an estimated 20% of the relative level of cohort exposure of members of group A. The relative value of cohort exposure to C. abortus was reduced to 10.4 (approximately 20% of the cohort challenge value in the initial and the first validation experiment [experiment A]) by reducing the number of heifers (n = 7) that were uterus inoculated with C. abortus and by reducing the inoculation dose (to 6.67 × 106 IFU) per heifer. The uterine inoculum dose was either 6.67 × 106 IFU C. abortus strain B577 or a placebo challenge. The results of these experiments are consistent with a reduction of bovine fertility subsequent to cohort challenge. Reduction of the estimated relative cohort challenge by 80% appears to have abolished the cohort-induced depression of fertility in heifers that did not receive uterine challenge, but this cohort challenge still negatively influenced fertility in heifers that also received a uterine challenge.
In the second validation experiment the relative cohort challenge with Chlamydophila spp. was reduced by 80% compared to the results seen with the initial experiment. A total of 1 of 7 (14%) or 5 of 8 (63%) heifers without cohort challenge became pregnant with or without uterine Chlamydophila challenge, respectively. In contrast, 0 of 8 (0%) or 8 of 8 (100%) heifers with cohort challenge became pregnant when challenged or not challenged with uterine Chlamydophila spp., respectively. These results suggest that cattle that are cohort challenged with Chlamydophila spp. (at a level below a discrete threshold value) will resist reinfection. However, when cattle are simultaneously inoculated with uterine Chlamydophila spp., this cohort challenge threshold value is breeched and the fertility (which is already reduced by the uterine challenge) of these cattle will be further reduced by the cohort challenge. These results further suggest that the herd dynamics of infection (such as total herd density, ratio of Chlamydophila-shedding versus nonshedding animals, and level of Chlamydophila shedding) strongly influence Chlamydophila-mediated depression of bovine fertility.
DISCUSSION
Investigations conducted over the last 30 years have suggested that C. abortus causes ubiquitous genital infection in cattle (6, 7, 18, 31, 32, 42). Recently, we detected with high frequency the presence of low levels of C. abortus strain B577 DNA in vaginal mucosal samples collected from virgin heifers (6, 7). These data also suggest that nonsexual transmission of the organism (most likely by ingestion from a contaminated environment or by direct contact with shedding herd mates) is an important mode of transmission for this organism in cattle (6, 7). The study reported here provides additional evidence that C. abortus is nonsexually transmitted to the reproductive tract and that this transmission has the potential to cause infertility. The present study confirms that environmental exposure and direct cohort exposure may both serve as important sources of infection and subsequent infertility caused by the presence of C. abortus in cattle.
C. abortus may cause infertility in a number of ways. Bulls with natural or experimentally induced C. abortus genital infection often have pyospermia with a high level of occurrence of morphological abnormalities (3, 38, 39). Cows and heifers with C. abortus genital infection may also have reduced levels of fertility as a result of direct infection of the embryo after loss of the zona pellucida and/or as a result of local infection and related inflammation that creates a uterine environment that is not conducive to embryo implantation. Experimental evidence strongly suggests that uterine inflammation, and not direct infection of the fertilized egg, is the dominant factor in bovine female infertility caused by C. abortus (3). Our observation of extended postinsemination estrus cycles of infertile heifers in this study supports this concept.
The highest possible level of fertility for all heifers was essential for meaningful results in this investigation. The combination of estrus synchronization with prostaglandin F2 and electronic estrus detection provided optimum information for the best time of insemination. Precise identification of the onset of estrus and a high plane of nutrition were important factors that contributed to the high conception rate of heifers that were not challenged with uterine C. abortus in this study.
Logistic regression models for the data from our study indicate that a uterine challenge with 2.4 × 105 C. abortus IFU would reduce the first-service conception rate of highly fertile cattle from 100 to 50% if the cattle were exposed to C. abortus shed by the herd mates. When cattle experience low-level environmental and cohort exposure to C. abortus, in contrast, the increased uterine dose of C. abortus required for a 50% reduction in the first-service conception rate is 2 × 106 IFU (an 8.5-fold difference). Similarly, the dose required to reduce fertility by 50% increases 17-fold (from 1.0 × 106 to 1.7 × 107 IFU) for cattle with high anti-C. abortus IgM antibody levels compared to the results seen with cattle with low antibody levels. Similar ranges of C. abortus challenge doses are typically used in mouse studies (irrespective of a more than 10,000-fold difference in body weight between cattle and mice), suggesting a high natural susceptibility of cattle to C. abortus infection and disease. Doses of C. abortus that caused infertility in this study correspond well to natural challenges that are likely to occur in commercial cattle operations (31). Vaginal infection and fertility rates commonly observed in commercial cattle operations are consistent with low-dose exposure to Chlamydophila spp. In the second confirmation experiment (using a reduced cohort challenge) of this study, a cohort-induced depression of fertility was not observed in heifers that did not receive a uterine challenge. However, the cohort-induced reduction in fertility was still evident in heifers that received uterine challenge. This result suggests that a threshold of infection needs to be overcome before fertility is depressed in heifers reinfected with Chlamydophila spp. We assume that this threshold might be lowered in cattle that experience C. abortus infection concurrent with reduced immune function due to dietary deficiencies, stressors, and/or concurrent disease (all of which occur frequently in commercial cattle operations).
The high concentrations of serum antibodies against C. abortus suggest that all heifers in this study experienced active infection with C. abortus or had recently recovered from infection. Thus, the heifers most likely had a competent naturally acquired immune response to C. abortus. In this study, an above-median IgM concentration was identified as a predictor of subsequent fertility in cattle following challenge with C. abortus. The association of improved fertility with above-median prechallenge IgM concentrations is an important finding with several possible explanations. One possible explanation is that IgM protects animals directly from infection by neutralizing circulating C. abortus organisms or by mediating lysis of infected cells, resulting in decreased incidences of diseases such as infertility. Epidemiological data suggesting functional effects of antibodies against C. abortus are limited (1, 11). Data from Chlamydiaceae disease models and epidemiological investigations of human disease suggest that humoral antibodies against C. abortus provide only limited protection from disease (1, 2, 11, 14, 16, 19, 24-28, 40).
In this study, infertile heifers with below-median prechallenge serum IgM levels still had considerable concentrations of IgM. This fact, and the lack of significant differences for other antibody isotypes, argues against causality of antibodies, including IgM, in protection from infertility and suggests an associative relationship of prechallenge IgM levels with protection. The primary mechanism of this protection is most likely based on Th1 cellular immunity, as suggested by a large body of evidence from human epidemiological and animal model studies (1, 2, 11-14, 16, 19, 24-28, 40). Above-median anti-C. abortus IgM levels may indicate a recent episode of C. abortus infection that elicits transient protection from disease. Thus, elevated IgM may provide a surrogate marker of recently stimulated anti-C. abortus cellular immunity, indicating the presence of circulating specific effector T cells or high levels of memory T cells. This explanation is consistent with our observation that elevated IgM levels (but not elevated total IgG or IgG1 or IgG2 antibody levels, which are less indicative of recent infection) predicted protection.
The strong association of an increased antibody trend for IgM with infertility (Fig. 2) suggests a common mechanism that results in both infertility and elevated anti-C. abortus serum IgM levels. We assume that that this mechanism is associated with reduced elimination kinetics of C. abortus from tissues, including those of the uterus. This would result in a prolonged uterine inflammatory response in animals with below-median IgM levels that prevents implantation of the embryo and represents a stronger immune stimulus than the stimulus of an infection that is rapidly eliminated by an effective immune response. This concept is consistent with the high-level fertility of heifers in this study that were bred a second time after failing to conceive 60 days earlier following the initial breeding and challenge. These heifers most likely mounted effective cellular immune responses subsequent to the earlier C. abortus challenge so that infection-associated inflammation was reduced, resulting in a high pregnancy rate.
The results of the present investigation clearly demonstrate that previous exposure to C. abortus does not provide complete protection from disease in a subsequent challenge (even in the absence of clinical signs of disease). Relative immune protection can be accomplished, however, as indicated by the association of elevated anti-C. abortus serum IgM levels with reduced disease. Simple models with a total of three predictor variables accounted for the variation in fertility observed in a herd of dairy cattle that was preexposed to and then rechallenged with C. abortus. These models addressed the main factors that impact the fertility of cattle infected with C. abortus and provided valuable insight into the population dynamics of infection with Chlamydophila. One of the logistic regression models clearly specifies profound effects on fertility of both sexual and nonsexual transmission of C. abortus from herd mates. Overall size and density of breeding and Chlamydophila-shedding cohort populations influence the frequency of sexual and cohort transmission and are therefore likely critical determinants of Chlamydophila-mediated fertility disorders.
Another major determinant of C. abortus-mediated fertility disorders is the time-dependent response to boost cycles of Chlamydophila-specific host immunity by natural reinfection. Serum anti-C. abortus IgM levels (relative to the herd average) provide a surrogate marker for immunity to Chlamydophila infection, as specified in the second logistic regression model. Aside from being influenced by the type and dose of reinfection, the immune response is influenced by a wide range of herd-specific immunosuppressive factors, including quantitative and qualitative malnutrition, numerous well-characterized herd stressors, and/or concurrent disease (all frequently observed in commercial cattle operations). Thus, the quality of herd management will likely affect Chlamydophila-mediated bovine fertility disorders. Another profound influence on any immune response is the genetic variation of the host that is characteristic of an outbred population. This resistance would most likely be expressed through immune function but could also be expressed as behavioral change, such as altered social interaction with herd mates shedding Chlamydophila.
Chronic infections with C. abortus are ubiquitous in cattle, and subtle manifestations of these infections are essentially not recognized and thus are not subject to prophylactic or therapeutic measures. This study demonstrates that these subclinical infections may have a profoundly negative effect on bovine fertility and that these losses may be reduced with appropriate herd management practices. Data from this study and C. abortus prevalence data (6, 7) suggest a steady-state equilibrium of C. abortus infection (at the herd level) which is driven and maintained by asynchronous cycles of relative resistance and the susceptibility of individual animals and by the kinetics of sexual and cohort transmission as determined by breeding regimen, herd size, and population density. This equilibrium may be induced to shift by a variety of mechanisms, such as a nutrition-related decrease in overall herd immunity, which can lead to increased susceptibility to infection and enhanced transmission, resulting in a proportional reduction in fertility.
The risk factors identified in this study may provide insight for the development of viable prophylactic and therapeutic measures. Numerous herd management practices (most importantly, high-quality nutrition) need to ensure high-level immunocompetence of herds. Routine monitoring of sires for the presence of C. abortus in semen and/or the prepuce would provide useful information for reducing sexual transmission. Cohort transmission may be decreased by reducing group size and density and by the elimination or isolation of debilitated animals that chronically shed Chlamydophila spp. Vaccination may also serve as a component of a prophylactic or therapeutic program by protecting individual animals from disease (9) and by reducing Chlamydophila transmission within the herd. The results of this investigation provide encouraging evidence that effective vaccines against Chlamydophila spp. can be constructed. It is likely that this protection will require circulating Chlamydophila-specific Th1 cells; consequently, this protection is likely to be transient, with frequent vaccination required to maintain continuous protection.
Acknowledgments
We thank Joe Galic for excellent technical support.
This study was supported by Bayer AG, Monheim, Germany.
Editor: D. L. Burns
REFERENCES
- 1.Bailey, R. L., M. Kajbaf, H. C. Whittle, M. E. Ward, and D. C. Mabey. 1993. The influence of local antichlamydial antibody on the acquisition and persistence of human ocular chlamydial infection: IgG antibodies are not protective. Epidemiol. Infect. 111:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batteiger, B. E., and R. G. Rank. 1987. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect. Immun. 55:1767-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen, R. A., P. Spears, J. Storz, and G. E. Seidel, Jr. 1978. Mechanisms of infertility in genital tract infections due to Chlamydia psittaci transmitted through contaminated semen. J. Infect. Dis. 138:95-98. [DOI] [PubMed] [Google Scholar]
- 4.Cody, R. P., and J. K. Smith. 1997. Multiple-regression analysis, p. 235-247. In Applied statistics and the SAS programming language, 4th ed. Prentice-Hall, Inc., Upper Saddle River, N.J.
- 5.Dawson-Saunders, B., and R. G. Trapp. 1994. Statistical methods for multiple variables, p. 222-223. In Basic & clinical biostatistics, 2nd ed. Appleton & Lange, Norwalk, Conn.
- 6.DeGraves, F. J., D. Gao, H. R. Hehnen, T. Schlapp, and B. Kaltenboeck. 2003. Quantitative detection of Chlamydia psittaci and C. pecorum by high-sensitivity real-time PCR reveals high prevalence of vaginal infection in cattle. J. Clin. Microbiol. 41:1726-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeGraves, F. J., D. Gao, and B. Kaltenboeck. 2002. Frequent natural chlamydial infection in cattle found with high-sensitivity high-through-put quantitative PCR platform, p. 127-130. In J. Schachter (ed.), Chlamydial infections. Proceedings of the Tenth International Symposium on Human Chlamydial Infection, Antalya, Turkey. International Chlamydia Symposium, San Francisco, Calif.
- 8.DeGraves, F. J., K. Stemke-Hale, J. Huang, S. A. Johnston, K. F. Sykes, T. Schlapp, H. R. Hehnen, and B. Kaltenboeck. 2002. Vaccine identified by in vivo genomic screening enhances fertility in cattle during environmental challenge with Chlamydia, p. 265-268. In J. Schachter (ed.), Chlamydial infections. Proceedings of the Tenth International Symposium on Human Chlamydial Infection, Antalya, Turkey. International Chlamydia Symposium, San Francisco, Calif.
- 9.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 10.Herring, A. J., I. E. Anderson, M. McClenaghan, N. F. Inglis, H. Williams, B. A. Matheson, C. P. West, M. Rodger, and P. P. Brettle. 1987. Restriction endonuclease analysis of DNA from two isolates of Chlamydia psittaci obtained from human abortions. Br. Med. J. 295:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland, M. J., R. L. Bailey, L. J. Hayes, H. C. Whittle, and D. C. Mabey. 1993. Conjunctival scarring in trachoma is associated with depressed cell-mediated immune responses to chlamydial antigens. J. Infect. Dis. 168:1528-1531. [DOI] [PubMed] [Google Scholar]
- 12.Huang, J., F. J. DeGraves, S. D. Lenz, D. Gao, P. Feng, D. Li, T. Schlapp, and B. Kaltenboeck. 2002. The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc. Natl. Acad. Sci. USA 99:3914-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, J., M. D. Wang, S. Lenz, D. Gao, and B. Kaltenboeck. 1999. IL-12 administered during Chlamydia psittaci lung infection in mice confers immediate and long-term protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J. Immunol. 162:2217-2226. [PubMed] [Google Scholar]
- 14.Igietseme, J. U., and R. G. Rank. 1991. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect. Immun. 59:1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, G. E. 1999. Chlamydial diseases of the reproductive tract of domestic ruminants, p. 293-309. In P. J. Hitchcock, H. T. MacKay, and J. N. Wasserheit (ed.), Sexually transmitted diseases and adverse outcomes of pregnancy. ASM Press, Washington, D.C.
- 16.Jones, G. E., A. Donn, J. Machell, B. Biolatti, and S. Appino. 1998. Experimental infections of the genital tract of cattle with Chlamydia psittaci and Chlamydia pecorum, p. 446-449. In R. S. Stephens (ed.), Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection, San Francisco. International Chlamydia Symposium, San Francisco, Calif.
- 17.Jorgensen, D. 1997. Gestational psittacosis in a Montana sheep rancher. Emerg. Infect. Dis. 3:191-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaltenboeck, B., D. Heard, F. J. DeGraves, and N. Schmeer. 1997. Use of synthetic antigens improves detection by enzyme-linked immunosorbent assay of antibodies against abortigenic Chlamydia psittaci in ruminants. J. Clin. Microbiol. 35:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee, D. M., J. U. Igietseme, J. G. Smith, C. A. Bleicker, B. G. Grubbs, J. Schachter, R. G. Rank, and D. M. Williams. 1993. Chlamydia trachomatis pneumonia in the severe combined immunodeficiency (SCID) mouse. Reg. Immunol. 5:305-311. [PubMed] [Google Scholar]
- 20.Papp, J. R., and P. E. Shewen. 1996. Localization of chronic Chlamydia psittaci infection in the reproductive tract of sheep. J. Infect. Dis. 174:1296-1302. [DOI] [PubMed] [Google Scholar]
- 21.Papp, J. R., P. E. Shewen, and C. J. Gartley. 1994. Abortion and subsequent excretion of chlamydiae from the reproductive tract of sheep during estrus. Infect. Immun. 62:3786-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp, J. R., P. E. Shewen, and C. J. Gartley. 1993. Chlamydia psittaci infection and associated infertility in sheep. Can. J. Vet. Res. 57:185-189. [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Martinez, J. A., and J. Storz. 1985. Antigenic diversity of Chlamydia psittaci of mammalian origin determined by microimmunofluorescence. Infect. Immun. 50:905-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey, K. H., W. J. T. Newhall, and R. G. Rank. 1989. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect. Immun. 57:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey, K. H., and R. G. Rank. 1991. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 59:925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey, K. H., L. S. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rank, R. G., and A. L. Barron. 1983. Effect of antithymocyte serum on the course of chlamydial genital infection in female guinea pigs. Infect. Immun. 41:876-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rank, R. G., K. H. Ramsey, and A. J. Hough, Jr. 1988. Antibody-mediated modulation of arthritis induced by Chlamydia. Am. J. Pathol. 132:372-381. [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. 1989. The LOGISTIC procedure, p. 1071-1126. In SAS/STAT user's guide, fourth ed., vol. 2. SAS Institute Inc., Cary, N.C.
- 30.Schachter, J., J. Banks, N. Sugg, M. Sung, J. Storz, and K. F. Meyer. 1974. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect. Immun. 9:92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachter, J., J. Banks, N. Sugg, M. Sung, J. Storz, and K. F. Meyer. 1975. Serotyping of Chlamydia: isolates of bovine origin. Infect. Immun. 11:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmeer, N., K. L. Schnorr, J. A. Perez-Martinez, and J. Storz. 1987. Dominance of Chlamydia psittaci-specific IgG2 subclass in the humoral immune responses of naturally and experimentally infected cattle. Vet. Immunol. Immunopathol. 15:311-322. [DOI] [PubMed] [Google Scholar]
- 33.Shewen, P. E. 1980. Chlamydial infection in animals: a review. Can. Vet. J. 21:2-11. [PMC free article] [PubMed] [Google Scholar]
- 34.Spears, P., and J. Storz. 1979. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect. Immun. 24:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steel, R. G. D., and J. H. Torrie. 1980. Nonparametric statistics, p. 533-553. In J. W. Maisel (ed.), Principles and procedures of statistics, 2nd ed. McGraw-Hill, Inc., New York, N.Y.
- 36.Stokes, M. E., C. S. Davis, and G. G. Koch. 1995. Logistic regression I: dichotomous response, p. 165-216. In Categorical data analysis using the SAS System. SAS Institute Inc., Cary, N.C.
- 37.Storz, J. 1966. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J. Comp. Pathol. 76:351-362. [DOI] [PubMed] [Google Scholar]
- 38.Storz, J., E. J. Carroll, L. Ball, and L. C. Faulkner. 1968. Isolation of a psittacosis agent (Chlamydia) from semen and epididymis of bulls with seminal vesiculitis syndrome. Am. J. Vet. Res. 29:549-555. [PubMed] [Google Scholar]
- 39.Storz, J., E. J. Carroll, E. H. Stephenson, L. Ball, and A. K. Eugster. 1976. Urogenital infection and seminal excretion after inoculation of bulls and rams with chlamydiae. Am. J. Vet. Res. 37:517-520. [PubMed] [Google Scholar]
- 40.Williams, D. M., B. G. Grubbs, E. Pack, K. Kelly, and R. G. Rank. 1997. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect. Immun. 65:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winer, B. J., D. R. Brown, and K. M. Michels. 1991. Principals of estimation and inference: means and variances, p. 12-73. In J. M. Morris (ed.), Statistical principles in experimental design, third ed. McGraw-Hill, Inc., New York, N.Y.
- 42.Wittenbrink, M. M., H. Horchler, and W. Bisping. 1988. Investigations into the incidence of Chlamydia psittaci in the genital tract and feces of female cattle. J. Vet. Med. Ser. B 35:237-246. [PubMed] [Google Scholar]