Abstract
Previous observations demonstrated that the delivery of recombinant Salmonella enterica serovar Dublin strains to mice via mucosal routes did not efficiently activate systemic and secreted antibody responses to either type d flagellin or genetically fused heterologous B-cell epitopes, thus reducing the usefulness of the protein as a carrier of epitopes for vaccine purposes. In this work, we investigated murine systemic and mucosal flagellin immunogenicity after oral immunization with attenuated Salmonella strains. The reduced anti-type d flagellin antibody responses in mice immunized via mucosal routes with three doses of flagellated S. enterica serovar Dublin strains were not caused by oral tolerance and could not be restored by coadministration of a mucosal adjuvant. The induction of antibody responses to Salmonella flagellins was shown to differ according to the genetic background, but not the haplotype, of the mouse lineage. Moreover, BALB/c mice orally immunized with S. enterica serovar Typhimurium strains developed anti-type i flagellin sera and secreted antibody responses, which indicated that the serovar of the Salmonella vaccine strain also affected flagellin immunogenicity. Analyses of cytokine responses of BALB/c mice immunized with three oral doses of flagellated S. enterica serovar Dublin vaccine strains showed that, in spite of the lack of antibody responses, elevated type d flagellin-specific CD4-cell-activation-dependent gamma interferon (IFN-γ) and interleukin-10 responses were elicited after the administration of the vaccine strains via either parenteral or mucosal routes. Similar cytokine production patterns were detected to a T-cell heterologous epitope, derived from the CFA/I fimbriae of enterotoxigenic Escherichia coli (ETEC), in mice orally immunized with a Salmonella vaccine strain expressing hybrid flagella. These results indicate that the immunogenicities of Salmonella flagellins can differ significantly, depending on the murine host and on the bacterial vector used, and demonstrate that the induction of CD4-cell-activation-dependent IFN-γ production represents a major immune response triggered by flagellin and in-frame fused heterologous T-cell epitopes after the oral administration of recombinant S. enterica serovar Dublin vaccine strains.
The Salmonella-flagellin system has been designed to activate immune responses to heterologous linear epitopes genetically fused to flagellin, the structural subunit of the flagellar filament, expressed by live Salmonella enterica serovar Dublin vaccine strains (31). The vaccine strategy was based on the cloning of the S. enterica serovar Müenchen fliC gene, which belongs to the same serological type (d) of the human pathogen S. enterica serovar Typhi, followed by the removal of an internal 48-bp EcoRV-EcoRV fragment located in hypervariable region IV which spans 350 bp at the central region of the gene (31, 39). The resulting plasmid, pLS408, allowed the in-frame fusion of heterologous sequences encoding up to 45 amino acids, in most cases without abolishing the motility function of the recombinant flagella (31-33, 39). The introduction of pLS408 into a nonflagellated attenuated aroA S. enterica serovar Dublin strain, SL5928, able to express a somatic antigen (O antigen) similar to that found in S. enterica serovar Typhi, represented a further step toward the development of live bivalent Salmonella vaccine strains that are suitable for human use (31, 39).
The first peptide to be expressed as a genetic fusion with Salmonella flagellin was a B-cell epitope derived from the cholera toxin B subunit (31). After that, several other B-cell epitopes, such as those derived from the hepatitis B virus surface protein, the Streptococcus pyogenes type 5 M protein, the human immunodeficiency virus gp160 surface protein, the structural subunit of the enterotoxigenic Escherichia coli (ETEC) colonization factor antigen I (CFA/I) fimbriae, influenza virus hemagglutinin, the surface antigen of Schistosoma mansoni, the class I outer membrane protein of Neisseria meningitidis, the VP7 protein of rotavirus, and the Plasmodium CS protein, among others, were expressed as fusions with the type d Salmonella flagellin (2, 3, 5, 26-28, 32, 33, 45). Most of the live vaccines were able to elicit specific serum antibody responses after parenteral immunization of mice. Nonetheless, attempts to immunize mice with the same strains via mucosal routes were frustrated by the reduced systemic and secreted antibody responses to flagellin-fused heterologous epitopes (5, 26, 27, 32, 39).
Recently we showed that the low-level antibody responses to peptides genetically fused to flagellin might be attributed, in contrast to the case for mice immunized via parenteral routes, to the reduced flagellin immunogenicity after delivery via mucosal routes (10, 36). The contrasting immunological behaviors of flagellin expressed by Salmonella vaccine strains delivered via different immunization routes did not reflect reduced gene expression or deficient host colonization-invasion by the bacterial vaccine strains (36). The elucidation of host and bacterial factors affecting the systemic and secreted immunogenicity of Salmonella flagellin still needs to be done, therefore, as an important step toward the more rational use of flagellin as a carrier of epitopes in vaccine formulations.
In the last few years, bacterial flagellin has been shown to possess strong immunostimulatory properties. Indeed, Salmonella flagellin is a potent inducer of proinflammatory mediators, such as NO, interleukin-1β (IL-1β), IL-8, IL-6, and tumor necrosis factor alpha, both in vitro and in vivo, in either rodents or humans (6-8, 12, 13, 24, 41, 46). Flagellin also represents a major antigen for activated CD4+ T cells in mice after oral infections with attenuated S. enterica serovar Typhimurium aroA strains, leading to CD4+-dependent gamma interferon (IFN-γ) production, a step required for the mounting of protective immunity against a challenge with virulent Salmonella strains (9, 29).
For this work, we investigated the immunogenicity of Salmonella flagellin following the administration of attenuated bacterial strains via mucosal or parenteral routes to mice of different lineages. The present observations show that the ability to mount systemic and secreted antibody responses to flagellin after oral immunization with Salmonella vaccine strains is affected by several factors concerning the genetic background of both the mammalian host and the bacterial vector. Moreover, we demonstrate that the type d Salmonella flagellin, as well as a genetically fused heterologous T-cell epitope, can elicit strong CD4-cell-activation-dependent IFN-γ and IL-10 production, irrespective of the administration route, in mice immunized with a live S. enterica serovar Dublin strain, which suggests that T-cell-dependent responses constitute a major aspect of the antiflagellin responses elicited in mice orally immunized with Salmonella vaccine strains. Such an observation should contribute to a better use of the Salmonella-flagellin expression system as a bivalent mucosal vaccine approach and to a more detailed knowledge of Salmonella-induce immune responses in mouse models.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. enterica serovar Dublin SL5928 is a nonflagellated derivative of the S. enterica serovar Dublin aroA SL1438 strain, while the SL5930 strain is SL5928 transformed with pLS408, which promotes the expression of a type d flagellin encoded from an S. enterica serovar Müenchen fliC gene carrying a 48-bp deletion at the central hypervariable region of the gene (31). S. enterica serovar Dublin strain ICB5 is a flagellated derivative of the aroA SL7379 strain carrying a single copy of the same deleted version of the fliC (d) gene integrated into the chromosome (36). S. enterica serovar Dublin strain FLAII is a derivative of the SL5928 strain transformed with plasmid pLG302, which encodes a hybrid flagellin genetically fused to a 15-amino-acid peptide, representing the major T-cell epitope of the structural subunit (CfaB) of the CFA/I fimbriae produced by some ETEC strains (26, 27). S. enterica serovar Typhimurium strain SL3261, a flagellated aroA strain, was kindly supplied by B. Stocker. S. enterica serovar Typhimurium strain LM408 was generated after the electroporation of pLS408 into strain SL3261. ETEC strain 258909-3 (CFA/I, O128:H?, ST/LT) and the corresponding isogenic CFA/I-negative strain, 258909-3 M, were kindly offered by A. M. Svennerholm (Göteborg University, Göteborg, Sweden). All Salmonella strains were grown in Luria-Bertani (LB) broth at 37°C with aeration. Cultures of strains harboring pLS408 or pLG302 were supplemented with ampicillin (100 μg/ml). The ETEC strains were cultivated on CFA agar plates (25). All strains were stored at −70°C in LB medium containing 20% glycerol. Bacteria used as stimulants in cell cultures were inactivated by suspension in 70% ethanol and were kept overnight at 4°C before being added to cell cultures, at which time the suspensions were washed three times with excess sterile 0.01 M phosphate-buffered saline (PBS) in order to remove the ethanol.
Mice and immunization protocols.
Specific-pathogen-free isogenic female mice of 8 to 12 weeks old from lineages BALB/c (H2d), C57BL/6 (H2b), B10A (H2a), DBA/2 (H2d), A/J (H2a), CBA/J (H2k), and C3H/HePas (H2k) were supplied by the Isogenic Mouse Breeding Facility of the Department of Immunology, Institute of Biomedical Sciences, São Paulo University (USP). Biozzi albino mice, genetically selected (selection III) from outbred populations for high and low levels of antibody responsiveness to Salmonella antigens, were described previously (4, 37) and were kindly supplied by O. Ibanez from the Laboratory of Immunogenetics at the Butantan Institute (São Paulo, Brazil). All procedures were done in accordance with the principles of the Brazilian code for the use of laboratory animals and were approved by the Ethics Committee on Use of Laboratory Animals from the Institute of Biomedical Sciences, USP. Immunizations were carried out using viable bacterial strains harvested during the exponential growth phase (optical density at 600 nm of 0.8). Bacteria were washed once by centrifugation with PBS and were suspended in sterile saline solution, for intraperitoneal (i.p.) and intranasal (i.n.) inoculations, or in 0.1 M sodium bicarbonate, for intragastric (p.o.) inoculations, to final concentrations ranging from 5 × 108 CFU ml−1 to 2 × 1010 CFU ml−1. Mice were immunized p.o. with 0.5-ml aliquots of bacterial suspensions containing approximately 1010 CFU by use of a stainless steel round-tip gavage cannule on days 0, 21, and 35. The same immunization schedule was followed for the i.p. immunizations, but the bacterial loads were reduced to 107 CFU per dose. When performed, i.n. immunizations were performed in mice under light anesthesia, with 20 μl of bacterial suspension containing 107 CFU applied into the nostrils with the aid of a micropipette on days 0, 21, and 35. Blood collected from the retro-orbital plexus, feces, and lung washes were harvested 1 week after the last immunization dose. Fecal homogenates and lung washes were prepared as previously described (36). Serum, fecal, and lung wash samples were collected individually, but samples belonging to the same immunization groups (with 5 to 10 individuals) were pooled before the determination of antibody titers. All harvested biological samples were stored at −20°C until testing.
Detection of Salmonella in mouse tissues.
Groups of three female BALB/c or C3H/HePas mice, with ages ranging from 8 to 12 weeks, were inoculated p.o. with a single dose of 1010 CFU of S. enterica serovar Dublin (strains ICB5 and SL5930) or S. enterica serovar Typhimurium (strains LM408 and SL3261). The animals were sacrificed 7 days after the inoculation and the small intestines and spleens were removed under aseptic conditions. Five Peyer's patches (PP) and the whole spleen from each mouse were homogenized and serially diluted in PBS. Aliquots were plated on novobiocin-Luria plates and bacterial colonies were counted after 24 h of incubation at 37°C. pLS408 stability in the different bacterial hosts was evaluated after the inoculation of bacterial colonies onto L agar plates with or without added ampicillin (100 μg/ml). Flagellin expression in strain ICB5 was monitored by Western blotting of isolated bacterial colonies, using antiflagellin H1-d serum raised in rabbits and peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibodies, as previously described (36).
Determination of oral tolerance and LTR192G adjuvant effects.
The induction of immunological tolerance to Salmonella flagellin was monitored in groups (5 to 10 animals) of BALB/c mice orally immunized with three doses (1010 CFU) of S. enterica serovar Dublin ICB5 on days 0, 21, and 35. Two weeks later, mice were injected i.p. with 10 μg of purified flagellin combined with 1 mg of aluminum hydroxide or with 107 CFU of the Salmonella ICB5 strain, and this was repeated once after 2 weeks. Serum (IgG) and mucosal (IgA) antiflagellin antibody levels were measured 1 week after the last i.p. boost. Control groups included p.o. immunized animals receiving three doses of live Salmonella and animals that had been immunized i.p. with two doses of purified flagellin or Salmonella cells. The nontoxic derivative of the ETEC heat-labile toxin, LTR192G, was obtained by site-specific mutagenesis (11) and was kindly supplied by J. Clements (Tulane University). BALB/c mice were immunized with Salmonella cell suspensions containing 25 or 1 μg of LTR192G for p.o. or i.n. immunizations, respectively. Sera, fecal extracts, and lung washes were collected 1 week after the last immunization and stored at −20°C until they were tested by enzyme-linked immunosorbent assay (ELISA) for antiflagellin and anti-LT antibody responses.
Detection of antibody responses.
All reagents and antisera for ELISAs were obtained from Sigma. Microtiter plates (Nalge Nunc, Roskilde, Denmark) were coated with purified Salmonella flagellins (0.1 μg/well) and Salmonella group D or group B lipopolysaccharide (LPS) (100 ng/well) in 0.05 M sodium bicarbonate buffer at pH 9.6 and were incubated overnight at 4°C. The plates were blocked with 1% gelatin in PBS for 1 h at 37°C, and serum, fecal extract, and lung wash pools, previously diluted in PBS with 0.05% Tween 20 (PBST), were added to the plates in series of twofold dilutions, followed by incubation for a further 2 h at 37°C. The plates were washed twice with PBST and incubated for 1 h with mouse-specific IgG or IgA isotypes. After being washed with PBST, plates were developed with O-phenylenediamine dihydrochloride, with H2O2 as a substrate. Reactions were stopped with H2SO4 and absorbance values were measured at 492 nm in a microtiter plate spectrophotometer (Multiscan MS; LabSystem). Endpoint titers were automatically calculated with the Microcal Origin 6.0 Professional program as the reciprocal values of the last dilutions with an optical density of 0.1. Results are expressed as arithmetic means ± standard errors of the means (SEM) of the endpoint titers of at least duplicate determinations.
Purification of Salmonella flagellin and ETEC CFA/I fimbriae.
Salmonella flagellins expressed by S. enterica serovar Dublin ICB5 (type d flagellin) and S. enterica serovar Typhimurium SL3261 (type i and FljB flagellins) were purified by mechanical shearing and/or the acid monomerization procedure (23, 38). The CFA/I fimbriae were harvested from ETEC cells grown on CFA plates and were purified by ammonium sulfate precipitation, followed by DEAE-cellulose chromatography, performed according to previously described procedures (14).
T-cell assays.
Spleens from immunized BALB/c mice were removed and single-cell suspensions were prepared in RPMI 1640 medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 0.05% 2-mercaptoethanol, penicillin (100 U/ml), and streptomycin (100 μg/ml) as previously described (16). Spleen cells were distributed (5 × 106/well) in 24-well cell culture plates (Costar, Cambridge, Mass.) and cultured at 37°C in a 5% CO2 enriched atmosphere in duplicate for 24 h (for IL-2 or IL-4 determinations) or 72 h (for IFN-γ or IL-10 determinations) in the presence of purified Salmonella type d flagellin (25 μg/ml), ethanol-inactivated Salmonella or ETEC cells (107 bacteria/well), or purified CFA/I fimbriae (25 μg/ml). For some experiments, polymyxin B (40 μg/ml), an inhibitor of LPS activity, was added to the cultures together with the purified flagellin preparations in order to demonstrate the lack of LPS contamination. At the end of the incubation periods, the supernatants were removed and stored at −20°C for the determination of cytokine levels by ELISA.
Cytokine ELISAs.
The IFN-γ, IL-10, IL-2, and IL-4 levels were determined by using two-site sandwich ELISAs with duplicate splenocyte culture supernatants, as previously described (1). Detection limits for the cytokine ELISAs were 0.78 ng/ml for IFN-γ, 3.125 U/ml for IL-10, 0.156 ng/ml for IL-4, and 0.391 ng/ml for IL-2. The results from representative experiments are expressed as the arithmetic means of determinations from duplicate cultures ± SEM. Each experiment was performed independently at least three times.
T-cell population involved in cytokine responses elicited by flagellin.
The identification of CD4- or CD8-cell-activation-dependent IFN-γ and IL-10 production in spleen cell cultures from mice immunized with flagellated Salmonella strains was done with specific neutralizing monoclonal antibodies (MAbs) to mouse CD4 (MAb GK 1-5, isotype IgG2b) or CD8 (MAb TIB105, isotype IgG2a). Anti-CD4 and anti-CD8 MAbs, as well as a control anti-β-galactosidase MAb (GL 117, isotype IgG2a), were used at a final concentration of 20 μg/ml. Splenocyte cultures were prepared in RPMI medium, as described above, added to the MAbs, and incubated for a further 24 h (for IL-2 and IL-4) or 72 h (for IFN-γ and IL-10). Culture supernatants were harvested by centrifugation and kept frozen at −20°C until the determination of their cytokine contents.
Statistical analyses.
Results were analyzed by Student's t test. Statistical significance was defined as occurring when P values were <0.05.
RESULTS
Immunization with attenuated S. enterica serovar Dublin strains did not induce oral tolerance to flagellin.
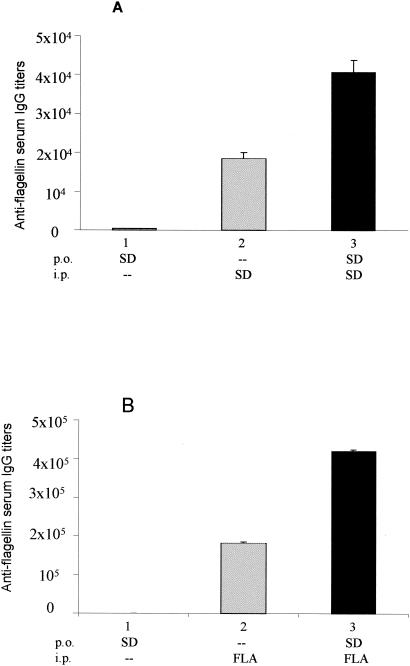
In contrast to the case for parenterally immunized mice, the administration of attenuated aroA S. enterica serovar Dublin strains to BALB/c or C57BL/6 mice via mucosal routes did not elicit significant serum (IgG) or secreted (IgA) antibody responses to type d flagellin, even with the stabilization of flagellin expression following integration of the fliC gene into the chromosome of the vaccine strain (36). One possible explanation for the reduced antiflagellin antibody responses elicited in mice immunized via mucosal routes with attenuated Salmonella strains could be the induction of an unresponsive immunological state, or tolerance, to specific bacterial antigens such as flagellin. To evaluate whether oral tolerance was the cause of the apparent low level of immunogenicity of Salmonella flagellin after delivery via mucosal routes, we first immunized BALB/c mice p.o. with three doses of the flagellated S. enterica serovar Dublin ICB5 strain and then immunized them i.p. with purified flagellin or live ICB5 cells. As shown in Fig. 1, control groups of mice that were only immunized i.p. with purified flagellin or live bacteria developed elevated systemic antiflagellin IgG titers (Fig. 1A, group 2), whereas mice that were only immunized p.o. with live Salmonella failed to develop serum antiflagellin IgG responses (Fig. 1, group 1). In a third group, mice that were previously immunized p.o. with the ICB5 strain generated robust anti-type d flagellin-specific IgG responses in serum after parenteral immunization with purified flagellin or with the ICB5 strain (Fig. 1, group 3). Similar results were obtained for the antiflagellin IgA responses in fecal extracts collected from the same mouse groups (data not shown), thus indicating that immunological tolerance does not contribute to the reduced immunogenicity of type d Salmonella flagellin as observed in mice immunized via the p.o. route with flagellated S. enterica serovar Dublin vaccine strains.
FIG. 1.
Evaluation of whether immunological tolerance to flagellin occurs in BALB/c mice immunized by the oral route with S. enterica serovar Dublin ICB5. Mouse groups 1 and 3 were immunized with three doses of 1010 CFU of the ICB5 strain on days 0, 21, and 35. Mouse group 2 was maintained as a control and was not immunized by the oral route. One week after the last immunizing dose, mice in groups 2 and 3 were injected i.p. with two doses of the ICB5 strain (SD) (107 CFU) (A) or two doses of 10 μg of purified flagellin (FLA) (B) separated by a 2-week interval (group 3). Antiflagellin (IgG) antibody responses in sera were measured 1 week after the last i.p. immunization. Five animals were used for each immunization group. Values are expressed as means of end point titers ± SEM for serum pools prepared from each mouse group.
Coadministration of a mucosal adjuvant (LTR192G) did not increase flagellin immunogenicity expressed by orally delivered S. enterica serovar Dublin strains.
LT and cholera toxin are the most potent and deeply studied mucosal adjuvants described thus far (44). LT enhances secreted and systemic antibody responses to coadministered soluble or particulate antigens, such as live Salmonella cells, delivered via the oral route (19). In an attempt to enhance flagellin immunogenicity relative to its ability to induce secreted and systemic antibody responses, we orally or nasally immunized BALB/c mice with three doses of live ICB5 strain coadministered with LTR192G, a nontoxic LT derivative with preserved immunomodulatory properties (11). None of the mouse groups that were coimmunized with LTR102G and strain ICB5 showed a significant enhancement of antiflagellin IgG in serum or of fecal or lung wash IgA responses (Table 1). The detection of high levels of serum LT-specific IgG and IgA in samples collected from the same mouse groups confirmed that the immunizations were correctly performed. These results suggest that, unlike the case for other antigens, the reduced immunogenicity of type d flagellin expressed by flagellated S. enterica serovar Dublin strains delivered via the oral route cannot be efficiently rescued by the incorporation of a mucosal adjuvant.
TABLE 1.
Adjuvant effects of LTR192G on the serum anti-flagellin IgG and secreted IgA responses in BALB/c mice immunized with attenuated S. enterica serovar Dublin ICB5 delivered via different routes
| Vaccine | Immunization routea | Antibody titerb
|
|||||
|---|---|---|---|---|---|---|---|
| Serum IgG
|
Fecal IgA
|
Lung wash IgA
|
|||||
| FLA | LT | FLA | LT | FLA | LT | ||
| ICB5 | i.p. | 32,180 ± 2,574 | ND | ND | ND | ND | ND |
| Oral | 50 ± 6 | <2 | <2 | <2 | ND | ND | |
| Nasal | 16 ± 2 | <2 | ND | <2 | 82 ± 9 | ND | |
| ICB5 plus LT | Oral | 51 ± 4 | 10,683 ± 961 | 3 ± 1 | 41 ± 4 | ND | ND |
| Nasal | 10 ± 2 | 2 × 106 ± 1.6 × 104 | ND | ND | 104 ± 12 | 8,000 ± 796 | |
Immunizations were carried out with three doses of 1010 CFU (p.o.) or 107 CFU (i.n. or i.p.) on days 0, 21, and 35. Serum, fecal, and lung wash samples were collected 1 week after the last immunization dose.
Titers were determined in ELISA plates treated with type d flagellin (FLA) or purified LTR192G (LT). Values are means of endpoint titers ± SEM of serum pools prepared from each mouse group. Data are based on two independent experiments. ND, not done.
Role of mammalian host and Salmonella strain on flagellin immunogenicity.
For an evaluation of the contribution of the genetic background of the murine host to the immunogenicity of Salmonella flagellin, mice belonging to seven isogenic strains, expressing different haplotypes (B10A [H2a], A/J [H2a], CL57BL/6 [H2b], BALB/c [H2d], DBA/2 [H2d], CBA/J [H2k], and C3H/HePas [H2k]) and carrying Ity (Nramp) alleles conferring susceptibility or resistance to Salmonella infection, were immunized with three doses of live S. enterica serovar Dublin ICB5 via the i.p. or p.o. route. Upon parenteral immunization with the ICB5 strain, flagellin was shown to be a strong immunogen to all tested mouse lineages, as measured by the specific IgG responses in sera, with reverse titers ranging from 2 × 105 (A/J) to 2.5 × 104 (C57BL/6) (Table 2). On the other hand, one priming dose followed by two oral boosting doses with the same Salmonella strain gave variable results. Most of the tested mouse strains expressed reduced or no systemic IgG responses to flagellin, but C3H/HePas mice developed significant antiflagellin serum IgG responses (reverse titer of 2,312 ± 221) (Table 2). Similar results were also found for the secreted antiflagellin IgA responses in feces of mice immunized with S. enterica serovar Dublin ICB5 (data not shown). All tested mouse strains developed anti-LPS IgG responses after immunization with strain ICB5 via either the i.p. or p.o. route (Table 2). Such evidence indicates that flagellin immunogenicity can be affected by the genetic composition of the vaccinated mouse strains. Since the C3H/HePas strain expresses the same haplotype (H2k) and Ityr allele as other mouse strains that failed to respond to flagellin, we conclude that type d flagellin immunogenicity following the delivery of attenuated S. enterica serovar Dublin strains via mucosal routes relies on the action of different genetic loci. The polygenic nature of the immune response to Salmonella type d flagellin in mice was further demonstrated by similar immunization experiments using outbred Biozzi mouse lines specifically selected for high (H) and low (L) levels of immunological responsiveness to Salmonella antigens (4, 37). The hyperresponsive outbred mouse H strain developed high-level systemic anti-type d flagellin IgG responses (reciprocal titer of 10,613 ± 1,230) after p.o. immunization with the ICB5 strain, while the hyporesponsive L strain developed meager anti-type d flagellin serum IgG responses (reciprocal titer of 56 ± 8) under the same conditions (Table 2). Similar results were found for fecal IgA responses to type d flagellin in the outbred Biozzi mice after oral immunization with the S. enterica serovar Dublin ICB5 vaccine strain (data not shown).
TABLE 2.
Genetic backgrounds of different mouse strains affect the serum (IgG) responses to flagellin and LPS after immunization with attenuated S. enterica serovar Dublin ICB5 delivered by the oral or i.p. route
| Mouse strain | Ity allelea | Haplotype | IgG antibody titer in serumb
|
|||
|---|---|---|---|---|---|---|
| Oral route
|
i.p. route
|
|||||
| LPS | FLA | LPS | FLA | |||
| C57BL/6 | S | H2b | 10,376 ± 1,350 | 345 ± 20 | 9,108 ± 978 | 25,053 ± 2,455 |
| BALB/c | S | H2d | 5,155 ± 495 | <2 | 40,930 ± 6,300 | 32,180 ± 2,574 |
| B10A | S | H2a | 3,638 ± 436 | <2 | 7,315 ± 790 | 13,977 ± 1,257 |
| CBA/J | R | H2k | 8,605 ± 798 | 160 ± 14 | 11,585 ± 1,056 | 150,132 ± 16,564 |
| DBA/2 | R | H2d | 4,770 ± 548 | <2 | 6,653 ± 678 | 152,568 ± 13,731 |
| A/J | R | H2a | 6,936 ± 716 | <2 | 14,655 ± 1,575 | 207,522 ± 20,863 |
| C3H/HePas | R | H2k | 33,147 ± 3,140 | 2,312 ± 221 | 54,064 ± 6,100 | 108,948 ± 12,560 |
| Biozi/High | R | Outbred | 42,675 ± 4,050 | 10,613 ± 1,230 | 24,976 ± 2,789 | 312,837 ± 25,890 |
| Biozi/Low | R | Outbred | 7,634 ± 852 | 56 ± 8 | 1,081 ± 124 | 78,591 ± 9,683 |
Allele of the Ity (Nramp) gene conferring resistance (R) or susceptibility (S) to Salmonella infection.
Immunizations were carried out with three doses of 1010 CFU (p.o.) or 107 CFU (i.n. or i.p.) on days 0, 21, and 35. Serum samples were collected 1 week after the last immunization dose. Titers were determined in ELISA plates treated with type d flagellin (FLA) or group D Salmonella LPS. Values are means of endpoint titers ± SEM of serum pools prepared from each mouse group. Data are based on two independent experiments.
To test the role of the Salmonella vaccine strains on the immunogenicity of flagellin, we immunized unresponsive BALB/c mouse groups via different routes with S. enterica serovar Typhimurium strain LM408, a pLS408-transformed derivative of the aroA S. enterica serovar Typhimurium SL3261 vaccine strain, which simultaneously expresses type d flagellin and type I (type i) and type II (FljB) flagellins, encoded by chromosomal genes of the strain. As shown in Table 3, mice immunized via the i.n. or p.o. route with S. enterica serovar Typhimurium LM408 were capable of developing high-level systemic and secreted antibody responses to S. enterica serovar Typhimurium flagellins, but not to the type d flagellin encoded by pLS408. Curiously, antibodies raised in mice immunized with S. enterica serovar Dublin SL5930 did not react with flagellins isolated from the S. enterica serovar Typhimurium SL3261 strain, which shows that, despite their significant amino acid similarities, the different Salmonella flagellin types do not share immunologically dominant B-cell epitopes. Irrespective of that observation, these results show that the immunogenicities of Salmonella flagellins in the murine model are affected by the genetic background of the flagellated vaccine strain delivered via mucosal routes.
TABLE 3.
Immunogenicity of flagellins expressed by different S. enterica serovar Dublin and S. enterica serovar Typhimurium strains, as measured by antiflagellin systemic (IgG) and secreted (IgA) antibody responses elicited in BALB/c mice immunized via the parenteral or mucosal route
| Bacterial strain | Immunization route | Antibody titera
|
|||||
|---|---|---|---|---|---|---|---|
| Serum IgG
|
Fecal sIgA
|
Lung wash sIgA
|
|||||
| FLA-SD | FLA-ST | FLA-SD | FLA-ST | FLA-SD | FLA-ST | ||
| S. enterica serovar Dublin SL5930 | i.p. | 24,454 ± 2,689 | <2 | ND | ND | ND | ND |
| Nasal | 64 ± 7 | <2 | ND | ND | 46 ± 5 | <2 | |
| Oral | 36 ± 4 | <2 | 15 ± 3 | <2 | ND | ND | |
| S. enterica serovar Typhimurium LM408 | i.p. | 20 ± 3 | 61,078 ± 7329 | ND | ND | ND | ND |
| Nasal | 30 ± 4 | 15,409 ± 1694 | ND | ND | <2 | 48 ± 5 | |
| Oral | 25 ± 2 | 6,051 ± 617 | 4 ± 1 | 64 ± 7 | ND | ND | |
Immunizations were carried out with three doses of 1010 CFU (p.o.) or 107 CFU (i.n. or i.p.) on days 0, 21, and 35. Serum, fecal, and lung wash samples were collected 1 week after the last immunization dose. Titers were determined in ELISA plates treated with type d flagellin isolated from strain ICB5 (FLA-SD) or with flagellins isolated from strain SL3261 (FLA-ST). Values are means of endpoint titers ± SEM of serum pools prepared from each mouse group. Data are representative of two experiments. ND, not done.
Colonization and invasive properties of S. enterica serovar Dublin and S. enterica serovar Typhimurium strains.
The distinct immunogenicities of Salmonella flagellins were not ascribed to different colonization and invasion abilities of the bacterial strains. The results presented in Table 4 indicate that the S. enterica serovar Dublin SL5930 strain was recovered from PP and spleens of inoculated BALB/c mice in higher numbers than the S. enterica serovar Typhimurium SL3261 strain after a single oral administration of the bacterial strains. Moreover, S. enterica serovar Dublin SL5930 was detected for up to 45 days in mouse tissues, while under our assay conditions, S. enterica serovar Typhimurium LM408 was detected for a maximum of 21 days (data not shown). Similar colonization (PP) and invasion (spleen) numbers were obtained with C3H/HePas mice, which were able to develop anti-type d flagellin antibody responses upon oral immunization with flagellated S. enterica serovar Dublin and S. enterica serovar Typhimurium strains (Table 4). Approximately 80% of the colonies recovered from PP and spleens of mice 7 days after inoculation with S. enterica serovar Dublin SL5930 were able to express flagellin, as measured by the presence of pLS408, while <10% of the colonies recovered from mice inoculated with S. enterica serovar Typhimurium LM408 maintained the plasmid (Table 4). Moreover, S. enterica serovar Dublin ICB5, which carries the fliC gene (type d) integrated into the chromosome, does not lose the ability to express flagellin after several passages in mouse tissues (data not shown). Taken together, these results indicate that the reduced B-cell responses to Salmonella flagellins are not due to an impaired ability of the Salmonella strains to infect and/or persist in host tissues. Moreover, the low level of immunogenicity of type d flagellin in the S. enterica serovar Dublin genetic background is not a consequence of the unstable nature of flagellin expression in this bacterial host.
TABLE 4.
Colonization and invasive properties of S. enterica serovar Dublin and S. enterica serovar Typhimurium vaccine strains and pL408 stability after oral administration to mice
| Bacterial straina | Mouse lineageb | Colonization (CFU/5 PP)/invasion (CFU/spleen)c | % flagellin expression4 |
|---|---|---|---|
| SL5930 | BALB/c | 3.5 × 103/1.1 × 102 | 83 |
| C3H/HePas | 1.1 × 103/9.5 × 101 | 79 | |
| LM408 | BALB/c | 4.1 × 102/8 × 101 | 5 |
| C3H/HePas | 9.1 × 102/2.5 × 101 | 7 |
The Salmonella strains were given as a single oral dose (1010 CFU).
The C3H/HePas and BALB/c mouse strains were chosen for their ability to develop antibody responses to type d flagellin after p.o. immunization with S. enterica serovar Dublin vaccine strains.
Colonization was measured as the number of colonies detected on Luria-novobiocin plates inoculated with five homogenized PP collected 7 days after oral inoculation of the animals with one of the tested Salmonella strains. Similarly, invasion was determined after plating of the spleen homogenates of inoculated mice. Numbers represent the total number of bacteria recovered from five PP (colonization) or the whole spleen (invasion) and are the means for three inoculated female mice.
Flagellin expression was measured indirectly by measuring pLS408 stability in the two Salmonella hosts. Colonies recovered from plates inoculated with spleen or PP homogenates were replica plated in ampicillin-containing LB agar plates. Values are percentages of ampicillin-resistant colonies compared to the total number of tested colonies.
Flagellin-specific cytokine responses elicited in mice orally immunized with S. enterica serovar Dublin vaccine strains.
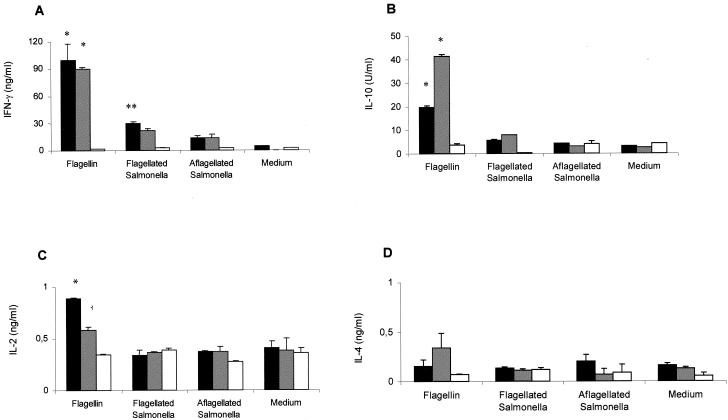
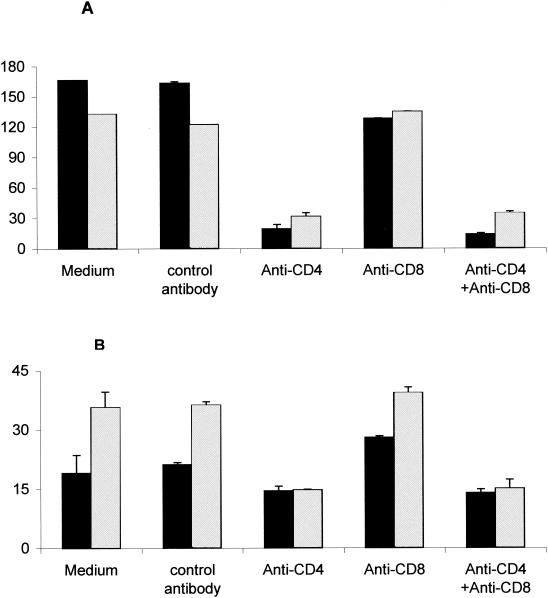
Previous reports have described the activation of flagellin-specific CD4+ T lymphocytes after oral administration of attenuated S. enterica serovar Typhimurium strains to BALB/c and C57BL/6 mice (9, 29). To determine whether attenuated S. enterica serovar Dublin ICB5 could also promote the activation of cell-mediated cytokine responses in vaccinated mice, we measured IFN-γ, IL-10, IL-2, and IL-4 production by splenocytes isolated from BALB/c mice immunized p.o. or i.p. with S. enterica serovar Dublin ICB5. As shown in Fig. 2, splenocytes isolated from BALB/c mice immunized via parenteral or mucosal routes with the S. enterica serovar Dublin ICB5 strain produced high levels of IFN-γ and IL-10 and moderate amounts of IL-2, but very little IL-4, when stimulated with purified type d flagellin. The amounts of IFN-γ production after flagellin stimulation were similar for the i.p. and p.o. immunized groups, whereas IL-10 levels were twofold higher in splenocyte cultures derived from i.p. immunized mice (Fig. 2). Similar results were observed for cultures treated with polymyxin B, confirming that the cytokine responses to flagellin preparations were not due to residual LPS contamination (data not shown). When inactivated bacteria were used as an antigenic stimulus, IFN-γ production, but not that of IL-10 or IL-2, was significantly enhanced in splenocyte cultures stimulated with flagellated Salmonella compared to the levels obtained in cultures stimulated with nonflagellated bacteria (Fig. 2). Identical results were observed in assays performed in the presence of polymyxin B to avoid possible splenocyte stimulation by LPS contamination in the flagellin preparations (data not shown). In order to determine the T-lymphocyte population involved in the activation of IFN-γ production as a recall response to flagellin, we treated splenocyte cultures with MAbs that block either CD4- or CD8-dependent T-cell activation. The addition of an anti-CD4 MAb (GK 1-5) to the splenocyte cultures suppressed IFN-γ production about 90%, while an anti-CD8 MAb (TIB105) did not modify the IFN-γ production pattern (Fig. 3A). Regarding IL-10 production, the results depicted in Fig. 3B show that the GK1-5 MAb treatment reduced IL-10 levels about 50%, whereas the TIB105 MAb did not have an effect. Therefore, IFN-γ production in ICB5-immunized mice was primarily CD4-cell-activation dependent, whereas 50% of the IL-10 production was independent of CD4 cell activation. Taken together, these results indicate that, in spite of the reduced antibody responses, mice immunized via the oral route with S. enterica serovar Dublin ICB5 develop strong flagellin-specific CD4-dependent IFN-γ and IL-10 responses.
FIG. 2.
Flagellin-specific cytokine responses elicited in BALB/c mice immunized with three doses of S. enterica serovar Dublin ICB5 via different administration routes. IFN-γ (A), IL-10 (B), IL-2 (C) and IL-4 (D) production was measured in supernatants of splenocyte cultures kept in RPMI medium or stimulated with inactivated nonflagellated (SL5928) or flagellated (ICB5) bacteria at 107 cells/ml or with purified type d flagellin (25 μg/ml). Mice were immunized via the p.o. (black bars) or i.p. (gray bars) route or were not immunized (white bars). Data from a representative experiment are expressed as means ± SEM of duplicate cultures. *, significant differences (P < 0.05) between immunized and nonimmunized mouse groups; **, significant differences (P < 0.05) between stimulation levels with flagellated and nonflagellated Salmonella.
FIG. 3.
CD4 cell activation dependence of flagellin-specific IFN-γ and IL-10 production in BALB/c mice immunized with three doses of S. enterica serovar Dublin ICB5 via the oral route. IFN-γ (A) and IL-10 (B) were measured in supernatants from splenocyte cultures stimulated with purified flagellin (25 μg/ml), and an anti-CD4 MAb (GK 1.5), an anti-CD8 MAb (TIB105), or both were added at the beginning of the culture. Controls were splenocyte cultures without an added antibody (medium) and cultures treated with an anti-β-galactosidase MAb (GL 117) (control antibody). Mice were immunized via the p.o. (black bars) or i.p. (gray bars) route. Data from a representative experiment are expressed as means ± SEM of duplicate cultures.
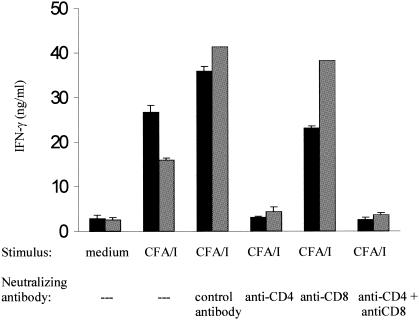
Salmonella flagellin has been intensively used as a carrier of heterologous B-cell epitopes, either as a purified protein in acellular formulations or as a particular antigen expressed by attenuated vaccine strains. The present results indicate that the production of IFN-γ and IL-10 prevails as the immune response elicited against flagellin in mice orally immunized with S. enterica serovar Dublin strains and suggest that similar immunological responses could be triggered by heterologous T-cell epitopes genetically fused to flagellin. To test this hypothesis, we immunized BALB/c mice with S. enterica serovar Dublin FLA II, which expresses a hybrid flagellin genetically fused to a 15-amino-acid T-cell epitope derived from the structural subunit of the CFA/I fimbriae produced by some ETEC strains (26). As shown in Fig. 4, splenocytes harvested from mice immunized with S. enterica serovar Dublin FLA II delivered i.p. or p.o. produced significant IFN-γ levels in cultures stimulated with purified CFA/I fimbriae. Similar to the results obtained with flagellin, IFN-γ production secondary to in vitro stimulation with CFA/I fimbriae was primarily CD4-cell-activation dependent since the addition of an anti-CD4 MAb almost completely suppressed it, whereas no significant suppression was detected in cultures treated with an anti-CD8 MAb. These results indicate that CD4-cell-activation-dependent IFN-γ production after exposure to flagellin and fused heterologous T-cell epitopes represents a major immune response elicited in mice immunized via the oral route with flagellated S. enterica serovar Dublin vaccine strains.
FIG. 4.
Determination of CFA/I-induced IFN-γ production and CD4 cell activation dependence in mice immunized with S. enterica serovar Dublin FlaII. IFN-γ production was measured in supernatants of splenocyte cultures derived from BALB/c mice immunized via the i.p. (gray bars) or p.o. (black bars) route with S. enterica serovar Dublin FlaII. Splenocyte cultures were kept in RPMI medium or stimulated with 25 μg of purified CFA/I fimbriae/ml. Cultures were further treated with neutralizing MAb GK 1.5 (anti-CD4), TIB105 (anti-CD8), or both (anti-CD4 + anti-CD8). Control cultures were treated with the anti-β-galactosidase GL 117 MAb (control antibody). Data from a representative experiment are expressed as means ± SEM of duplicate cultures.
DISCUSSION
Flagellin represents one of the most active bacterial immunogens described thus far, both for innate and adaptive mammalian immune systems (6-8, 12, 13, 17, 18, 21, 46). Reports on the reduced systemic and secreted antibody-inducing properties of Salmonella flagellin following mucosal administration of flagellated attenuated S. enterica serovar Dublin strains to isogenic mice were thus intriguing (10, 36). In the present report, we have shown that the ability of mice to elicit secreted and systemic antibody responses to flagellin after mucosal administration of attenuated Salmonella strains is affected by both host and bacterial factors. Moreover, we showed that, in contrast to the humoral responses elicited by flagellin and heterologous B-cell epitopes genetically fused to it, mice orally immunized with attenuated S. enterica serovar Dublin strains expressing a hybrid type d flagellin genetically fused with a T-cell epitope derived from the CFA/I fimbriae of ETEC developed a strong induction of CD4-dependent IFN-γ and IL-10 production against both flagellin and the heterologous epitope. Although at the moment we cannot explain the genetic and molecular bases of specific mechanisms involved in the control of B-cell immunogenicity of Salmonella flagellin in the murine model, the present work has unveiled so far unnoticed complex host-parasite relationships affecting the development of specific immune responses to flagellin expressed by vaccine strains after the oral administration of live bacterial cells. Therefore, these results have important implications for the understanding of Salmonella pathogenicity and the development of vaccines based on flagellin as a carrier protein of heterologous epitopes.
Orally administered vaccines have numerous advantages over parenterally delivered formulations. Nonetheless, the development of a T-cell tolerance state, measured as unresponsiveness to the antigen delivered via parenteral routes in animals previously immunized with the same antigen via the oral route, usually restricts the development of effective mucosal vaccines (43). In fact, recent results indicated that a previous exposure of cultured human monocytes to purified flagellin reduces the activation of the innate immune response to a subsequent exposure to the same antigen due to a stabilization of binding between the flagellin receptor TLR-5 and the interleukin-1 receptor-associated kinase (30). The reduced flagellin-specific antibody responses elicited in mice immunized via mucosal routes with live Salmonella strains could therefore reflect continuous exposure to flagellin due to the maintenance of Salmonella cells in host tissues favoring the establishment of immunological tolerance. Nonetheless, our experiments showed that oral administration of live S. enterica serovar Dublin strains did not reduce the immunogenicity of parenterally delivered flagellin, either as a purified protein or in flagellated Salmonella cells, thus indicating that the tolerogenic state was not established in the immunized animals. The recent finding that the S. enterica serovar Enteritidis flagellin can induce efficient systemic and secreted antibody responses when incorporated into biodegradable microparticles administered orally to mice further demonstrates that the Salmonella flagellin by itself does not lead to oral tolerance (40). Taken together, the available evidence indicates that the low antibody levels induced by orally delivered type d flagellin are not due to a tolerogenic state to this protein, but rather may reflect the activation of an immunological response pattern which would favor immune responses other than the production of systemic and secreted antibodies.
The discovery of mucosal adjuvants, such as the cholera or heat-labile toxins, has led to a renewed interest in the development of effective oral vaccines based either on acellular formulations or whole live or inactivated microorganisms (11, 19, 44). In the present work, the incorporation of LTR192G, an LT derivative with reduced toxic effects but preserved adjuvant effects, did not enhance type d flagellin immunogenicity with regard to the production of systemic and secreted antibody responses in mice immunized via mucosal routes with flagellated S. enterica serovar Dublin strains. Such a finding further supports the assumption that low-level antibody responses after flagellin delivery by mucosal routes are not a consequence of low antigen loads or an inefficient activation of mucosa-associated lymphoid tissues but rather reflect an intrinsic characteristic of the immune response by the murine host. These results also emphasize that, in contrast to the case for other antigens, coadministration of a mucosal adjuvant most probably would not overcome the reduced immunogenicity of recombinant flagellins genetically fused to heterologous B epitopes expressed by orally delivered bivalent S. enterica serovar Dublin vaccine strains.
Previous evidence indicated that the genetic background of the murine host can modulate the immunological responsiveness to heterologous antigens expressed by parenterally delivered Salmonella vaccine strains (15). In an attempt to evaluate the role of the genetic composition of the murine host on the immunogenicity of type d flagellin expressed by attenuated S. enterica serovar Dublin strains delivered via mucosal routes, we tested seven mouse strains expressing four different haplotypes and harboring alleles of the Ity (Nramp) gene conferring resistance or sensitivity to Salmonella infection. Our results confirmed the role of the host genetic background on the immunogenicity of flagellin delivered by attenuated Salmonella strains. Our data indicate that the ability to mount secreted and systemic antibody responses to the Salmonella type d flagellin after the oral delivery of attenuated strains is not related to specific H2 mouse haplotypes or the presence of Ity alleles, but rather reflects the interplay of genes located outside the H2 complex. An additional piece of evidence of the polygenic nature of antibody responsiveness to Salmonella flagellin in the murine model came from experiments using selection III Biozzi mice, which are outbred lines genetically selected for high- and low-level antibody responsiveness to the Salmonella flagellar antigen (4, 37). Originally derived from Swiss albino mice, such mouse lines are considered homozygous for the relevant alleles involved with the generation of antibody responses, which are considered to comprise 4 to 10 independently segregating loci responsible for the interline differences (35). As expected, high- and low-level responders showed distinct immunological behaviors toward type d flagellin after the administration of attenuated S. enterica serovar Dublin strains via mucosal and parenteral routes. Although the genetic basis of the distinct antibody responsiveness of the two Biozzi strains is still unknown, previous evidence indicates that the T-cell proliferative responses and altered T-helper functions, leading to different types of regulation of T-helper subsets under antigenic stimulation, could be responsible for the interline difference in antibody responsiveness to flagellin (34). These data also stress the importance of a careful interpretation of results based on the use of murine models for the evaluation of vaccine formulations based on attenuated Salmonella strains administered via different inoculation routes.
The various Salmonella serological flagellin types, although sharing significant amino acid homologies, have specific sequence domains that are able to elicit non-cross-reacting antibodies, which are the basis of the immunological typing of numerous Salmonella serovars (22). Indeed, antibodies raised in mice immunized i.p. with type d or type i (or FljB) flagellin did not show a significant cross-reaction with each other, further supporting the notion that the immunodominant B-cell epitopes of Salmonella flagellins lie at the hypervariable central domain of the protein. The detection of secreted and systemic antiflagellin antibody responses in BALB/c mice orally immunized with a derivative of the S. enterica serovar Typhimurium SL3261 strain showed that bacterium-associated factors can also affect flagellin immunogenicity in the mouse model. Attempts to evaluate the role of the bacterial host on the immunogenicity of type d flagellin were not conclusive due to the unstable behavior of pLS408 in S. enterica serovar Typhimurium. However, our results clearly demonstrate that the distinct immunogenicities of type d, type i, and FljB flagellins do not correlate with the colonization and invasive abilities of the Salmonella serovars. Further investigations of the differential immunogenicities of the different Salmonella flagellin types and the role of the bacterial host on the antibody responses elicited in mice immunized with different vaccine strains are currently under way.
BALB/c mice immunized p.o. with S. enterica serovar Dublin strains had enhanced CD4-cell-activation-dependent IFN-γ and IL-10 production upon stimulation with purified flagellin or flagellated bacterial cells. Previous work demonstrated that flagellin represents a major antigen that stimulates murine CD4+-T-cell clones after p.o. immunization with S. enterica serovar Typhimurium SL3261 (9, 29). Flagellin is also known to be an important antigen for the generation of protective responses in mice orally immunized with attenuated Salmonella strains (20, 29). Indeed, the induction of IL-2 production has also been detected against a T-cell epitope inserted at the hypervariable region of Salmonella flagellin delivered parenterally to mice (42). These results further support the hypothesis that antiflagellin cell-mediated immune responses prevail in most mouse lineages exposed to attenuated S. enterica serovar Dublin strains via the intestinal mucosa. The observation that orally delivered S. enterica serovar Dublin strains can elicit strong CD4-cell-activation-dependent IFN-γ responses to type d flagellin or genetically fused heterologous epitopes suggests that T-cell epitopes derived from antigens of pathogens requiring cell-dependent protective responses would be particularly interesting to test by the Salmonella-flagellin bivalent vaccine approach.
Our results showed that the predominant antiflagellin response in BALB/c mice immunized with S. enterica serovar Dublin vaccine strains via the p.o. route is the activation of IFN-γ production, which may lead to macrophage activation, and not a B-cell-mediated antibody response. However, an intense antibody response to the T-cell-independent antigen LPS was observed in these vaccinated mice, indicating that the B-cell compartment is also activated by this oral immunization protocol. Further experiments to evaluate the protective immune responses leading to protection against Salmonella challenges in the murine model are necessary and will contribute to the elucidation of the mechanisms involved in the protection of animals immunized via the natural port of entry of the pathogen.
During the last three decades, the remarkable immunological properties of bacterial flagellins have contributed to the understanding of different aspects of mammalian innate and adaptive immune systems. Some of the evidence reported in the present work will contribute to the elucidation of new aspects of bacterial flagellins as immune system modulators and will contribute to a more rational use of Salmonella flagella as carriers of heterologous epitopes in bivalent vaccine approaches.
Acknowledgments
This work was supported by grants supplied by Fundação de Amparo à Pesquisa do Estado de São Paulo. T. Mosca was part of the undergraduate scientific training program from CNPq and FAPESP.
We thankfully acknowledge K. Higuchi, M. N. de Lima, and A. T. Barbosa for technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immunol. 155:3955-3963. [PubMed] [Google Scholar]
- 2.Ben-Yedidia, T., H. Marcus, Y. Reisner, and R. Arnon. 1999. Intranasal administration of peptide vaccine protects human/mouse radiation chimera from influenza infection. Intern. Immunol. 11:1043-1051. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Yedidia, T., R. Tarrab-Hazdai, D. Schechtman, and R. Arnon. 1999. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect. Immun. 67:4360-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biozzi, G., D. Mouton, O. A. Sant'Anna, H. C. Passos, M. Gennari, M. H. Reis, V. C. A. Ferreira, A. M. Heumann, Y. Bouthillier, O. M. Ibanez, C. Stiffel, and M. Siqueira. 1979. Genetics of immunoresponsiveness to natural antigens in the mouse. Curr. Top. Microbiol. Immunol. 85:31-98. [PubMed] [Google Scholar]
- 5.Brey, R. N., G. S. Bixler, J. P. Fulginiti, D. A. Dilts, and M. I. J. Sabara. 1991. Oral delivery of antigens in live bacterial vectors, p. 169-184. In M. Z. Atassi (ed.), Immunology of proteins and peptide, vol. VI. Plenum Press, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 6.Ciacci-Woolwine, F., I. C. Blonfield, S. H. Richardson, and S. B. Mizel. 1998. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect. Immun. 66:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciacci-Woolwine, F., P. F. McDermott, and S. B. Mizel. 1999. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect. Immun. 67:5176-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciacci-Woolwine, F., L. S. Kucera, S. H. Richardson, N. P. Iyer, and S. B. Mizel. 1997. Salmonella activates tumor necrosis factor alpha production in a human promonocytic cell line via a released polypeptide. Infect. Immun. 68:4624-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cookson, B. T., and M. J. Bevan. 1997. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158:4310-4319. [PubMed] [Google Scholar]
- 10.De Almeida, M. E. S., S. M. C. Newton, and L. C. S. Ferreira. 1999. Antibody responses against flagellin in mice orally immunized with attenuated Salmonella vaccine strains. Arch. Microbiol. 172:102-108. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virág, G. Ross, F. G. Soriano, C. Szabó, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation of systemic inflammation: IkBa degradation, induction of nitric oxide synthase, induction of proinflamatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 13.Eaves-Pyles, T., H. R. Wong, K. Odoms, and R. B. Pyles. 2001. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxy regions of the protein. J. Immunol. 167:7009-7016. [DOI] [PubMed] [Google Scholar]
- 14.Evans, D. G., D. J. Evans, Jr., S. Clegg, and J. A. Pauley. 1979. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect. Immun. 25:738-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayolle, C., D. O'Callaghan, P. Martineau, A. Charbit, J. M. Clement, M. Hofnung, and C. Leclerc. 1994. Genetic control of antibody responses induced against an antigen delivered by recombinant attenuated Salmonella strain. Infect. Immun. 62:4310-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastão da Silva, A. P., J. F. Jacysyn, and I. A. Abrahamsohn. 2003. Resistant mice lacking interleukin-12 become susceptible to Trypanosoma cruzi infection but fail to mount a T helper type 2 response. Immunology 108:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz, A. T., P. O. Simon, C. K. Schimitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillobel, H. C., J. I. Carinhanha, L. Cárdenas, J. D. Clements, D. F. Almeida, and L. C. S. Ferreira. 2000. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica serovar Typhimurium vaccine strain. Infect. Immun. 68:4349-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett, J., S. Attridge, and D. Rowley. 1988. Oral immunization with live, avirulent fla+ strains of Salmonella protects mice against subsequent oral challenge with Salmonella typhimurium. J. Infect. Dis. 157:78-84. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Undehill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 22.He, X. S., M. Rivkina, B. A. D. Stocker, and W. S. Robinson. 1994. Hypervariable region IV of Salmonella gene fliCd encodes a dominant surface epitope and a stabilizing factor for functional flagella. J. Bacteriol. 176:2406-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim, G. F., G. H. Fleet, M. J. Lyons, and R. A. Walker. 1985. Method for the isolation of highly purified Salmonella flagellins. J. Clin. Microbiol. 22:1040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liaudet, L., K. G. K. Murthy, J. G. Mabley, P. Pacher, F. G. Soriano, A. L. Salzman, and C. Szabó. 2002. Comparison of inflammation, organ damage, and oxidant stress induced by Salmonella enterica serovar Muenchen flagellin and serovar Enteritidis lipopolysaccharide. Infect. Immun. 70:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Vidal, Y., P. Kelmm, and A. M. Svennerholm. 1988. Monoclonal antibodies against different epitopes on colonization factor antigen I of enterotoxin-producing Escherichia coli. J. Clin. Microbiol. 26:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luna, M. G., L. C. S. Ferreira, D. F. Almeida, and A. Rudin. 1997. Peptides 14VIDLL18 and 96FEAAAL101 defined as epitopes of antibodies raised against amino acid sequences of enterotoxigenic Escherichia coli colonization factor antigen I fused to Salmonella flagellin. Microbiology 143:3201-3207. [DOI] [PubMed] [Google Scholar]
- 27.Luna, M. G., M. M. Martins, S. M. C. Newton, S. O. P. Costa, D. F. Almeida, and L. C. S. Ferreira. 1997. Cloning and expression of colonization factor antigen I (CFA/I) epitopes of enterotoxigenic Escherichia coli (ETEC) in Salmonella flagellin. Res. Microbiol. 148:217-228. [DOI] [PubMed] [Google Scholar]
- 28.McEwen, J., R. Levi, R. J. Horwitz, and R. Arnon. 1992. Synthetic recombinant vaccine expressing haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine 10:405-411. [DOI] [PubMed] [Google Scholar]
- 29.McSorley, S. J., B. T. Cookson, and M. K. Jenkins. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 164:986-993. [DOI] [PubMed] [Google Scholar]
- 30.Mizel, S. B., and J. A. Snipes. 2002. Gram-negative flagellin-induced self tolerance is associated with a block in interleukin-1 receptor-associated kinase release from Toll-like receptor 5. J. Biol. Chem. 277:22414-22420. [DOI] [PubMed] [Google Scholar]
- 31.Newton, S. M. C., C. O. Jacob, and B. A. D. Stocker. 1989. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 244:70-72. [DOI] [PubMed] [Google Scholar]
- 32.Newton, S. M. C., M. Kotb, T. P. Poirier, B. A. D. Stocker, and E. H. Beachey. 1991. Expression and immunogenicity of a streptococcal M protein epitope inserted in Salmonella flagellin. Infect. Immun. 59:2158-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton, S. M. C., T. M. Joys, S. A. Anderson, R. C. Kennedy, M. Hovi, and B. A. D. Stocker. 1995. Expression and immunogenicity of a 18-residue epitope of HIV-gp41 as insert in the flagellar protein of a Salmonella live vaccine. Res. Microbiol. 146:203-216. [DOI] [PubMed] [Google Scholar]
- 34.Reis, M. H., O. M. Ibanez, W. H. Cabrera, O. G. Ribeiro, D. Mouton, M. Siqueira, and J. Couderc. 1992. T-helper functions in lines of mice selected for high and low antibody production (selection III): modulation by anti-CD4+ monoclonal antibody. Immunology 75:80-85. [PMC free article] [PubMed] [Google Scholar]
- 35.Sant'Anna, O. A., V. C. A. Ferreira, M. H. Reis, M. Gennari, O. M. Ibanez, M. B. Esteves, D. Mouton, and G. Biozzi. 1982. Genetic parameters of the polygenic regulation of Ab responsiveness to flagellar and somatic Ag of Salmonella. J. Immunogenet. 9:191-201. [DOI] [PubMed] [Google Scholar]
- 36.Sbrogio-Almeida, M. E., and L. C. S. Ferreira. 2001. Flagellin expressed by live Salmonella vaccine strains induces distinct antibody responses following delivery via systemic or mucosal immunization routes. FEMS Immunol. Med. Microbiol. 30:203-208. [DOI] [PubMed] [Google Scholar]
- 37.Siqueira, M., A. Bandieri, M. H. Resi, O. A. Sant'Anna, and G. Biozzi. 1976. Selective breeding of mice for antibody responsiveness to flagellar and somatic antigens of Salmonellae. Eur. J. Immunol. 6:241-248. [DOI] [PubMed] [Google Scholar]
- 38.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, A. J. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli express a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stocker, B. A. D. 1990. Aromatic-dependent Slamonella as live vaccine presenters of foreign epitopes as inserts in flagellin. Res. Microbiol. 141:787-796. [DOI] [PubMed] [Google Scholar]
- 40.Strindelius, L., L. D. Wikingsson, and I. Sjöholm. 2002. Extracellular antigens from Salmonella enterica serovar Enteritidis induce effective immune response in mice after oral vaccination. Infect. Immun. 70:1434-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sztein, M. B., S. S. Wasserman, C. O. Tacket, R. Edelman, D. Hone, A. A. Lindberg, and M. M. Levine. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J. Infect. Dis. 170:1508-1517. [DOI] [PubMed] [Google Scholar]
- 42.Verma, N. K., H. K. Ziegler, B. A. D. Stocker, and G. K. Schoolnik. 1995. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. Vaccine 13:235-244. [DOI] [PubMed] [Google Scholar]
- 43.Weiner, H. L. 2001. Oral tolerance: immune mechanisms and the generation of TH3-type TGF-beta secreting regulatory cells. Microb. Infect. 3:947-954. [DOI] [PubMed] [Google Scholar]
- 44.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 45.Wu, J. Y., S. Newton, A. Judd, B. Stocker, and W. S. Robbinson. 1989. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc. Natl. Acad. Sci. USA 86:4726-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyant, T. L., M. K. Tanner, and M. B. Sztein. 1999. Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect. Immun. 67:1338-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]