Abstract
The purpose of the present study was to examine the impact of drug-paired cues on methamphetamine reinstatement. Three groups of rats were trained to self-administer 0.1 mg/kg/infusion methamphetamine. Each methamphetamine infusion was accompanied by a 6 s flashing light + tone stimulus (cues). After training, the groups were then given 12, daily extinction sessions either with or without response-contingent drug-paired cues and then tested for 1 mg/kg i.p. methamphetamine priming-induced reinstatement either with or without cues. Methamphetamine priming significantly reinstated drug-appropriate responding regardless of whether response-contingent cues were omitted during both extinction and testing, presented during both extinction and testing, or omitted during extinction but presented during reinstatement testing. The group in which cues were omitted during extinction and presented during reinstatement exhibited significantly greater reinstatement than did the other two groups. A separate group of rats was also tested demonstrating that response-contingent presentation of previously methamphetamine-paired cues alone, without methamphetamine priming, significantly reinstated drug-appropriate responding. These data show that methamphetamine priming produces a robust reinstatement effect which can be influenced by drug-paired cues.
Keywords: methamphetamine, reinstatement, rat, self-administration, relapse, contingencies, animal model, stimulus, priming
Introduction
A high percentage of detoxified drug abusers will relapse to drug use one or more times before maintaining sustained abstinence (Haynes 1998, Hunt et al. 1971). There could be any number of retrospective reasons given by individual drug abusers for relapse but the experimental literature, both human and animal, has focused on three main factors. These include exposure to a single dose of the previously self-administered drug or a related drug (priming), exposure to environmental stimuli which have been paired with drug use (cues) and stressors [for review see (Epstein and Preston 2003, Epstein et al. 2006, Shaham et al. 2002)]. It is probable in relapsing humans that stressful events or stimuli which have previously been paired with drug could be encountered without exposure to the drug itself. However, except in a carefully controlled laboratory environment, it is unlikely that exposure to the previously self-administered drug could occur in the absence of co-exposure to drug cues. For this reason pre-clinical studies which attempt to understand the mechanisms responsible for relapse and to develop effective treatments would likely benefit from a more complete understanding of the interaction between drug-induced and cue-induced relapse.
The most widely used preclinical model of relapse in humans is the drug reinstatement procedure (de Wit and Stewart 1981, 1983, Stewart 1983). In drug reinstatement studies animals are trained to self-administer a drug and then subjected to a period of extinction during which drug is withheld and responding diminishes over time. After responding has decreased, renewed responding can be evoked or reinstated by presentation of non-contingent administration of the previously self-administered drug, presentation of exteroceptive cues which have previously been paired with drug infusions or experimental stressors (Shaham et al. 2002). While there is considerable disagreement in the field if the reinstatement procedure accurately reflects the same processes as drug craving and relapse in humans [for review see (Epstein et al. 2006)] there is evidence that the procedure may mimic at least some aspects of these phenomena (Koob 2000, Meil and See 1996, Shaham et al. 2002).
With a few notable exceptions (Feltenstein and See 2006, Liu and Weiss 2002, Shelton and Beardsley 2005, Shelton et al. 2004), reinstatement studies in animals have focused on drug-, cue- or stress-induced reinstatement in isolation. This tactic is without doubt valid and important, but it would also seem relevant to examine these events in combinations which are likely to occur in the natural environment. Those studies which have been published have shown that reinstatement resulting from combining different classes of reinstating stimuli is often more robust than that produced by any one reinstating event. For instance, cocaine-paired stimuli which predict drug availability under second-order schedules of cocaine self-administration will augment priming-induced and cue-induced-reinstatement (Di Pietro et al. 2006, Kantak et al. 2002a, b). However, explicit training of these drug-paired cues as predictors of drug availability are not necessary for them to enhance both drug-induced reinstatement and experimental stressor-induced reinstatement (Di Ciano et al. 2001, Feltenstein and See 2006, Shelton et al. 2004, Spealman et al. 1999).
The majority of the reinstatement literature to date has focused on cocaine, opioids and ethanol. Considerably less attention has been devoted to methamphetamine, despite data suggesting that lifetime use rates are greater than those for a number of other abused drugs including heroin and crack cocaine (SAMHSA 2006). The methamphetamine reinstatement studies which have been published have produced results consistent with those generated using other drugs of abuse. For instance, non-contingent priming injections of methamphetamine will reinstate methamphetamine-appropriate responding following extinction (Anggadiredja et al. 2004a, Hiranita et al. 2004, Kruzich and Xi 2006, Yan et al. 2006a). Presentation of cues previously associated with methamphetamine self-administration results in renewed drug-appropriate responding (Anggadiredja et al. 2004b, Moffett and Goeders 2007, Yan et al. 2006a). Likewise experimental stressors, such as the administration of the anxiogenic drug yohimbine, will also reinstate extinguished methamphetamine lever-pressing behavior (Shepard et al. 2004). As of yet, no studies have yet explored the interaction between different classes of reinstating stimuli involved in methamphetamine reinstatement.
The major goal of the present study was to extend to methamphetamine reinstatement our prior findings with cocaine which showed that extinguish drug-paired cues facillitated priming-induced reinstatement.. We also wished to determine to what extent presentation of unextinguished response-contingent methamphetamine-paired cues would enhance methamphetamine-priming induced reinstatement. To achieve these goals four different reinstatement conditions were examined. One group of rats was tested for the ability of methamphetamine priming alone to produce reinstatement in the absence of drug-paired cues. A second group examined reinstatement produced by response-contingent cues which had previously been paired with methamphetamine infusions during self-administration. A third group examined reinstatement resulting from a combination of methamphetamine priming and drug-paired cues. A fourth and final condition examined if methamphetamine-paired cues which had been explicitly extinguished had any effect on subsequent methamphetamine-priming reinstatement.
Methods
Subjects
Subjects were 58 adult, male, experimentally naïve Long-Evans rats (Harlan Sprague-Dawley, USA) rats. The rats had continuous access to water except during the experimental sessions and were maintained at a body weight of 320 g for the duration of the study. The animals were individually housed in standard plastic rodent cages in a temperature-controlled (22° C) 12-h reversed light/dark cycle (lights off 7:00 AM) colony room. All training and testing was conducted during the dark portion of the cycle. Studies were approved by the Institutional Animal Care and Use Committee of VCU and conformed with NIH Guidelines for Care and Use of Laboratory Animals (1996).
Surgical procedure
Rats were anesthetized with a combination of 50 mg/kg s.c. ketamine and 8.7 mg/kg i.p. xylazine. A tapered catheter constructed from 3.5 French polyurethane tubing (Access Technologies, USA) was then implanted into each rat’s right jugular vein. The distal end of the catheter was passed subcutaneously to a cannula connector pedestal (Plastics One, USA) implanted subcutaneously in the mid-scapular region. The catheters were flushed with 0.2 ml heparinized normal saline before each experimental session. Following each self-administration session catheters were filled with 0.1 ml of a 50% glycerol/50% sterile saline solution to which was added 500 units/ml heparin, 250 mg/ml ticarcillin and 9 mg/ml clavulanic acid (Timentin, SmthKline Beacham, USA) to help maintain patency. Rats were permitted a minimum of five days of post-operative recovery before beginning self-administration training. If a catheter failed during methamphetamine self-administration training, it was removed, the left jugular vein was catheterized and the animal was returned to the study after a minimum of 5 days of surgical recovery.
Drugs
Methamphetamine HCL (Sigma-Aldrich, USA) was diluted in heparinized (5 units/ml) sterile saline for the intravenous self-administration solution. Methamphetamine for i.p. priming was diluted in sterile saline to a volume of 1 ml/kg of body weight.
Apparatus
Experiments were conducted in operant conditioning chambers housed inside individually-isolated and ventilated enclosures (Med Associates, USA). The front wall of each chamber was equipped with two retractable response levers with a white LED stimulus light above each lever. A 5-watt house light was located in the rear wall of the chamber and an adjustable Sonalert (Model ENV-223AM, Med Associates, USA) in the upper left wall of the chamber. The output of the sonalerts were equalized at 60dB using a Realistic model 33–2050 analog sound level meter placed in the bottom of the cage with the microphone equidistant between the two levers. During each session, infusion tubing, protected by a stainless steel spring tether (Plastics One, USA), connected the back-mounted pedestal implanted in each rat to a balanced liquid swivel suspended above each chamber (Lomir Biomedical, Canada). Infusions were delivered by a syringe pump located outside each chamber (Med Associates, USA). Schedule parameters were controlled by MED-PC IV software (Med Associates, USA) running on IBM PC compatible computers.
Training, Extinction and Testing
Methamphetamine self-administration training sessions were conducted five days per week (M– F) for two hours daily. Each response (fixed ratio 1) on the active lever resulted in delivery of a 0.1 mg/kg methamphetamine infusion (0.2 ml/6 s). At the onset of each infusion the houselight was extinguished, a 2900 Hz, 60 dB tone sounded and the stimulus lights above both levers flashed at 3 Hz (T+L cues) for 6 sec which was then followed by a 14 sec time-out during which the chambers were darkened. Active-lever responses during this 20 sec period as well as all inactive lever responses were recorded, but had no scheduled consequences. Rats were eligible for extinction and reinstatement testing only after they had received at least 12 self-administration training sessions, 125 total lifetime methamphetamine infusions and had received 15 or more drug infusions during each of the last four self-administration sessions.
Two hour extinction sessions were then conduced daily, 7 days/week, for 12 consecutive days. Thirty minutes prior to each of the last 4 extinction sessions each rat received a 1 ml/kg i.p. saline injection to habituate them to the injection process. The animals were then placed into the self-administration chambers as during methamphetamine self-administration training, but no infusions were given following each FR1 completion. One group of 22 rats (Ext Cues+/Test Cues+) received the 6 s tone + light stimulus and 14 sec time out (Cues+) after each completed FR1 during extinction. Responding had no scheduled consequences for rats in the, Ext Cues−/Test Cues+ (n=11), Ext Cues−/Test Cues− (n=13) and Cues+ Reinstatement (n=12) groups.
After 12 extinction sessions, each group of rats was exposed to a different test procedure to examine the reinstating efficacy of non-contingent methamphetamine priming, previously methamphetamine-paired exteroceptive cues and a combination of both priming and cues. Groups Ext Cues+/Test Cues+, Ext Cues−/Test Cues+ and Ext Cues−/Test Cues− received a priming injection of 1 mg/kg i.p. methamphetamine, 30 min prior to the start of a 2-hr reinstatement test session. The Cues+ Reinstatement group received only a 1 ml/kg injection of saline, 30 min prior to the start of the reinstatement test session. Groups Ext Cues+/Test Cues+, Ext Cues−/Test Cues+ and Cues+ Reinstatement were presented with a 6-sec tone + light stimuli followed by a 14 sec time out following each response on the lever which had been active during self-administration training. Responding had no scheduled consequences for group Ext Cues−/Test Cues− A summary of extinction and testing conditions for each group is shown in Table 1.
Table 1.
Extinction and reinstatement conditions in each test group
| Group | Light+tone during extinction | Light+tone during reinstatement | Methamphetamine Prime |
|---|---|---|---|
| Ext Cues+/Test Cues+ | yes | yes | yes |
| Ext Cues−/Test Cues+ | no | yes | yes |
| Ext Cues−/Test Cues− | no | no | yes |
| Cues+ Reinstatement | no | yes | no |
Data analysis
Active-lever (right lever) and inactive-lever (left lever) presses and drug infusions were recorded for each subject daily. Separate one-way analyses of variance tests (ANOVA) were used to compare responding on the final day of methamphetamine self-administration across groups to determine if the groups differed in their baseline self-administration rates and on the first day of extinction to determine if the groups differed in their initial response to extinction conditions. One-way ANOVA’s and Dunnett’s post-hoc tests were also conducted on active-lever and inactive-lever responses on the final day of self-administration and the twelve days of extinction in each group to assess whether significant changes in responding occurred as a result of cessation of methamphetamine infusions during extinction. A two-way ANOVA with extinction versus reinstatement test and group as factors was used to determine if the reinstatement procedures produced significant reinstatement and to compare the magnitude of reinstatement produced by each test condition. Tukey post-hoc tests were used to follow up on significant main effects and interactions.
Results
Methamphetamine self-administration and extinction
Mean number of self-administration days until the beginning of extinction were 18 (±1) for the Cues alone reinstatement group, 19 (±2) for the Ext Cues−/Test Cues− group, 19 (±) for the Ext Cues+/Test Cues+ group and 21 (±2) for the Ext Cues−/Test Cues+ group. Total mean number of lifetime methamphetamine infusions prior to extinction were 395 (±57) for the Cues alone reinstatement group, 361 (±42) for the Ext Cues−/Test Cues− group, 479 (±53) for the Ext Cues+/Test Cues+ group and 468 (±63) for the Ext Cues−/Test Cues+ group. Figure 1 (left panels) shows active-lever responding on the last day of self-administration and all twelve days of extinction in each of the four test groups. The right panels of Figure 1 show inactive-lever responding during the same period for all four test groups. Mean active-lever responses on the last day of self-administration ranged from a high of 51 responses/session in the Ext Cues+ Test Cues+ group to a low of 30 responses/session in the Ext Cues−/Test Cues− group. There were no significant differences between groups in active-lever responding on the last day of self-administration. Mean inactive-lever responding was low on the final day of self-administration in all four groups with a maximum of 6 inactive-lever responses in the Ext Cues+/Test Cues+ group.
Figure 1.
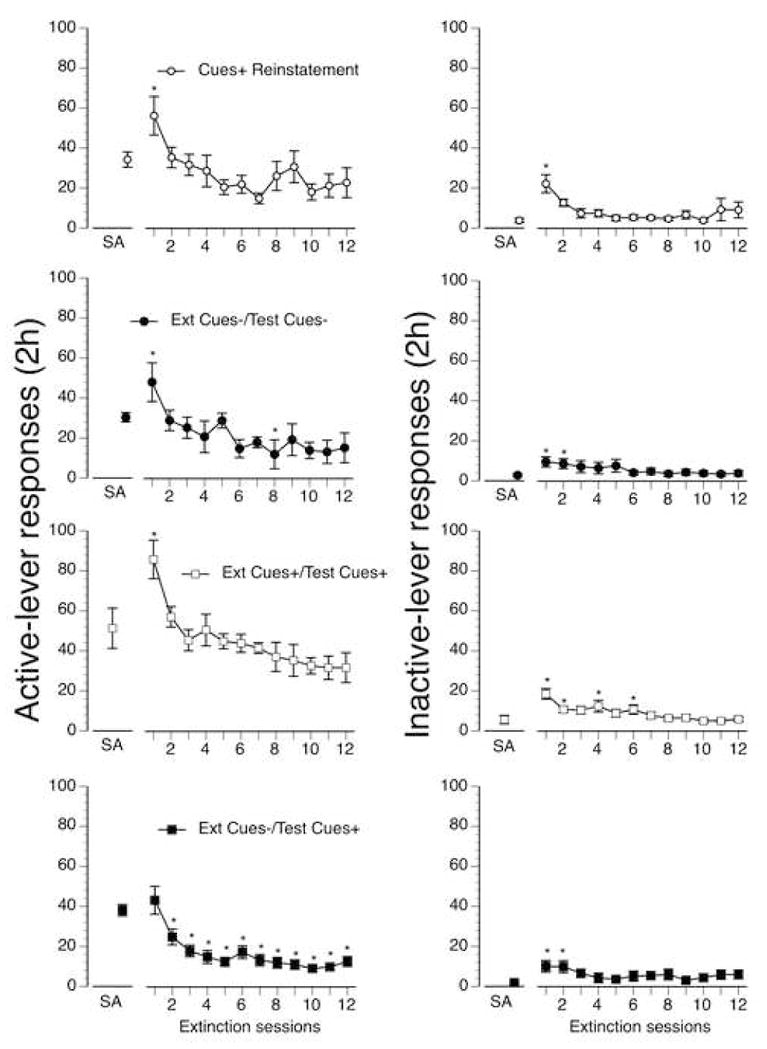
Self-administration and extinction responding: Left panels show responses (+/− SEM) on the methamphetamine-appropriate lever for each of the four test groups. Right panels show inactive-lever responding for each of the four groups. Symbols above SA show group mean responses per 2 h session on the final day of methamphetamine self-administration. Connected points show mean responses per 2 h session on all twelve extinction days for each of the four test groups. * denote significant differences (p < 0.05) in extinction responding compared to the final day of self-administration (SA).
There was a significant main effect of extinction condition on both first extinction day active-lever [F(3,57)=3.686, p=0.017] and inactive-lever [F(3,57)=3.6, p=0.019] responding. Post hoc analysis showed that active-lever responding was significantly higher on the first day of extinction in Ext Cues+/Test Cues+ group than the Ext Cues−/Test Cues+ group. In contrast, inactive-lever responding was significantly higher on the first day of extinction in the Cues+ group compared to the Ext Cues−/Test Cues− group.
There was a significant main effect of extinction days in all four groups: Ext Cues−/Test Cues− [F(12,144)=13.31, p<0.001], Ext Cues−/Test Cues+ [F(12,120)=14.51, p<0.001], Ext Cues+/Test Cues+ [F(12,252)=7.54, p<0.001] and Cues+ Reinstatement [F(12,132)=6.80, p<0.001]. Subsequent post-hoc analysis indicated that there was a significant increase in active-lever responding in groups Cues+ Reinstatement, Ext Cues−/Test Cues− and Ext Cues+/Test Cues+ on the first day of extinction (Figure 1, top three left side panels). In all groups, mean levels of lever pressing were lower on the last day of extinction than on the last day of self-administration but only significantly so for group Ext Cues−/Test Cues+. For this group, levels of responding on extinction days 2–12 were all significantly lower than on the last day of self-administration (Figure 1, bottom left panel). There was also a significant main-effect of extinction days on inactive-lever responding in all four groups: Ext Cues−/Test Cues− [F(12,144)=16.61, p=0.003], Ext Cues−/Test Cues+ [F(12,120)=7.99, p<0.001], Ext Cues+/Test Cues+ [F(12,252)=13.90, p<0.001] and Cues+ Reinstatement [F(12,132)=4.69, p<0.001]. Post hoc analysis indicated that for all four groups, inactive-lever responding significantly increased for one or more days at the onset of extinction testing (Figure 1, right panels) but was not significantly different from inactive-lever responding on the final day of self-administration for the majority of the extinction test sessions.
Methamphetamine reinstatement
Figure 2 (upper panel) shows previously active-lever responding on the final day of extinction and the reinstatement test session for all four groups. A two way ANOVA indicated a significant main effect of reinstatement test [F(1,54)=27.79, p<0.001] as well as significant group by reinstatement test interaction [F(3,54)=5.60, p=0.015] but no main effect of group [F(3,54)=4.32, p=0.110]. Individual Tukey’s tests comparing extinction to reinstatement showed that all four groups emitted significantly greater responding (p<0.05) on the reinstatement test compared to the final day of extinction. Tukey’s tests comparing reinstatement conditions indicated that the Ext Cues+/Test Cues+ group had significantly greater reinstatement responding (p < 0.05) than all of the other three other groups, which did not significantly differ from each other.
Figure 2.
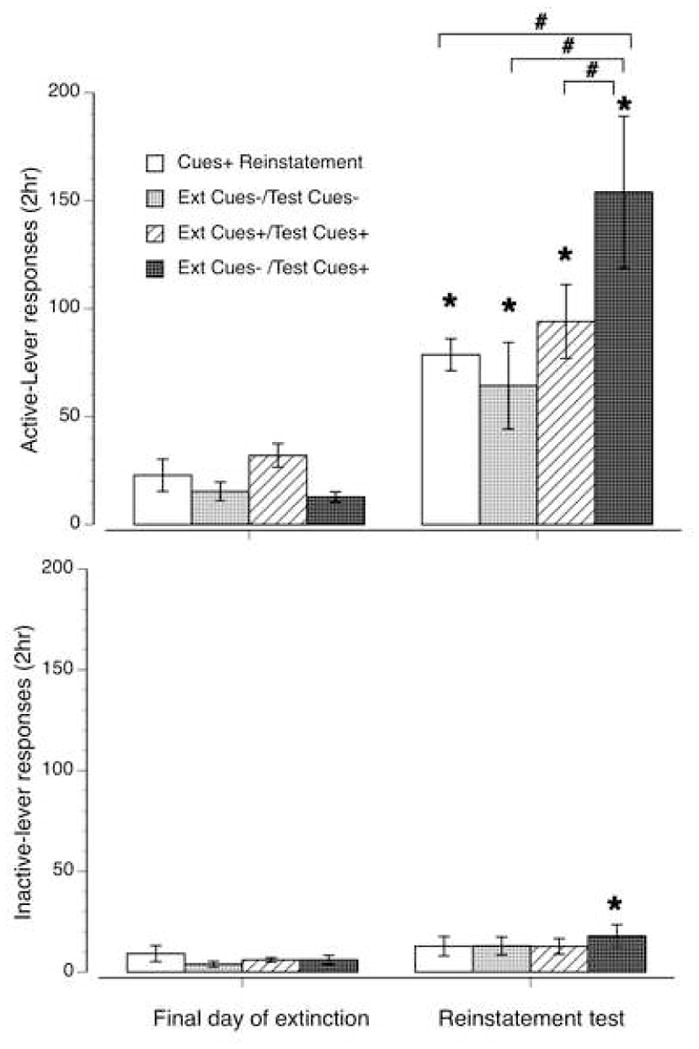
Reinstatement responding: The upper panel shows active-lever responses (+/− SEM) on the final day of extinction and the reinstatement test session for each of the four test groups. The bottom panel shows inactive-lever responses for the same period. * denote significant increases in (p < 0.05) in reinstatement responding for each group compared to their last final day of extinction. # denote significant differences (p<0.05) in the magnitude of reinstatement between the groups linked by brackets.
Figure 2 (bottom panel) shows previously inactive-lever responding on the final day of extinction and the reinstatement test session for all four groups. A two way ANOVA indicated a significant main effect of reinstatement test [F(1,54)=8.41, p<0.001] but no main effect of group [F(3,54)=0.73, p=0.892] or group by reinstatement test interaction [F(3,54)=0.91, p=0.597]. Individual Tukey’s tests comparing extinction to reinstatement showed that only the Ext Cues−/Test Cues+ group had a significant (p<0.05) increase in reinstatement test day responding compared to the final day of extinction.
Discussion
Dozens of studies have shown that following a period of drug self-administration and subsequent extinction, non-contingent administration of that drug can produce renewed lever-pressing behavior characteristic of the reinstatement phenomena. This response has been hypothesized to be a measure of drug seeking and may have some common characteristic with relapse in humans (Kalivas et al. 2006, Koob 2000, Meil and See 1996, Shaham et al. 2002). Likewise many studies have also demonstrated that presentation of exteroceptive stimuli, typically lights and/or tones, previously paired with self-administered drug will also reinstate responding that has been reduced by extinction (Alleweireldt et al. 2002, Davis and Smith 1976, Highfield et al. 2001, McFarland and Ettenberg 1997, Meil and See 1996, 1997). Fairly few have focused on drug- or cue-induced reinstatement of responding following methamphetamine self-administration (Anggadiredja et al. 2004a, Anggadiredja et al. 2004b, Hiranita et al. 2004, Hiranita et al. 2006, Kruzich and Xi 2006, Lu et al. 2003, Moffett and Goeders 2007, Yan et al. 2006a, Yan et al. 2006b). Those experiments which have been conducted indicate that both drug- and cue-induced methamphetamine reinstatement is comparable to reinstatement produced by other drugs of abuse such as cocaine, heroin and ethanol (Shaham et al. 2002, Shalev et al. 2002). The present results replicate and extend those experiments. Both non-contingent methamphetamine primes as well as response-contingent cues which had been paired with methamphetamine self-administration produced robust reinstatement. Indeed, it is interesting to note that significant reinstatement was generated in each of the test conditions, despite the fact that 12 days of extinction sessions did not suppress rates of responding significantly below prior rates of self-administration responding in three of the four groups of rats.
The ability of the light + tone stimulus compound to elevate rates of lever pressing relative to levels occurring at the end of the 12-day extinction period is likely attributable to their previous association with methamphetamine delivery, as well as their contingent relationship with lever pressing during training and testing. A limitation of the present studies which prohibits an unequivocal inference that associative processes were responsible for empowering the cues to reinstate responding was the absence of a control group for which cues were not presented concurrently with methamphetamine infusions during training, but were presented contingently upon lever pressing during testing. Few other reports have included such a control group, and when they had they were found ineffectual in reinstating responding (e.g., Smith and Davis, 1973). Another factor at least potentially influential in empowering the cues to reinstate responding was their response-contingent presentation during training. When stimuli are presented independently of behavior but concurrently with drug delivery during training they can come, through associative conditioning, to elicit effects opposite to the direct effects of the drug they are presented with (e.g., see Siegel, 1978a, b; Siegel et al., 1987). This type of associative conditioning was unlikely occurring in the present study given the response-contingent nature of the cues and given that co-presentation of the cues with methamphetamine primes increased levels of responding relative to either presented alone. If the cues had elicited effects opposite to those directly elicited by methamphetamine these opposing effects would have reduced, and not have increased the reinstatement effect. The response-contingent nature of the cues during testing was also a likely factor influencing their ability to increase response levels. When drug-associated stimuli have been presented independently of behavior in other studies response rates are poorly, if at all elevated (de Wit and Stewart, 1981; Grimm et al., 2000; Tran-Nguyen et al., 1998). Also, if the response-contingency was not important for the cues to elevate responding of the previously-reinforced lever, their presentation during testing would have elevated responding on the inactive lever as well which did not occur.
The present study explores several additional factors which may be important for the expression of reinstatement. Firstly we examined whether contingent presentation of extinguished drug-paired exteroceptive cues enhances reinstatement produced by methamphetamine priming. In reinstatement studies drug-paired stimuli are often presented during acquisition, extinction and reinstatement. These stimuli have been shown to facilitate acquisition and enhance maintenance levels of self-administration (Caggiula et al. 2002, Deroche-Gamonet et al. 2002, Panlilio et al. 2000). In a series of two cocaine reinstatement papers from our laboratory, one using non-contingent cocaine primes and a second using footshock stress, we found that statistically significant reinstatement behavior did not occur when cues, which had been present during self-administration, were omitted during extinction and reinstatement testing (Shelton and Beardsley 2005, Shelton et al. 2004). In contrast, in the present study there was no significant difference in reinstatement responding between groups in which extinguished, response-contingent, drug-paired cues were present during reinstatement testing or omitted during both extinction and reinstatement testing. These data are in general agreement with that from two recent methamphetamine reinstatement experiments in mice. In those studies there was no examination of whether presentation of extinguished methamphetamine-paired cues enhanced reinstatement, but it was demonstrate that cues were not necessary to elicit significant priming-induced reinstatement (Yan et al. 2006a, Yan et al. 2006b).
It is surprising that extinguished drug-paired cues did not have a greater effect on methamphetamine-primed reinstatement in the present study given the results of both our cocaine reinstatement experiments in which they seemed more critical to the expression of drug seeking (Shelton and Beardsley 2005, Shelton et al. 2004). The present methamphetamine reinstatement study and our prior cocaine priming and footshock reinstatement experiments were methodologically similar in many respects such as length of self-administration training, strain of rat, duration of extinction, type and number of stimulus presentations and session duration. These studies did, however, differ in a number of other aspects. In our prior cocaine priming reinstatement study each test group was treated with a different reinstating dose of cocaine (3, 10 and 17 mg/kg i.p.) but received sequential tests with different stimulus (cue) conditions (Shelton et al. 2004). In contrast, in the present study we examined a single reinstating dose of methamphetamine and each group was only examined in one stimulus condition. Given that reinstatement is a transient effect it could be that the order of test conditions could have been responsible for the differing results (Shaham et al. 2002). However, in our footshock-induced cocaine reinstatement study we only examined a single reinstatement test condition, therefore, it seems somewhat unlikely that treatment order alone could be responsible for the results.
It is also possible that the 1 mg/kg methamphetamine dose was simply a more efficacious reinstating dose in the present study compared to any of the three cocaine doses in the prior study. If such were the case, the methamphetamine prime may not have required the augmentation produced by presentation of extinguished drug-paired cues in order to produce significant reinstatement. This hypothesis is a possibility but might be unlikely, given that the 17 mg/kg i.p. dose used in our previous study was close to the 20 mg/kg i.p. dose which produced maximal reinstatement in an experiment which specifically measured the reinstatement dose-effect curve for cocaine (Schenk and Partridge 1999). Also, while reinstatement dose could potentially be responsible for the differences in the priming reinstatement results, it does not provide a unified explanation for why extinguished cues were essential for both priming- and stress-induced cocaine reinstatement but not necessary for priming-induced methamphetamine reinstatement.
Another potential difference in the present methamphetamine experiment and prior cocaine reinstatement studies may have been the relative unit self-administration dose. While there is no direct evidence that unit self-administration dose has any effect on subsequent reinstatement, it has been demonstrated that other factors related to self-administration can alter subsequent reinstatement. For instance, rats allowed long daily access to self-administered drugs exhibit more robust prime- and stress-induced reinstatement then do animals allowed shorter, daily access to drug (Ahmed et al. 2000, Mantsch et al. 2004). Several experiments from the drug discrimination literature suggest that methamphetamine is approximately 10 fold more potent than cocaine as a discriminative stimulus (Munzar et al. 2000, Schechter 1997, Suzuki et al. 2004). There was, however, only a 5 fold difference in methamphetamine (0.1 mg/kg/infusion) and cocaine self-administration doses (0.5 mg/kg/infusion) between our reinstatement studies. In the present experiment we chose a methamphetamine self-administration dose of 0.1 mg/kg/infusion based on literature reports of it serving as an effective reinforcer (Jun and Schindler 2000, Moffett and Goeders 2005, Ranaldi and Poeggel 2002, Shepard et al. 2006) but it appears based on the literature available that 0.1 mg/kg/infusion falls on the descending limb of the methamphetamine self-administration dose-effect curve (Jun and Schindler 2000, Moffett and Goeders 2005) and may therefore be a somewhat high unit self-administration dose relative to 0.5 mg/kg/infusion cocaine. This conjecture is strengthened by results from two progressive-ratio studies of methamphetamine-self-administration in rats. In those experiments peak numbers of methamphetamine responses were generated by 0.08 and 0.1 mg/kg/infusion methamphetamine doses, respectively (Clemens et al. 2006, Roth and Carroll 2004) indicating that a 0.1 mg/kg/infusion methamphetamine dose is a very high efficacy reinforcer. In contrast, progressive ratio schedules of cocaine self-administration indicate that unit doses of cocaine considerably greater than our 0.5 mg/kg/infusion dose are required to produce maximal responding on a progressive ratio schedule. This suggests that 0.5 mg/kg/infusion cocaine has a more moderate reinforcing efficacy, at least when compared to other cocaine doses (Roberts et al. 1989, Ward et al. 2005).
The mechanism by which unit self-administration dose might effect the ability of drug-paired cues to influence reinstatement is unclear. One possibility could be that the dose of the self-administered drug might effect the salience of the drug-paired cues. While there is some disagreement whether the reinstatement produced by drug priming is due to the ability of the prime to produce discriminative stimulus effects like the self-administered drug (Feltenstein and See 2006, Odum and Shahan 2004, Spealman et al. 1999) experiments have shown that the relative strength of components of a compound discriminative stimulus are dependent upon the salience of the individual constituents during training (Duncan 1986, Jarbe et al. 1989). It may also be the case that the strength of individual components of a compound reinstating stimulus, even if they are mediated by separate neurochemical mechanisms, may also be dependent upon their salience during training. If in the present study the methamphetamine self-administration dose was relatively high, as seems likely, one might predict that the salience of the methamphetamine component of the compound reinstating stimulus might also take on a more prominent role and could, thereby, alone have been sufficient to produce significant reinstatement in the absence of extinguished methamphetamine-paired cues. Additional studies with lower self-administered unit doses of methamphetamine will be required in order to definitively test this hypothesis.
A few studies in the literature with other drugs of abuse have shown that combining drug + exteroceptive cues, which have been paired with drug but not extinguished, can result in a dramatic enhancement of reinstatement responding compared to either drug or cues alone (Di Ciano et al. 2001, Di Pietro et al. 2006, Shelton et al. 2004, Spealman et al. 1999). The present study largely confirms and extends those findings to methamphetamine reinstatement. The reinstatement produced by prime + non-extinguished cues was greater than drug prime alone, cues alone or prime+ extinguished cues. In regards to absolute values, reinstatement on the first day in the prime + non-extinguished cue condition was almost twice as large as that in the cue alone condition and over twice as great as the drug prime alone condition.
In conclusion, the present study demonstrates that reinstatement of methamphetamine-seeking behavior in rats can be produced by methamphetamine priming as well as by contingent presentation of methamphetamine-paired exteroceptive cues. The data also show that combining methamphetamine prime + cues produces a more robust reinstatement effect than when either of these same reinstating stimuli are presented alone. These data, together with those involving the reinstatement of cocaine-seeking behavior [e.g. (Feltenstein and See 2006, Shelton and Beardsley 2005, Shelton et al. 2004)] continue to document the importance of the interaction between drug-associated cues with other reinstating events for precipitating renewed drug-seeking, and suggest conditions environmentally and pharmacologically likely more similar to relapse in the drug abuser which should be considered during the evaluation of potential pharmacotherapies.
Acknowledgments
This work was supported in by National Institute on Drug Abuse contract N01DA-0-8801. The authors wish to thank Elizabeth Hendrick, Aaron Loveland, Stephen Carter, Vanessa Davis and Kelly Kinzer for their excellent technical assistance.
The authors wish to thank Drs. David McCann and Jane Acri of the National Institute on Drug Abuse for their substantive scientific input regarding the execution of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–93. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004a;29:1470–8. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T. Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004b;1021:272–6. doi: 10.1016/j.brainres.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Intravenous methamphetamine self-administration in rats: Effects of intravenous or intraperitoneal MDMA co-administration. Pharmacol Biochem Behav. 2006;85:454–63. doi: 10.1016/j.pbb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11:222–36. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–70. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–58. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–98. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Duncan PM. The effect of training dose on discrimination of compound drug-exteroceptive stimuli. Psychopharmacology. 1986;90:543–7. doi: 10.1007/BF00174076. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Haynes P. Drug using offenders in south London. Trends and outcomes. J Subst Abuse Treat. 1998;15:449–56. doi: 10.1016/s0740-5472(97)00307-3. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–31. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Anggadiredja K, Fujisaki C, Watanabe S, Yamamoto T. Nicotine attenuates relapse to methamphetamine-seeking behavior (craving) in rats. Ann N Y Acad Sci. 2004;1025:504–7. doi: 10.1196/annals.1316.062. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–6. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jarbe TUC, Hiltunen AJ, Swedberg MDB. Compound drug discrimination learning. Drug Dev Res. 1989;16:111–22. [Google Scholar]
- Jun JH, Schindler CW. Dextromethorphan alters methamphetamine self-administration in the rat. Pharmacol Biochem Behav. 2000;67:405–9. doi: 10.1016/s0091-3057(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–44. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002a;22:1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology (Berl) 2002b;161:278–87. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. Animal models of craving for ethanol. Addiction. 2000;95 (Suppl 2):S73–81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2002 doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl) 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–63. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. Neither non-contingent electric footshock nor administered corticosterone facilitate the acquisition of methamphetamine self-administration. Pharmacol Biochem Behav. 2005;80:333–9. doi: 10.1016/j.pbb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–80. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Goldberg SR. Modulation of the discriminative stimulus effects of methamphetamine by 5-HT1A and 5-HT2A/2C receptors in rats. 2000;234:234. [Google Scholar]
- Odum AL, Shahan TA. D-Amphetamine reinstates behavior previously maintained by food: importance of context. Behav Pharmacol. 2004;15:513–6. doi: 10.1097/00008877-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Effects of compounding drug-related stimuli: escalation of heroin self-administration. J Exp Anal Behav. 2000;73:211–24. doi: 10.1901/jeab.2000.73-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13:1107–10. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–8. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–9. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2005 National Survey on Drug Use and Health. Department of Health and Human Services; 2006. [Google Scholar]
- Schechter MD. Discriminative characteristics of high and low cocaine administration: Effect of other psychostimulants. Pharmacol Biochem Behav. 1997;56:457–63. doi: 10.1016/s0091-3057(96)00301-2. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147:285–90. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2002;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev V, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmac Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int J Comp Psych. 2005;18:154–66. [Google Scholar]
- Shelton KL, Hendrick E, Beardsley PM. Interaction of noncontingent cocaine and contingent drug-paired stimuli on cocaine reinstatement. Eur J Pharmacol. 2004;497:35–40. doi: 10.1016/j.ejphar.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–9. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185:505–13. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Siegel S. The role of pavlovian conditioning in morphine tolerance. Psychopharmacology. 1978a:58. [Google Scholar]
- Siegel S. Tolerance to the hyperthermic effect of morphine in the rat is a learned response. J Comp Physiol Psychol. 1978b;92:1137–1149. doi: 10.1037/h0077525. [DOI] [PubMed] [Google Scholar]
- Siegel S, Krank MD, Hinson RE. Anticipation of pharmacological and nonpharmacological events: Classical conditioning and addictive behavior. J Drug Issues. 1987;17:83–110. [Google Scholar]
- Smith SG, Davis WM. Behavioral control by stimuli associated with acquisition of morphine self-administration. Behavioral Biology. 1973;9:777–780. doi: 10.1016/s0091-6773(73)80139-7. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–36. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:591–7. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–61. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DC. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology. 2005;30:286–95. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res. 2006a;168:137–43. doi: 10.1016/j.bbr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Nitta A, Nabeshima T. Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res. 2006b doi: 10.1016/j.bbr.2006.11.033. [DOI] [PubMed] [Google Scholar]


