Abstract
Objective
Post-traumatic knee osteoarthritis is prevalent after anterior cruciate ligament reconstruction. Biomarkers that identify individuals likely to develop osteoarthritis, especially symptomatic osteoarthritis, can help target preventative and therapeutic strategies. This study examined the magnitude and change over time in urinary CTX-II (uCTX-II) concentrations shortly after ACL reconstruction, and, secondarily, the associations with knee pain and function.
Design
Subjects were 28 patients with ACL reconstruction (ACLR) and 28 age- and sex-matched controls (CNTRL). Testing was conducted at 4 time points spaced 4 weeks apart (4, 8, 12 and 16 weeks post-operative in ACLR). Measures included demographics, urine samples, Numeric Pain Rating Scale (NPRS) and International Knee Documentation Committee Subjective Knee Form (IKDC-SKF). uCTX-II concentrations were determined with competitive ELISA. uCTX-II concentrations at each time point in ACLR were compared to the mean concentration over time in CNTRL, with and without adjustment for body mass index (BMI). Changes over time in each measure and correlations between the slopes of change were examined.
Results
uCTX-II concentrations were significantly higher in ACLR than CNTRL through 16 weeks post-operative when adjusted for BMI. In ACLR, uCTX-II concentrations significantly decreased over time, and the slope was associated with NPRS (r =.406, p=.039) and IKDC-SKF (r = −.402, p = .034) slopes.
Conclusion
uCTX-II concentrations shortly after ACL reconstruction were elevated compared to controls and declined over time. Decreasing uCTX-II concentrations were associated with decreasing knee pain and improving function. uCTX-II may have a role as a prognostic marker following ACL reconstruction and warrants further investigation.
Introduction
An estimated 200,000 anterior cruciate ligament (ACL) injuries occur in the United States annually, usually during sports participation,[1] and most are treated surgically with an ACL reconstruction.[2, 3] A prevalent, negative outcome of ACL reconstruction is post-traumatic knee osteoarthritis (OA) development. Within 15 years after ACL reconstruction, up to 80% of the population show radiographic signs of knee OA including joint space narrowing and osteophyte formation.[4-9] Almost half of those with radiographic signs of knee OA also have symptoms such as pain, swelling and stiffness.[4, 9] The development of post-traumatic knee OA has public health implications because the average age at the time of ACL injury is about 25 years.[10] Hence, knee symptoms and functional limitations will affect a substantial portion of the life span. On an individual level, the symptoms of knee OA may also reduce quality of life.[4, 11] Consequently, there is a need to implement strategies to post-traumatic knee OA in ACL reconstruction post-surgical care. A critical step towards this goal is achieving a better understanding of post-traumatic knee OA development after ACL reconstruction.
Knee OA affects all aspects of the joint, but its hallmark feature is degeneration of articular cartilage.[12] Articular cartilage structure is maintained when chondrocyte metabolism of extracellular matrix components (synthesis and degradation) is balanced. When degradation exceeds synthesis, articular cartilage structure degenerates.[12] Articular cartilage metabolism potentially can be assessed with biomarkers[13] including the crosslinked C-telopeptide fragments of type II collagen (CTX-II), which is a byproduct of articular cartilage degradation. CTX-II has been detected in both synovial fluid and urine. An advantage of urine analysis is urine can be obtained more easily than synovial fluid, which facilitates a stronger research design because samples can be collected from a comparable control group. However, urine concentrations do not directly represent the local environment of the joint and instead represent input from the entire body. In addition, urine concentrations may reflect cartilage activity at the bone-cartilage interface.[13] Despite these limitations, uCTX-II concentrations appear to have diagnostic and prognostic value for knee OA.[13] Studies have shown that uCTX-II concentrations are elevated in people with radiographic signs of knee OA,[14-17] and generally increase with the severity of knee OA.[14, 18-20] In addition, baseline uCTX-II concentrations have been variably associated with knee OA progression, [15, 16, 21, 22] while short-term increases in uCTX-II concentrations have been associated with both radiographic knee OA progression and loss of articular cartilage on magnetic resonance images.[21, 23, 24]
Concentrations of uCTX-II have been investigated for associations with the symptoms and functional limitations that can accompany knee OA. This is important because it would be ideal to identify people at risk for symptomatic knee OA (i.e. radiographic signs of OA and knee symptoms, especially pain) because they are most likely to have functional limitations and seek medical attention. In a population-based study, while the longitudinal trajectory of uCTX-II over time was positively associated with a knee stiffness rating, no significant association was found with the knee pain rating or score on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) physical function sub-scale. [23] Similarly, a study of people with knee OA reported no association between uCTX-II concentrations and the WOMAC index or sub-scale scores.[14] Conversely, subjects with early knee OA (Kellgren-Lawrence Grade 2) who also had knee pain demonstrated higher uCTX-II concentrations than those without knee pain.[25] Hence, there is some evidence that uCTX-II concentrations may be elevated in people with knee symptoms, making further examination worthwhile.
Previous research in a cross-section of people with ACL injury found CTX-II concentrations in synovial fluid to be elevated compared to uninjured controls and to decrease with time from injury.[26] However, to the best of our knowledge, uCTX-II concentrations have not been reported following ACL reconstruction. It is therefore unknown if elevated CTX-II concentrations can be detected in urine shortly after surgery and if concentrations change within a few months post-operatively. The aim of the current study was to examine the magnitude and change over time in uCTX-II concentrations shortly after ACL reconstruction, and, secondarily, the associations with knee pain and function. We hypothesized that following ACL reconstruction, uCTX-II concentrations would be elevated compared to controls but decrease over time, and uCTX-II concentrations would be positively associated with knee pain and negatively associated with knee function. The early post-operative period was chosen because it is a time when patients are in supervised rehabilitation and closely monitored. Therefore, study results can contribute to understanding how uCTX-II concentrations respond before structural damage is evident and provide information that can benefit rehabilitation protocols.
Methods
Subjects
Patients with an ACL reconstruction (ACLR) were recruited from Brooks Center for Sports Therapy, Jacksonville, FL, or the Orthopaedic and Sports Medicine Institute, Gainesville, FL. Eligible subjects were between 15 and 30 years of age, had surgery less than 1 year after injury, and participated at least 50 hours per year in Level I or II activities (i.e. sports that include cutting, jumping or pivoting)[27] prior to injury. Potential subjects were excluded from participating if any of the following were present: bilateral knee injury, prior knee ligament injury and/or surgery, concomitant ligamentous injury greater then Grade II, articular cartilage defect greater than Grade II (Outerbridge classification), or surgical complications requiring rehabilitation modification. The inclusion and exclusion criteria were meant to create a population of active subjects with an acute, unilateral and relatively isolated ACL injury with potential to make typical progress in rehabilitation.
A cohort of uninjured control subjects (CNTRL) was recruited from the local community. Subjects in CNTRL were matched to ACLR by age (± 3 years) and sex and were required to participate in high-demand sports for at least 50 hours per year. During the study, subjects in CNTRL were allowed to participate in up to 10 hours of high-impact activity per week (e.g. Level I or 2 sports or running and jumping exercises), and low-impact activity was unrestricted. These parameters were set to insure that subjects in CNTRL would not significantly increase their high-impact activity over the course of the study.
Subjects gave written consent or assent (minor subjects) to participate in this study on a form approved by the University of Florida Institutional Review Board.
Surgical Technique and Rehabilitation
Surgery for all subjects in ACLR except one was performed by one of two board-certified orthopaedic surgeons (P.A.W. and M.W.M.). Surgical procedures were performed using an arthroscopically assisted technique with anatomic placement of tunnels on the femur and tibia. After graft harvest and/or preparation, the knee underwent a diagnostic arthroscopy and meniscectomy or meniscal repair was completed, if applicable. In some cases, a small notchplasty was then performed. After drilling the tunnels, the graft was passed and fixation on the femur was obtained using a cortically based button (Arthrex or Smith and Nephew). Tibial fixation was obtained with a bio-absorbable interference screw and when indicated, backed up with a post or anchor.
Rehabilitation was not controlled in this study. However, the rehabilitation protocol used at both sites allowed for early weight bearing without a brace. Rehabilitation interventions were focused on pain and effusion control, restoration of knee range of motion, quadriceps muscle activation and normalization of gait until at least 12 weeks post-surgery. At 12 weeks post-surgery subjects were allowed to begin advanced activities such as running as long as they met clinical criteria for knee pain levels, knee range of motion and quadriceps strength.
Testing Protocol
Testing was conducted at 4 time points spaced 4 weeks apart. Testing time points corresponded to 4, 8, 12 and 16 weeks after surgery for ACLR. Testing was spaced at 4-week intervals to allow ample time for changes in uCTX-II concentrations. The first testing time point was 4 weeks after surgery for ACLR because these subjects were recruited after surgery, and it was feasible to have testing coordinated by that time. Demographic information was collected at the first test session. Urine samples and responses on the Numeric Pain Rating Scale (NPRS) and International Knee Documentation Committee Subjective Knee Form (IKDC-SKF) were collected at all time points. Subjects were asked to refrain from high-impact activity for 24 hours before data collection because high impact activity has the potential to influence uCTX-II levels.[28, 29]
Demographic Information
Demographic information included age, sex, height (cm), and weight (kg). Height and weight were measured with a standard measuring stick and digital bathroom scale, respectively, and used to calculate body mass index (BMI). Additional information collected for ACLR included the time from injury to surgery, graft type, graft source, and any surgical procedures to the meniscus. Graft type was categorized as “allograft” or “autograft”. The autograft sources were bone-patellar tendon-bone or semi-tendinosus/gracilis tendons. The allograft sources were tibilalis anterior or Achilles tendons.
Urinary CTX-II
Early morning (within 2 hours of waking),[30] second void, urine samples were collected. Samples were immediately put on dry ice and transported for storage at -20° C until analysis. uCTX-II concentrations were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) based on a mouse monoclonal antibody raised against the EKGPDP sequence of human type II collagen C-telopeptide (Urine CartiLaps®; Nordic Bioscience, Herlev, Denmark). Urine samples were diluted as needed, ranging from 1:2 to 1:60, and were analyzed in duplicate. uCTX-II concentrations were corrected for urinary creatinine concentration, which was also determined with ELISA methods (Cayman Chemical Company, Ann Arbor, MI), using the formula [corrected CTX-II value (ng/mmol) = 1000 × urine CartiLaps (ug/L)/creatinine (mmol/L)]. The intra-assay coefficient of variation was calculated across multiple subjects, and inter-assay coefficient of variation was calculated across the multiple assays. The average (± SD) intra-assay and inter-assay coefficients of variation were 8.5% ± 8.9% and 17.6% ± 14.5%, respectively.
Numeric Pain Rating Scale
Knee pain intensity in ACLR was measured with an 11-point numeric pain rating scale (NPRS). Subjects verbally rated their worst and least knee pain intensity in the past 24 hours, as well as their current knee pain intensity, on a scale from 0 (no pain) to 10 (worst pain imaginable) points. The ratings on these three items were averaged for analysis. The NPRS has been shown to be a reliable and valid method of measuring pain.[31, 32]
International Knee Documentation Committee Subjective Knee Form
The 18-item International Knee Documentation Committee Subjective Knee Form (IKDC-SKF) was used to assess knee symptoms and function..[33] Point values for each item vary based on the number of responses possible. Item responses are summed, divided by the total points possible, and multiplied by 100 to create a total score. Higher scores indicate less severe knee symptoms and better knee function. The psychometric properties of the questionnaire were analyzed in patients with knee problems, including ACL injury, and a test-retest reliability coefficient (intraclass correlation coefficient) of 0.94,[33] a minimal detectable change of 12.8 points,[33] and a standardized response mean of 0.94[34] have been obtained. Normative values have also been determined in a non-patient population.[35]
Sample Size
Power calculations were conducted for the primary objective with the intent on detecting a group main effect using a two-tailed test, power of 80%, and alpha of 0.05. Based on uCTX-II concentrations reported in older adults with and without severe knee OA,[36] a sample of 12 subjects per group was needed. However, articular cartilage degradation is likely to be less in patients shortly after ACL reconstruction, which could affect the magnitude of uCTX-II concentrations. Therefore, to assure that we would detect a smaller difference in uCTX-II concentrations, we conservatively aimed for a sample size of at least 24 subjects per group.
Statistical Analysis
Statistical analyses were performed with SPSS software, version 20.0 (IBM SPSS Statistics). Descriptive statistics were generated for all measures. uCTX-II data were log-transformed prior to analysis to limit the impact of outliers as has been done in previous reports.[17, 36] An alpha level of 0.05 was set for significance in all statistical tests.
Group differences in log uCTX-II concentrations at each testing time point were determined with students t-tests for paired samples. For ACLR, the log uCTX-II concentration at the testing time point was used; for CNTRL, the mean log uCTX-II concentration across testing time points was used because the testing time points had no clinical relevance. Group differences in log uCTX-II concentrations were also examined with adjustment for differences in BMI using linear regression models. The mean paired difference (ACLR-CNTRL) in log uCTX-II concentration was the dependent variable, and the mean paired difference (ACLR-CNTRL) in BMI was the independent variable. By fitting a least squares regression line, the fitted intercept estimates the mean ACLR-CNTRL difference for the log uCTX-II concentration when the difference in BMI between ACLR and CNTRL is zero. That is, this analysis adjusts for BMI differences. For ACLR, the log uCTX-II concentration and BMI at the testing time point were used; for CNTRL, the mean log uCTX-II concentration and mean BMI across testing time points were used. BMI was included in the model because it can influence the magnitude of uCTX-II concentrations.[37] Age and gender can also influence uCTX-II concentrations [37] but were not included because ACLR and CNTRL were matched on these characteristics.
Separate general linear models for repeated measures (time points) examined changes over time in log uCTX-II concentrations, NPRS scores, and IKDC-SKF scores. A regression line was fitted across time points for these measures, and the slope was computed. Correlations among these slopes were examined with Pearson's Product Moment correlation.
Exploratory analyses in ACLR examined the association of demographic variables with the mean uCTX-II concentration over time and the slope of log uCTX-II concentrations over time. Associations with age and time from injury to surgery were examined with Pearson's Product Moment correlation, and student's t-tests for independent samples examined differences based on sex, the presence of meniscal surgery (yes or no), and graft type.
Results
Demographic data for both groups can be found in Table 1. Both ACLR and CNTRL groups were comprised of 28 subjects, with equal representation between males and females. The mean (standard deviation, SD) difference in age between groups was .3 (1.5) years (Table 1) and between matched pairs was 1.2 (.94) years. The mean time from injury to surgery ranged from 17 to 278 days in ACLR. Weight and BMI values were noted to be higher in ACLR and so these were examined further. The mean weight across testing time points and the BMI at each testing time point were higher in ACLR compared to CNTRL (Table 1). Also, BMI values increased over time in ACLR (Table 1).
Table 1.
Demographic information for subjects with ACL reconstruction (ACLR) and age- and gender-matched controls (CNTRL).
| ACLR (n=28) | CNTRL (n=28) | ||
|---|---|---|---|
| Female | 14 (50%) | 14 (50%) | |
| Age (years) | 19.6 (4.5) | 19.9 (4.3) | |
| Height (cm) | 172.5 (9.6) | 172.7 (9.2) | |
| Mean weight across time points (kg) | 79.5 (18.1)* | 66.7 (12.4) | |
| Body mass index (kg/m2) | |||
| 4 weeks post-op or Time point 1 | 26.2 (5.2)* | 22.2 (3.0) | |
| 8 weeks post-op or Time point 2 | 26.6 (5.2)** | 22.2 (2.9) | |
| 12 weeks post-op or Time point 3 | 26.7 (5.0)** | 22.4 (3.2) | |
| 16 weeks post-op or Time point 4 | 26.9 (5.1)** | 22.2 (3.0) | |
| Time from injury to surgery (days) | 69.6 (64.3) | ||
| Graft type | Allograft | 11 (39%) 9 Achilles tendon, 2 anterior tibialis tendon | |
| Autograft | 17 (61%) 16 hamstring tendons, 1 patellar tendon | ||
| Surgical procedures to meniscus | 10 (36%) 1 medial meniscectomy 7 lateral menisectomy 1 medial and lateral menisectomy 1 medial meniscal repair | ||
Values are numbers (%) or mean (standard deviation).
ACLR is greater than CNTRL (p =.001)
ACLR is greater than CNTRL (p < .001)
In ACLR, the mean (SD) uCTX-II concentrations were 3521.2 (4217.5), 3332.8 (3795.1), 3190.5 (3317.9), and 2850.7 (3185.5) ng/mmol at 4, 8, 12 and 16 weeks post-operative, respectively. In CNTRL, the mean (SD) uCTX-II concentration across testing time points was 2827.8 (5033.7) ng/mmol. Log uCTX-II concentrations for both groups are shown in Figure 1. Log uCTX-II concentrations were significantly higher in ACLR at 4, 8, and 12 weeks post-operative compared to the mean log uCTX-II concentration across time points in CNTRL (p=.003, .006, .021, and .081; respectively). The magnitude of difference was .22 [95% confidence interval .08, .36], .18 [.06, .31], .16 [.03, .29], and .19 [−.02, .25], at 4, 8, 12 and 16 weeks post-operative; respectively. Log uCTX-II concentrations significantly declined from 4 to 16 weeks post-surgery in ACLR while no significant change over time was found in CNTRL (p = .045 and .682, respectively; Figure 1). After adjusting for BMI, log uCTX-II concentrations were significantly higher in ACLR at all time points compared to the mean log uCTX-II concentration across time points in CNTRL (p = .001, .029, .002, and .005; respectively). The BMI-adjusted magnitude of difference was 2.3 [1.7, 3.1], 2.0 [1.4, 2.7], 2.0 [1.5, 2.8], and 1.8 [1.3, 2.6] fold higher in ACLR at 4, 8, 12, and 16 weeks post-operative, respectively.
Figure 1.
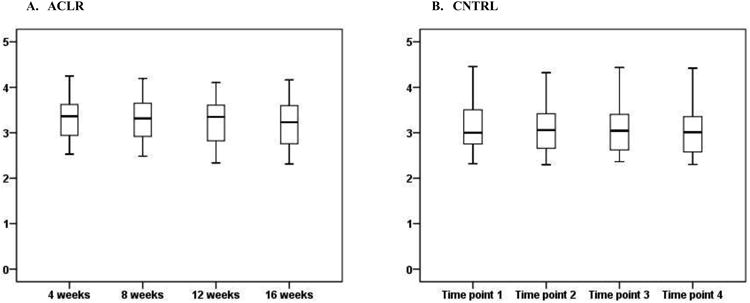
Box and whisker plot of log uCTX-II concentrations in subjects with ACL reconstruction (ACLR) from 4 to 16 weeks post-surgery and in age- and sex-matched control subjects (CNTRL) at 4 weeks intervals. The mean log uCTX-II concentrations decreased from 4 to 16 weeks post-op in ACLR (p =.045).
NPRS scores were not acquired for 2 subjects in ACLR, thus only 26 subjects were analyzed for this measure. Mean NPRS scores significantly decreased over time (p <.001) and point values were 2.3 (1.3), 1.2 (.9), .8 (.6), and 1.0 (.9) at 4, 8, 12 and 16 weeks post-operative; respectively. IKDC-SKF scores significantly improved over time in ACLR (p<.001), whereas the scores in CNTRL were close to the maximum value (100 points) at each time point (Table 2). In ACLR, the mean slope for uCTX-II concentrations was -.03 (.06); however, 8 subjects had a positive slope. The slope for log uCTX-II concentrations in ACLR was positively correlated with the slope for NPRS scores (r =.406, p = .039; Figure 2) and negatively correlated with the slope for IKDC-SKF scores (r = −.402, p = .034; Figure 3).
Table 2.
Scores on the International Knee Documentation Committee Subjective Knee Form (IKDC-SKF) in subjects with ACL reconstruction (ACLR) and matched controls (CNTRL). The questionnaire was completed at 4 week intervals (4 to 16 weeks post-operative in ACLR). Scores are reported as mean (SD). IKDC-SKF scores increased over time in ACLR, whereas scores for CNTRL were near the maximum of 100 at all time points.
| 4 weeks post-op/Time point 1 | 8 weeks post-op/Time point 2 | 12 weeks post-op/Time point 3 | 16 weeks post-op/Time point 4 | |
|---|---|---|---|---|
| ACLR* | 45.6 (9.5) | 57.6 (10.5) | 66.5 (10.1) | 73.7 (11.8) |
| CNTRL | 98.7 (3.8) | 99.4 (1.7) | 99.6 (1.0) | 99.2 (1.6) |
Values are mean ± standard deviation (range) points
IKDC-SKF scores increased over time (p < .001)
Figure 2.
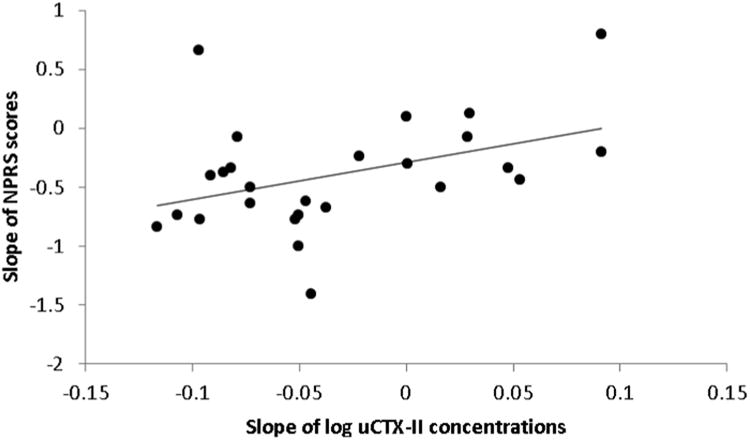
Slopes for log uCTX-II concentrations and Numeric Pain Rating Scale (NPRS) scores across 4, 8, 12 and 16 weeks post-surgery are positively correlated in subjects with ACL reconstruction (r = .406, p = .039).
Figure 3.
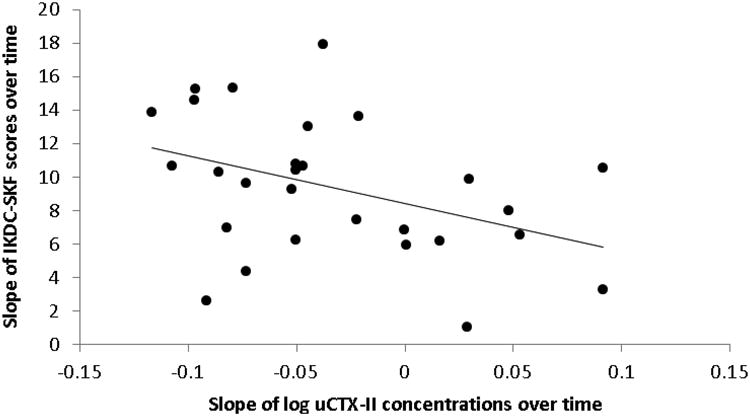
Slopes for log uCTX-II concentrations and International Knee Documentation Committee Subjective Knee Form (IKDC-SKF) scores across 4, 8, 12 and 16 weeks post-surgery are negatively correlated in subjects with ACL reconstruction (r = −.402, p = .034).
The exploratory analyses in ACLR revealed that the mean log uCTX-II concentration across time was significantly correlated with age (r= −.769, p < .001), but not time from injury to surgery (r= −.284, p = .143). The slope of log uCTX-II concentrations was not significantly associated with age (r = −.302, p = .118) or time from injury to surgery (r = .114, p = .564). There were no differences in the log uCTX-II concentration variables between sub-groups based on sex, the presence of meniscal surgery or graft type (Table 4).
Discussion
This short-term, longitudinal study provides knowledge about uCTX-II concentrations in subjects with ACL reconstruction, a population at high risk for developing post-traumatic knee OA. uCTX-II concentrations in ACLR were highest at the first testing time point, 4 weeks post-operative, and gradually declined until the last testing time point, 16 weeks post-operative. After adjustment for differences in BMI, log uCTX-II concentrations were found to be elevated in ACLR from 4 to 16 weeks post-operative compared to the mean concentration across 12 weeks in CNTRL. In ACLR, a greater decline in log uCTX-II concentrations was correlated with a greater decline in knee pain intensity and a greater improvement in IKDC-SKF scores. We interpret these results to indicate that uCTX-II concentrations are elevated shortly after ACL reconstruction, improve with time after surgery, and may be associated with changes in knee symptoms and function.
Our results indicate that elevated CTX-II concentrations may be detected in urine in the early period following ACL reconstruction. These results agree with previous findings of elevated CTX-II concentrations in synovial fluid after ACL reconstruction [26] and an increased percentage of denatured type II collagen at the femoral condyle after ACL injury.[38] Thus, uCTX-II concentrations may reflect local changes in knee articular cartilage metabolism. However, the implications of elevated uCTX-II concentrations on articular cartilage structure cannot be directly inferred from this study. Future research with both uCTX-II and articular cartilage structure measures is necessary to determine whether elevated uCTX-II concentrations are just a consequence of ACL injury and surgery or predict the development and progression of post-traumatic knee OA after ACL reconstruction.
Control subjects in this study were physically active in sports, and their activity level may have influenced study results. Group differences would possibly have been magnified with a more sedentary control group since higher uCTX-II concentrations have been reported in athletes compared to non-athletes.[28] Nonetheless, uCTX-II concentrations were maintained throughout the study in CNTRL, while in ACLR they demonstrated the capacity to decrease shortly after surgery. Another study of articular cartilage biomarkers similarly found a decrease over time after ACL injury.[39] However, uCTX-II concentrations increased in 8 subjects from the current study. It cannot be determined why this occurred, but the temporal pattern is worthy of further investigation because increasing uCTX-II concentrations have been associated with articular cartilage degradation in people with knee OA.[21, 23, 24] Subjects in ACLR likely increased their activity level over the study period and began running and jumping activities. Participation in sports, especially those that load the lower extremity, can raise uCTX-II concentrations.[28] It is also possible that increasing lower extremity loading after a period of relative unloading is detrimental to articular cartilage.[40] Therefore, monitoring the effect of increased activity in the advanced phase of rehabilitation should be considered in future research.
Ishijima and colleagues [25] reported higher uCTX-II concentrations in people with early knee OA who had knee pain. They speculated the mechanism for pain production might be 1) synovitis resulting from joint debris as articular cartilage degrades; or 2) pain in peri-articular tissues resulting from altered joint mechanics as joint structure changes.[25] We similarly found increasing knee pain with increasing uCTX-II concentrations in ACLR, but the mechanism for the association after ACL reconstruction is unclear. It may be due to synovitis that is still present after injury and/or surgery, or it may be that individuals with higher uCTX-II concentrations had worse injury, but this could not be confirmed. The association between uCTX-II concentrations and IKDC-SKF scores is difficult to interpret because the questionnaire contains items related to knee symptoms and function. The association may reflect higher knee pain and the effect of pain on function. Further research is needed to explore the mechanisms that link uCTX-II concentrations to these clinical outcomes.
Within the ACLR group, there was a negative association between age and the mean uCTX-II concentration, which agrees with a study of subjects aged 20 to 87 years.[37] The average age of subjects in our study was less than 20 years old, and 15 years was the youngest age. Because uCTX-II concentrations reflect input from the entire body, activity at the growth plate likely contributed to elevated concentrations in younger subjects. To the authors' knowledge, this is one of the youngest populations studied using uCTX-II. Therefore, ranges of uCTX-II for normal and diseased states in our study are not comparable to previous knee OA research because most subjects with knee OA are middle-aged or older. uCTX-II concentrations have ranged from 190 to 345 ng/mmol in middle-aged to older adult subjects without knee OA and 344 to 431 ng/mmol in those with advanced knee OA,[14, 23, 36] whereas the mean uCTX-II concentrations in our study were 2827 ng/mmol in CNTRL and 3223 ng/mmol in ACLR. With the study of post-traumatic knee OA in a younger population increasing, our study demonstrates the importance of age-matching for control subjects. While no associations between uCTX-II and other demographic variables were found, this should be examined in future research with larger samples.
The main strengths of this study include the inclusion of age- and sex-matched controls, longitudinal data collection across the early post-operative period, and a relatively homogeneous population of subjects with ACL reconstruction. However, there are limitations that should be considered. The coefficients of variation for uCTX-II analysis are higher than ideal because some samples were on the low end of the standard curve, amplifying any differences between calculated concentrations. Physical maturity was not assessed, thus the effect of growth plate status on uCTX-II concentrations and potential differences in growth plate status between matched pairs are unknown. This study did not include a measure of articular cartilage structure, so it is unknown if elevated uCTX-II concentrations in this early post-surgical timeframe are predictive of post-traumatic knee OA development. The baseline time point for ACLR was 4 weeks post-surgery and the time from injury to surgery varied, which means that uCTX-II concentrations were not measured acutely relative to injury in some subjects, and the differential effects of injury and surgery on uCTX-II concentrations cannot be distinguished. In addition, the study period only extended to 16 weeks post-surgery, which is well before radiographic signs of OA develop and before subjects resume pre-injury sport activities. We were unable to control rehabilitation, and although a common rehabilitation protocol was followed, it cannot be determined if subjects began advanced rehabilitation interventions (e.g. running and jumping) during the study period.
In conclusion, this study provides evidence that between 4 and 16 weeks after ACL reconstruction uCTX-II concentrations are elevated compared to controls and decline over time. The rate of decline in uCTX-II concentrations was shown to be associated with the rate of decline in knee pain and rate of improvement in knee symptoms and function. These findings provide initial support for further research of uCTX-II concentrations after ACL reconstruction, particularly related to the effect on articular cartilage structure and the response to rehabilitation interventions.
Table 3.
The mean log uCTX-II concentration and slope of log uCTX-II concentrations between 4 and 16 weeks after ACL reconstruction. No significant differences were found between sub-groups based on sex, the presence of meniscal surgery or graft type. Values are reported as mean (SD).
| Gender | Meniscal Surgery | Graft Type | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (n = 14) | Females (n = 14) | P-value | No (n = 18) | Yes (n = 10) | P-value | Allograft (n=11) | Autograft (n=17) | P-value | |
| Mean log uCTX-II concentration | 3.32 (.56) | 3.22 (.36) | .559 | 3.24 (.48) | 3.33 (.46) | .625 | 3.26 (.47) | 3.29 (.49) | .845 |
| Slope of log uCTX-II concentration | -.02 (.06) | -.04 (.06) | .560 | -.04 (.05) | -.01 (.07) | .202 | -.04 (.06) | -.02 (.06) | .378 |
Acknowledgments
The authors would like to acknowledge Chris Koenig, MS, ATC; Matt Walser, PA-C, ATC; Robert Coltman, PT, MPT, OCS, MTC; and Dawn Rosado, PT, MPT, COMT, CGFI; for their assistance with subject recruitment. The authors also acknowledge Melissa Cosgrave; DPT; Patty Hovis, MSESS; and Caroline Davis, BS; for their assistance with subject testing.
Role of the Funding Source: This study was funded by a grant from the Brooks Rehabilitation Research Endowment and supported in part by a grant from the National Institutes of Health (UL1-TR000064) from the National Center for Advancing Translational Sciences. Dr. Chmielewski's time and effort on this project were supported by a grant from the National Institutes of Health (K01-HD052713). The Metabolism and Biomarkers Core of The University of Florida's Institute on Aging and Claude D. Pepper Older Americans Independence Center (1P30AG028740) was utilized for biomarker analysis. The study sponsors had no involved in the study design, collection, analysis, interpretation of the data, or writing of the manuscript.
Footnotes
Author Contributions: All authors assisted with drafting of the article and critically appraising it for important intellectual content as well as providing final approval of the version to be submitted.
TLC: conception and design of the study, acquisition of the data, and analysis and interpretation of the data.
TNT, AMJ: analysis and interpretation of the data, including technical support for uCTX-II data.
JS: analysis and interpretation of the data, including statistical expertise.
MWM and PAI: provision of patients and interpretation of the data.
FMC: analysis and interpretation of the data.
CL: conception and design of the study and analysis and interpretation of the data.
Terese L. Chmielewski (tchm@ufl.edu) takes responsibility for the integrity of this work from inception to the finished manuscript.
Competing Interest Statement: None of the authors declare any competing interests in relation to this study.
Contributor Information
Terese L. Chmielewski, Department of Physical Therapy, University of Florida, Gainesville, FL.
Troy N. Trumble, Department of Veterinary Population Medicine, Veterinary Medical Center, University of Minnesota, St. Paul, MN.
Anna-Maria Joseph, Institute on Aging, University of Florida, Gainesville, FL.
Jonathan Shuster, Department of Health Outcomes and Policy, University of Florida, Gainesville, FL.
Peter A. Indelicato, Department of Orthopaedics and Rehabilitation, University of Florida, Gainesville, FL.
Michael W. Moser, Department of Orthopaedics and Rehabilitation, University of Florida, Gainesville, FL.
Flavia M. Cicuttini, Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
Christiaan Leeuwenburgh, Department of Aging and Geriatric Research, University of Florida, Gainesville, FL.
References
- 1.Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005;33:1579–1602. doi: 10.1177/0363546505279913. [DOI] [PubMed] [Google Scholar]
- 3.Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18:502–509. doi: 10.1053/jars.2002.30649. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 5.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 6.Hui C, Salmon LJ, Kok A, Maeno S, Linklater J, Pinczewski LA. Fifteen-year outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft for “isolated” anterior cruciate ligament tear. Am J Sports Med. 2011;39:89–98. doi: 10.1177/0363546510379975. [DOI] [PubMed] [Google Scholar]
- 7.Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 8.Andersson D, Samuelsson K, Karlsson J. Treatment of anterior cruciate ligament injuries with special reference to surgical technique and rehabilitation: an assessment of randomized controlled trials. Arthroscopy. 2009;25:653–685. doi: 10.1016/j.arthro.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 9.Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010;38:2201–2210. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 10.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: Part 1, outcomes. Am J Sports Med. 2008;36:40–47. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oiestad BE, Holm I, Engebretsen L, Risberg MA. The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10-15 years after anterior cruciate ligament reconstruction. Br J Sports Med. 2011;45:583–588. doi: 10.1136/bjsm.2010.073130. [DOI] [PubMed] [Google Scholar]
- 12.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 13.De Ceuninck F, Sabatini M, Pastoureau P. Recent progress toward biomarker identification in osteoarthritis. Drug Discov Today. 2011;16:443–449. doi: 10.1016/j.drudis.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17:384–389. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46:2613–2624. doi: 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 17.Cibere J, Zhang H, Garnero P, Poole AR, Lobanok T, Saxne T, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60:1372–1380. doi: 10.1002/art.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan KM, Syddall HE, Garnero P, Gineyts E, Dennison EM, Sayer AA, et al. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis. 2006;65:871–877. doi: 10.1136/ard.2005.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsdal MA, Byrjalsen I, Bay-Jensen AC, Henriksen K, Riis BJ, Christiansen C. Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis--the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation. BMC Musculoskelet Disord. 2010;11:125. doi: 10.1186/1471-2474-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanishi N, Yamagiwa H, Hayami T, Mera H, Koga Y, Omori G, et al. Relationship between radiological knee osteoarthritis and biochemical markers of cartilage and bone degradation (urine CTX-II and NTX-I): the Matsudai Knee Osteoarthritis Survey. J Bone Miner Metab. 2009;27:605–612. doi: 10.1007/s00774-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 21.Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050–1054. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharif M, Kirwan J, Charni N, Sandell LJ, Whittles C, Garnero P. A 5-yr longitudinal study of type IIA collagen synthesis and total type II collagen degradation in patients with knee osteoarthritis--association with disease progression. Rheumatology (Oxford) 2007;46:938–943. doi: 10.1093/rheumatology/kel409. [DOI] [PubMed] [Google Scholar]
- 23.Sowers MF, Karvonen-Gutierrez CA, Yosef M, Jannausch M, Jiang Y, Garnero P, et al. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17:1609–1614. doi: 10.1016/j.joca.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnero P, Aronstein WS, Cohen SB, Conaghan PG, Cline GA, Christiansen C, et al. Relationships between biochemical markers of bone and cartilage degradation with radiological progression in patients with knee osteoarthritis receiving risedronate: the Knee Osteoarthritis Structural Arthritis randomized clinical trial. Osteoarthritis Cartilage. 2008;16:660–666. doi: 10.1016/j.joca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. 2011;13:R22. doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48:3130–3139. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
- 27.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 28.O'Kane JW, Hutchinson E, Atley LM, Eyre DR. Sport-related differences in biomarkers of bone resorption and cartilage degradation in endurance athletes. Osteoarthritis Cartilage. 2006;14:71–76. doi: 10.1016/j.joca.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Niehoff A, Muller M, Bruggemann L, Savage T, Zaucke F, Eckstein F, et al. Deformational behaviour of knee cartilage and changes in serum cartilage oligomeric matrix protein (COMP) after running and drop landing. Osteoarthritis Cartilage. 2011;19:1003–1010. doi: 10.1016/j.joca.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54:2496–2504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]
- 31.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 32.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 34.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Neyret P, Richmond JC, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34:1567–1573. doi: 10.1177/0363546506288855. [DOI] [PubMed] [Google Scholar]
- 35.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34:128–135. doi: 10.1177/0363546505280214. [DOI] [PubMed] [Google Scholar]
- 36.Jung M, Christgau S, Lukoschek M, Henriksen D, Richter W. Increased urinary concentration of collagen type II C-telopeptide fragments in patients with osteoarthritis. Pathobiology. 2004;71:70–76. doi: 10.1159/000074419. [DOI] [PubMed] [Google Scholar]
- 37.Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62:332–336. doi: 10.1136/ard.62.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson F, Billinghurst RC, Pidoux I, Reiner A, Langworthy M, McDermott M, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Dahlberg L, Friden T, Roos H, Lark MW, Lohmander LS. A longitudinal study of cartilage matrix metabolism in patients with cruciate ligament rupture--synovial fluid concentrations of aggrecan fragments, stromelysin-1 and tissue inhibitor of metalloproteinase-1. Br J Rheumatol. 1994;33:1107–1111. doi: 10.1093/rheumatology/33.12.1107. [DOI] [PubMed] [Google Scholar]
- 40.Chmielewski TL. Asymmetrical lower extremity loading after ACL reconstruction: more than meets the eye. J Orthop Sports Phys Ther. 2011;41:374–376. doi: 10.2519/jospt.2011.0104. [DOI] [PubMed] [Google Scholar]


