Abstract
Alcohol consumption has been described as a risk factor for infection with Mycobacterium tuberculosis, but its contribution to tuberculosis has been difficult to isolate from other adverse socioeconomic factors. Our objective was to evaluate the impact of alcohol consumption on pulmonary infection with M. tuberculosis in a murine model. BALB/c mice were maintained on the Lieber-DeCarli liquid ethanol diet or a liquid control diet and infected intratracheally with low-dose M. tuberculosis H37Rv. Lung organism burdens, lung and lung-associated lymph node CD4+- and CD8+- lymphocyte numbers and rates of proliferation, and CD4+-lymphocyte cytokine production levels were compared between the groups. The alcohol-consuming mice had significantly higher lung organism burdens than the control mice, and the CD4+- and CD8+-lymphocyte responses to pulmonary infection with M. tuberculosis were blunted in the alcohol group. Lymphocyte proliferation and production of gamma interferon were decreased in the CD4+ lymphocytes from the alcohol-consuming mice. Additionally, lung granulomas were significantly smaller in the alcohol-consuming mice. In conclusion, murine alcohol consumption is associated with decreased control of pulmonary infection with M. tuberculosis, which is accompanied by alterations in the region-specific CD4+- and CD8+-lymphocyte responses and defective lung granuloma formation.
For many years, the medical literature worldwide has suggested an association between alcohol consumption and active tuberculosis in humans (7, 10, 16, 19, 23, 24, 28, 44). Screening alcohol-abusing subjects in an urban setting for tuberculous infection revealed rates of active tuberculosis that were 28-fold greater than those of age-matched residents and rates of positive tuberculin tests that were 1.5-fold greater than those of age-matched residents of the same locale (19). In another setting, heavy alcohol consumption conferred a twofold-heightened risk for developing active tuberculosis (10). Additionally, a person with active tuberculosis who routinely frequented a neighborhood bar was found to have infected 41 persons who were frequent clients of the bar, one-third of whom developed active tuberculosis (24). Similar reports of increased prevalence of tuberculosis in alcohol-consuming subjects exist outside of the United States. In London, England, regular alcohol use increased the risk of contracting tuberculosis more than twofold (28), and alcohol abuse was found to be a risk factor in the acquisition of multidrug-resistant tuberculosis in Russia (44).
However, the bulk of the evidence of an association between alcohol consumption and tuberculosis is circumstantial, and not all studies consistently demonstrate this association (34, 42). Among homeless persons in San Francisco, despite very high rates of tuberculosis, alcohol abuse was not found to be a significant risk factor (34). Regular consumers of alcohol frequently have confounding lifestyle factors, such as other substance (i.e., illicit drugs or tobacco) abuse, low socioeconomic status, homelessness, and lack of compliance with medical follow-up and/or treatments. Thus, it has been very difficult to evaluate the specific contribution of alcohol consumption to increased rates of tuberculosis in human studies. Limited data have been published regarding the adverse effects of alcohol on rodent models of mycobacterial infections, exclusively with nontuberculous mycobacterial strains (4, 25, 30, 31). In order to evaluate the effect of alcohol consumption on infection with M. tuberculosis, we used a murine model of chronic alcohol administration. Alcohol worsened pulmonary infection with a virulent strain of M. tuberculosis, which was accompanied by alterations in the region-specific lymphocytes in the alcohol-consuming animals. This demonstrates that alcohol can contribute to failure to control infection with M. tuberculosis.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free BALB/c mice (Hilltop Labs, Scottdale, Pa.) were used for these experiments. The animals were housed in the Louisiana State University Health Sciences Center (LSUHSC) Pulmonary/Critical Care Medicine Biocontainment Level-3 Laboratory, and experiments were performed in the Biocontainment Level-3 Laboratory in accordance with the appropriate safety precautions recommended by the Centers for Disease Control and Prevention (36). All animal procedures were approved by the LSUHSC Institutional Animal Care and Use Committee. The experiments were performed in duplicate, and representative results are presented. All data represent groups of five mice unless otherwise noted.
Chronic alcohol diet.
Mice consumed the nutritionally complete Lieber-DeCarli liquid ethanol diet (LED) (Dyets catalog number 710260; Bethlehem, Pa.), which supplies 36% of caloric intake as ethanol, or were fed an isocaloric liquid control diet (LCD) (Dyets catalog number 710027). The animals were fed the respective liquid diets for 5 of 7 days and given chow ad lib for 2 of 7 days. Animals in the LED group were given water containing 20% (wt/vol) ethanol on the two chow diet days. The weights of the mice were recorded weekly. Serum alcohol concentrations (random morning sample) were measured. The mice were fed the Lieber-DeCarli LED or the LCD for 2 weeks prior to intratracheal inoculation with M. tuberculosis H37Rv, and the LED or LCD was continued on the described schedule until the mice were sacrificed. All groups included five mice unless otherwise specified.
M. tuberculosis H37Rv infection.
M. tuberculosis H37Rv was obtained from the American Type Culture Collection (Rockville, Md.; ATCC 27294) and was grown in Middlebrook 7H11 broth at 37°C for 14 days. This culture was concentrated by centrifugation, gently sonicated at 95 W for 10 s in a cup horn sonicator, and stored in 0.1-ml aliquots at −80°C. At the time of inoculation, an aliquot was thawed, gently sonicated, and diluted in endotoxin-free phosphate-buffered saline to a concentration of 102 organisms/ml. Mice were lightly anesthetized intramuscularly with ketamine (200 mg/kg of body weight) and xylazine (10 mg/kg), the ventral surface of the neck was swabbed with isopropyl alcohol, and a midline skin incision was performed in a sterile fashion. The soft tissues of the neck were gently retracted laterally to expose the trachea. Mycobacteria were sonicated at 95 W for 10 s in a cup horn sonicator prior to injection to achieve uniform single-organism dispersion. Direct intratracheal injection was performed by injecting 100 μl of bacterial suspension (20 CFU) through a 30-gauge needle followed by 0.3 ml of air. The incision was closed with a stainless-steel surgical clip, and the animal was allowed to recover. At serial time points (days 7, 14, 21, 28, and 35) after injection, the animals were sacrificed and the lungs were homogenized, serially diluted, and plated in quadruplicate on Middlebrook 7H10 agar plates. The plates were incubated at 37°C for 17 days. At the end of the incubation period, the number of CFU present on plates containing between 20 and 200 CFU per quadrant was counted and multiplied by the appropriate dilution ratio in order to determine the number of CFU in the initial tissue homogenate. The M. tuberculosis organism burdens of the LED and LCD groups were then compared.
Isolation of lung and LALN lymphocytes.
In additional groups of animals, lung and lung-associated lymph node (LALN) lymphocytes were isolated according to the following methods (22). Lungs were removed from mice in both groups at serial times after sacrifice (days 14, 21, 28, and 35), and the conducting airways were removed. The lung tissue was minced and placed in RPMI 1640 containing collagenase (150 U/ml) and DNase (50 U/ml) for enzymatic digestion. Ten milliliters of the enzymatic solution was used for every 600 mg of lung tissue, and the mixture was incubated at 37°C for 90 min with constant stirring. After incubation, the mixture was passed through a 70-μm-pore-size nylon mesh, placed briefly in NH4Cl lysis buffer, and resuspended in indomethacin (1 μg/ml) and catalase (250 U/ml). The cells were counted, their viability was checked, and then they were diluted to the desired concentration. LALNs were isolated from hilar lymph nodes and mediastinal lymph nodes dissected after sacrifice. Single-cell suspensions were prepared by gently disrupting the tissue on a 70-μm-pore-size nylon filter. The cells were centrifuged prior to resuspension in NH4Cl lysis buffer, to which Hanks balanced salt solution was added. The cells were centrifuged again, counted with a hemacytometer, and resuspended at a concentration of 106 cells/ml. CD4+ lymphocytes were isolated from both lung cells and LALN cells by magnetic-bead selection (Dynal, Inc., Lake Success, N.Y.) (1, 14, 29). CD4+ lymphocytes isolated by the magnetic-bead technique were >95% pure for the LALN cell population and >90% pure for the lung cell population as determined by flow cytometry (data not shown). Differential cell counts were performed with cytospin monolayers of isolated lung and LALN cells, and the total lymphocyte counts from each site were determined from the total cell count and the percentage of lymphocytes. The total CD4+- and CD8+-lymphocyte counts were calculated as described below and compared between the LED and LCD groups. Additional groups of mice maintained on the LED and LCD diets were studied for LALN and lung CD4+-lymphocyte numbers in the absence of infection with M. tuberculosis.
Analysis of CD4+-and CD8+-lymphocyte proliferation.
LALN lymphocytes (in single-cell suspensions prepared as described above) were stained for CD4 or CD8 surface marker expression with either anti-CD4 monoclonal antibody (MAb; clone RM4-5) or anti-CD8 MAb (clone 53-6.7) conjugated with peridinin chlorophyll a protein (PerCP) (BD Pharmingen, San Diego, Calif.). A total of 106 cells were incubated with 1 μg of anti-CD4 or anti-CD8 MAb for 20 min at 4°C, and then the cells were washed and pelleted (200 × g for 5 min). The cells were fixed and permeabilized by incubation with paraformaldehyde (1%) and saponin (0.1%). After incubation and washing, the cells were incubated with a fluorescein isothiocyanate-conjugated anti-human Ki67 antibody (clone B56; BD Pharmingen), an intracellular nuclear proliferation marker expressed exclusively by cells in all but the resting phase of the cell cycle (15, 32). The anti-Ki67 antibody cross-reacts with murine Ki67. Appropriate isotype MAbs were used as controls for each step of the staining process. After the final washing, the cells were analyzed with a FACSCalibur flow cytometer. The lymphocyte population was determined based on light scatter characteristics (forward versus 90° scatter). CD4+ or CD8+ lymphocytes were identified by log signal fluorescence, and the percentage of each subset was multiplied by the total lymphocyte count from each site to determine the absolute number of CD4+ and CD8+ lymphocytes for the LALNs and lungs. Additionally, the percentage and absolute number of each lymphocyte subset that expressed Ki67 were determined.
CD4+-lymphocyte cytokine production.
Cytokine elicitation was performed in vitro with M. tuberculosis-infected peritoneal macrophages (PMs) (21, 29). PMs were elicited from naive BALB/c mice with concanavalin A (100 μg, type IV; Sigma, St. Louis, Mo.) injected intraperitoneally 96 h prior to peritoneal lavage with Dulbecco's modified Eagle medium (DMEM) at 4°C. The harvested cells were washed, counted (with a differential count), and checked for viability, and the red blood cells were lysed. PMs were then placed in complete DMEM-10-10% fetal bovine serum, adjusted to a concentration of 2 × 106 cells/ml, plated in 96-well plates at 2 × 105 cells/well, and allowed to adhere for 2 h at 37°C. The cells were then washed three times to remove nonadherent cells. Next, M. tuberculosis H37Rv was added to the macrophage monolayer at a concentration of 106 CFU/well (multiplicity of infection, 5:1) and incubated for 18 to 24 h at 37°C. After incubation, extracellular bacteria were removed three times by aspiration with DMEM, complete DMEM-10 was replaced, and the infected PMs were maintained in culture for an additional 24 h. Aliquots of isolated CD4+ lymphocytes from LALNs and lungs from both the LED and LCD groups of animals were suspended in DMEM-10 at 37°C, and 105 cells were added to each well of PMs and incubated at 37°C for 48 h. Cells from the LED mice were incubated with 25 mM ethanol added to the wells in an incubator which maintained a 25 mM ambient concentration of ethanol. Control experiments were performed with uninfected PMs incubated with lymphocytes from LCD or LED mice as well as with infected PMs incubated in the absence of lymphocytes (with and without in vitro ethanol). After the incubation, the supernatants were harvested, spun, saved at −80°C, and subsequently assayed for gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-2 by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.). The lower limits of detection for these assays were 2 pg/ml for IFN-γ, 2 pg/ml for IL-4, and 3 pg/ml for IL-2. Cytokine levels were then compared between the LED and LCD groups.
Lung histopathology.
After sacrifice (on days 21, 28, and 35), the lungs from two mice from each diet group were excised and gently inflated with 10% formalin after tracheal cannulation. The tracheas were then ligated to maintain the inflated state, and the lungs were immersed in formalin. The lungs were then embedded in paraffin, sagittal sections were prepared, and the sections were stained with hematoxylin and eosin and Fite stains (the latter for demonstration of acid-fast bacilli). The sections were examined by light microscopy under a magnification of ×100, and granulomas were evaluated. The granulomas identified in the sections from each mouse were assessed with a Leica DMRXA microscope, and the granuloma area (in square micrometers) was determined with Slidebook software (Spectra Services, Inc., Webster, N.Y.). The mean granuloma area for each mouse for each time point was determined, and the values were compared between the groups.
Statistical analysis.
Differences between the LED and LCD groups were analyzed by t testing for each time point studied. A difference with a P value of ≤0.05 was considered significant (8).
RESULTS
The experiments were performed in duplicate with consistent results. Data from representative experiments are presented.
Chronic alcohol diet.
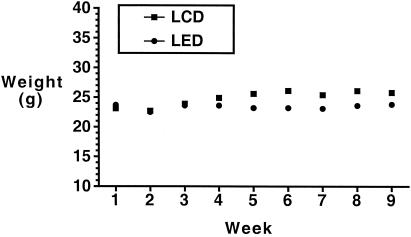
The animals on the LED consumed ∼8 ml of liquid diet per day and gained weight throughout the course of the experiments, though more slowly than the mice on the LCD (Fig. 1). Serum alcohol levels (from random morning samples) for representative animals (n = 15) on the LED for 4 to 6 weeks were measured at the LSUHSC Alcohol Research Center Core Laboratory with an AM1 alcohol analyzer (Analox Instruments, Lunenburg, Mass.), with a median concentration of 39 mg/dl (range, 19 to 130 mg/dl).
FIG. 1.
Weights of mice in the groups consuming the LED and the LCD over 9 weeks.
Impact of chronic alcohol consumption on infection with M. tuberculosis H37Rv.
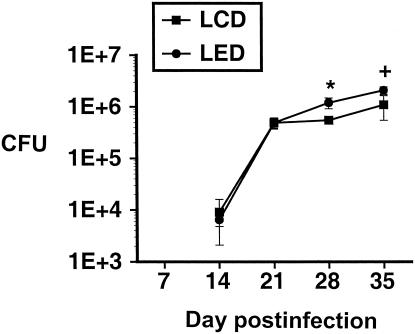
After infection with M. tuberculosis, mice in the LED groups had a higher lung organism burden than the LCD mice did at days 28 and 35 after M. tuberculosis inoculation (Fig. 2), indicating that alcohol consumption led to failure to control the infection at those time points.
FIG. 2.
Lung burden of M. tuberculosis H37Rv in mice in the LED and LCD groups at serial time points after inoculation with M. tuberculosis. There are significantly more organisms recovered from the lungs of the LED mice than from the LCD mice at day 28 (*, P < 0.05) and a trend toward a greater burden at day 35 (+, P = 0.06). Error bars indicate standard errors of the means.
Effect of alcohol consumption on region-specific CD4+- and CD8+-lymphocyte responses to M. tuberculosis.
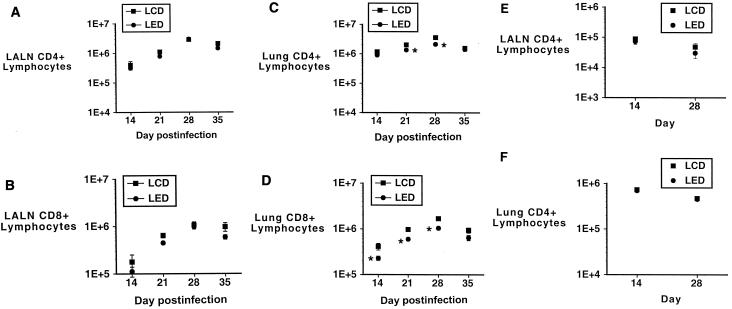
At the serial time points at which the lungs were cultured for M. tuberculosis burden, lung and LALN cells were harvested and lymphocytes were isolated as described above. No samples were collected on day 7 because previous work revealed no differences from uninfected mice and because insufficient numbers of CD4+ and CD8+ T cells are retrieved at this time (especially from the LALNs). Total, CD4+, and CD8+ lymphocytes were counted for each tissue site for each animal. As shown in Fig. 3A through D, there were no differences between the LED and LCD animals with respect to the CD4+- or CD8+-lymphocyte counts in the LALNs, but there were significantly fewer CD4+ and CD8+ lymphocytes present in the lungs of the LED animals at days 21 and 28 (and day 14 for the CD8+ subset). The LCD group's CD4+ counts compare favorably with the results of a previous study in which infected mice were maintained on a chow diet (29). Figure 3E and F show numbers of LALN and lung CD4+ lymphocytes from the LED and LCD groups in the absence of infection with M. tuberculosis at days 14 and 28. There are no differences in cell numbers between the two diet groups at either time point for either the LALN or lung CD4+ lymphocytes. The numbers of total lung and LALN lymphocytes were also not different between the two diet groups of uninfected mice at these times (data not shown).
FIG. 3.
(A and B) Numbers of LALN CD4+ (A) and CD8+ (B) lymphocytes recovered at serial time points after M. tuberculosis infection in the LED and LCD groups. There are no differences between the groups at any time point for LALN CD4+ or CD8+ lymphocytes. (C and D) Numbers of lung CD4+ (C) and CD8+ (D) lymphocytes recovered at serial time points after M. tuberculosis infection in the LED and LCD groups. There are significantly fewer lung CD4+ lymphocytes in the LED group than in the LCD group at days 21 and 28 and fewer lung CD8+ lymphocytes at days 14, 21, and 28 (*, P < 0.05). (E and F) Numbers of LALN (E) and lung (F) CD4+ lymphocytes in mice consuming the LED and LCD diets, but not infected with M. tuberculosis, at days 14 and 28. There were no differences between the groups at either site at either time point. Error bars indicate standard errors of the means.
As only the surface markers CD4 and CD8 were utilized to identify CD4+ or CD8+ lymphocytes, the contribution of other CD4+ or CD8+ cell types cannot be excluded with certainty. However, in naive BALB/c mice (n = 4), >99% of CD4+ or CD8+ lung or LALN cells in the lymphocyte gate stained positively for CD3 (the pan-T cell marker), with less than 1% being CD3− CD4+ or CD3− CD8+ (data not shown).
CD4+- and CD8+-lymphocyte proliferation.
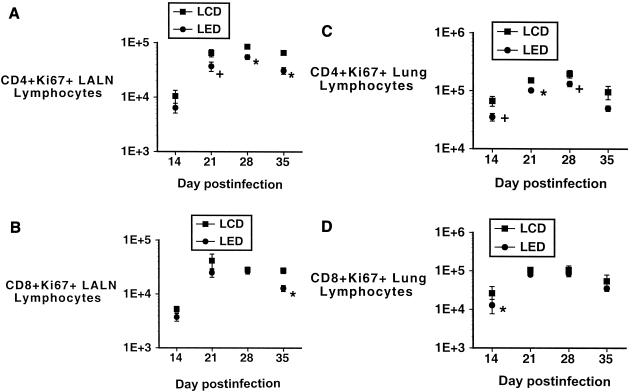
The results of the analysis of the Ki67+ population of the CD4+ and CD8+ LALN and lung lymphocytes are shown in Fig. 4. On day 14, the number of mice in each group (LCD, n = 3; LED, n = 4) is due to low numbers of LALN lymphocytes at that time point. There were fewer CD4+ Ki67+ LALN lymphocytes in the LED group than in the LCD group, but there were no differences in Ki67 expression in the CD8+ LALN lymphocytes between the LED and LCD groups (with the exception of the day 35 time point). Similar profiles are present with respect to lymphocytes from the lungs (using four mice for the day 14 analyses), with fewer CD4+ Ki67+ lung lymphocytes in the LED group (at days 21 and 28), but no differences in the CD8+ Ki67+ lung lymphocytes between the two groups.
FIG. 4.
(A and B) Numbers of Ki67+ CD4+ (A) and CD8+ (B) LALN lymphocytes recovered at serial time points after M. tuberculosis infection in the LED and LCD groups. There are significantly fewer Ki67+ CD4+ lymphocytes in the LED group than in the LCD group at days 28 and 35 (*, P < 0.05; results for four mice at day 14) and a trend to fewer Ki67+ lymphocytes in the LED group at the day 21 time point (+, P = 0.057) (A). There are no differences in the numbers of Ki67+ CD8+ LALN lymphocytes between the groups except at the day 35 time point, when there are fewer Ki67+ CD8+ lymphocytes in the LED group than in the LCD group (*, P < 0.05; results for three mice at day 14 and four mice at day 35). (C and D) Numbers of lung Ki67+ CD4+ (C) or CD8+ (D) lymphocytes recovered at serial time points after M. tuberculosis infection in the LED and LCD groups. (C) There are significantly fewer Ki67+ CD4+ lung lymphocytes in the LED group than in the LCD group at day 21 (*, P < 0.05) and trends to fewer Ki67+ cells at days 14 and 28 (+, P < 0.08; results for four mice at day 14). (D) There are no differences in the numbers of Ki67+ CD8+ lung lymphocytes between the groups except at the day 14 time point, when there are fewer Ki67+ CD8+ lymphocytes in the LED group than in the LCD group (*, P < 0.05; results for four mice at day 14). Error bars indicate standard errors of the means.
CD4+-lymphocyte cytokine production.
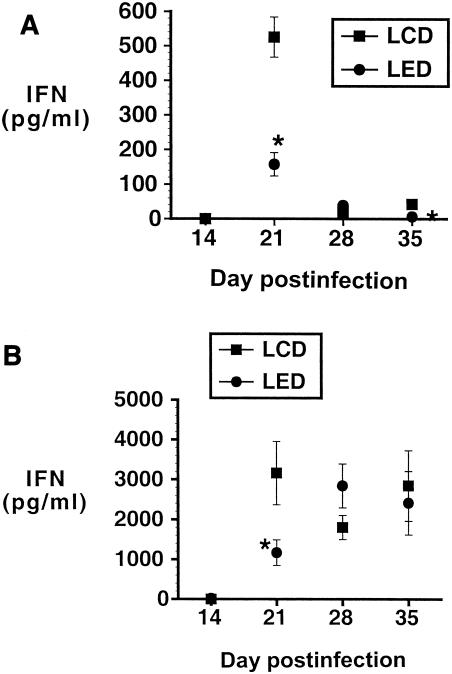
CD4+ lymphocytes isolated from LALNs and lungs from both the LED and LCD groups were assayed for cytokine production after stimulation with M. tuberculosis-infected PMs as described above. Figure 5 shows IFN-γ production from LALN and lung CD4+ lymphocytes for the LED and LCD groups. At the day 21 time point, there was significantly less IFN-γ elicited from the LALN and lung CD4+ lymphocytes from the LED group than from the LCD group. All control wells had <42 pg of IFN-γ/ml at all time points (data not shown). In a repeat experiment in which lung lymphocytes from the LED mice were incubated with M. tuberculosis-infected macrophages either in an ethanol (25 mM) incubator or a conventional incubator, there were no significant differences in elicited production of IFN-γ between the two sets of LED group lymphocytes at any time point. The peak IFN-γ value from the LED group lymphocytes maintained under ethanol incubation was 5,562 ± 1,048 pg/ml, and that from the LED group lymphocytes incubated without ethanol was 7,278 ± 1,511 pg/ml (P, not significant), both levels being below the peak IFN-γ value for the LCD group. Thus, while ethanol incubation results in minor decrements in elicited IFN-γ, the differences in IFN-γ between the LED and LCD groups (Fig. 5) are not likely the result of in vitro ethanol effects.
FIG. 5.
(A and B) IFN-γ production elicited from LALN (A) and lung (B) CD4+ lymphocytes by 48 h of in vitro incubation with M. tuberculosis-infected PMs. For both the LALN and lung lymphocytes, there is significantly less IFN-γ produced at the day 21 time point by lymphocytes from the LED group than from the LCD group (*, P < 0.05). Error bars indicate standard errors of the means.
IL-4 concentrations were <9 pg/ml at all time points for both LALNs and lungs. IL-2 concentrations are shown in Table 1. There was significantly less IL-2 detected in the CD4+ lymphocytes isolated from both the LALNs and the lungs in the alcohol-consuming (LED) group than in those from the LCD group at 21 days.
TABLE 1.
IL-2 levels in supernatants of LALN and lung CD4+ lymphocytes after stimulation with M. tuberculosis-infected PMs
| Lymphocyte source | Day postinfection | IL-2 levela (pg/ml) (mean ± SEM)
|
|
|---|---|---|---|
| LCD group | LED group | ||
| LALNsb | 14 | 13.6 ± 0.3 | 11.8 ± 0 |
| 21 | 26.3 ± 2.0 | 20.4 ± 1.5* | |
| 28 | 16.9 ± 1.5 | 17.5 ± 1.6 | |
| 35 | 18.2 ± 2.1 | 19.7 ± 1.3 | |
| Lungsc | 14 | 14.6 ± 1.1 | 17.5 ± 2.5 |
| 21 | 62.9 ± 13.9 | 26.2 ± 2.1* | |
| 28 | 27.1 ± 2.4 | 31.2 ± 2.9 | |
| 35 | 38.3 ± 5.6 | 29.5 ± 2.5 | |
*,Significantly different from value for LCD mice at a P of <0.05.
LALN lymphocytes were pooled for day 14 because of low numbers of cells (for LCD, n = 2, for LED, n = 1). For all other times, n = 5 per group.
n = 4 mice per group for day 14 time point (all other times, n = 5).
Lung histopathology.
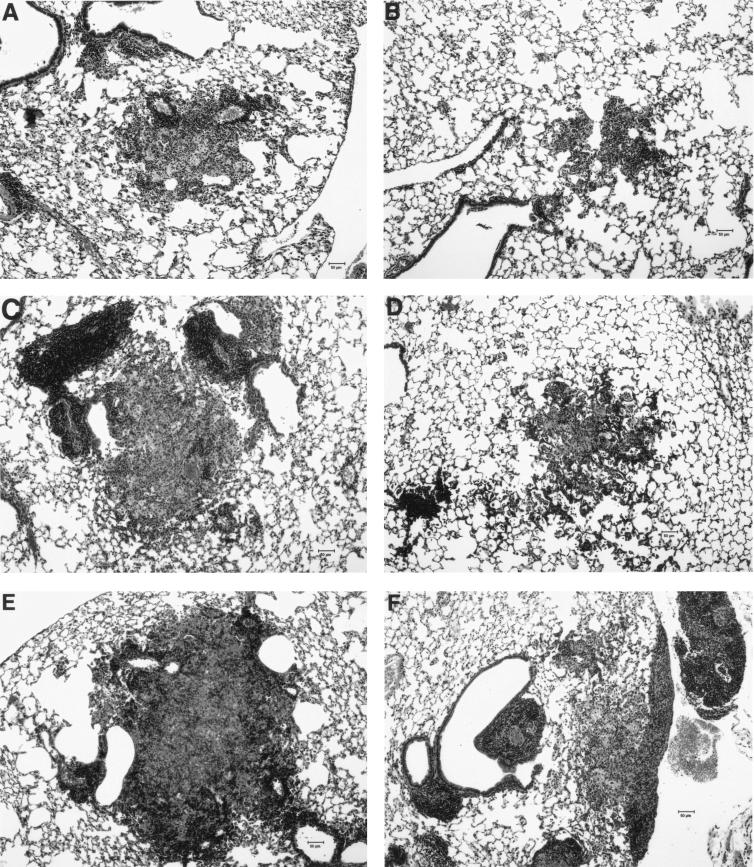
Lung sections stained with hematoxylin and eosin and Fite stains were examined at a magnification of ×100. Fite staining revealed abundant acid-fast bacilli within the granulomas of mice from both groups without obvious differences between the groups. Hematoxylin-and-eosin-stained sections from the mice in the LCD group revealed progressively larger granulomas from day 21 to day 35. The granulomas from mice from the LED group had smaller areas than those from the LCD mice at each of the three time points (Table 2 and Fig. 6). The areas of the granulomas from the LED group were 29, 36, and 25% of those from the LCD group for days 21, 28, and 35, respectively.
TABLE 2.
Mouse granuloma size after infection with M. tuberculosis
| Day | Granuloma area (μm2 × 104)
|
LED/LCD (%)b | |
|---|---|---|---|
| LCDa | LEDa | ||
| 21 | 21.1 ± 4.9 | 6.2 ± 0.9 | 29 |
| 28 | 30.5 ± 0 | 11.1 ± 5.1 | 36 |
| 35 | 39.9 ± 3.1 | 9.9 ± 5.3 | 25 |
Values are means ± standard errors of the means (n = 1 for LCD group on day 28).
LED/LCD, granuloma area for LED mice divided by granuloma area for LCD mice.
FIG. 6.
Representative lung sections from mice in the LCD and LED groups stained with hematoxylin and eosin. All magnifications, ×100. (A) Day 21 LCD mouse; (B) day 21 LED mouse; (C) day 28 LCD mouse; (D) day 28 LED mouse; (E) day 35 LCD mouse; (F) day 35 LED mouse.
DISCUSSION
We have shown that chronic alcohol consumption worsens pulmonary infection with M. tuberculosis in a murine model. In this model, alcohol consumption results in lower lymphocyte IL-2 production, diminished CD4+-lymphocyte proliferation in the regional lymph nodes, and fewer CD4+ lymphocytes in the lungs of the infected mice. Lung CD8+-lymphocyte numbers are also reduced in the alcohol-consuming mice. Additionally, IFN-γ production elicited by LALN and lung CD4+ lymphocytes is lower in the LED group than in the LCD group. Lung histopathology added confirmatory information, in that the granulomas of the alcohol-consuming mice are significantly smaller than those of the control mice, being approximately one-third of the area of the granulomas from the control group. These results suggest that the lower number of CD4+ lymphocytes in the lungs and the defective granuloma formation combined with lower IFN-γ production (on a per-cell basis) contribute to the lack of control of M. tuberculosis growth in the lungs of the alcohol-consuming mice.
The importance of CD4+ lymphocytes in protection against tuberculosis and in maintenance of latency is evident in human and murine studies. Human immunodeficiency virus-infected individuals with resultant CD4+-lymphocyte depletion have rates of tuberculosis progression, reactivation, and reinfection that are markedly higher than those of persons uninfected with HIV (2, 41). CD4+ lymphocytes are normally prominently featured in granulomas and play key roles in maintaining granuloma structure and preventing unrestricted growth and dissemination of infecting mycobacteria (20, 37, 38). Control of the infection may take place by local interaction of CD4+ lymphocytes with, and activation of, macrophages for mycobactericidal activity by IFN-γ produced by the CD4+ lymphocytes. The production of reactive nitrogen intermediates is required for mycobacterial growth restriction by macrophages, and these substances are IFN-γ dependent (12, 17). Mice rendered CD4+ lymphocyte deficient are unable to contain an acute challenge or latent infection with M. tuberculosis despite compensatory IFN-γ production by CD8+ lymphocytes as well as persistent production of reactive nitrogen intermediates (11, 38). This finding implies that the timing of the CD4+-lymphocyte responses to infection is critical and/or that the CD4+ lymphocyte performs other (non-IFN-γ-related) key roles in the setting of murine tuberculosis. Possible roles include alternate mechanisms of macrophage activation for mycobactericidal function, maintenance of the local CD8+ lymphocyte population, and mononuclear cell recruitment and retention for proper granuloma structure and function (37).
Traditional models hold that effector lymphocytes expand from naive T cells in the regional lymph tissue after encountering antigen presented by the antigen-presenting cells (18). IL-2, produced by activated lymphocytes, facilitates effector lymphocyte expansion. The effector lymphocytes then traffic to the site of the ongoing infection and/or inflammation. We have shown that the alcohol-consuming animals have lower levels of IL-2 production elicited by the LALN lymphocytes and depressed CD4+-lymphocyte proliferation in the regional lymph nodes (by Ki67 expression) but that no differences in proliferation are discernible for CD8+ lymphocytes. Interestingly, we also demonstrated this same pattern in the lungs of the infected mice. Thus, in our model, defective proliferation preferentially affects CD4+ lymphocytes.
We detected low-level expression of Ki67 among the CD4+ lung lymphocytes, which is suppressed in the LED group. No group differences were found for the CD8+ lung lymphocytes. Possible explanations for this finding of Ki67+ T cells in the lung include that the Ki67+ lymphocytes are recent arrivals from the regional lymph tissue via the circulation or that low-level proliferation occurs in the lungs of mice with tuberculosis. However, others refute this latter possibility (40). Lymphocyte-trafficking studies would be required to differentiate these possibilities. We did detect lower levels of IL-2, a cytokine integrally involved in lymphocyte proliferation, at day 21 in the LED mice. This finding is likely related to the lower numbers of CD4+ lymphocytes found in the lungs of our LED mice. Alcohol has been reported to have variable effects on IL-2, but in some models, alcohol or its metabolites play a role in the suppression of this cytokine and in diminished lymphocyte proliferation (9, 13, 39). In our model, the depression of IL-2 levels occurs at day 21, correlating with the time point at which there are significantly fewer CD4+ lymphocytes in the lungs of the LED mice.
The number of lung CD8+ lymphocytes in the infected LED mice was also diminished compared to that in the mice in the LCD group. As for the CD4+ subset, the numbers of CD8+ T cells in the LALNs from the LED and LCD group mice were not different, suggesting that the effect of alcohol was not simply global depression of lymphocyte numbers. No alcohol-induced alterations in CD8+-lymphocyte proliferation were present in either the LALNs or the lungs, suggesting that other mechanisms are responsible for the reduced numbers of lung CD8+ T cells in the LED group. Decreased recruitment of the lymphocytes to the lungs or increased CD8+-cell death in the lungs may explain the difference and provide avenues for future study.
CD8+ T cells play a role (albeit a lesser one than that of CD4+ lymphocytes) in the control of mycobacterial growth, as mice lacking functional CD8+ lymphocytes have higher organism burdens (by ∼1 log) than those with intact CD8+ T cells (33, 35). Evidence is emerging that the CD8+-lymphocyte subset plays a greater role in the maintenance of the latent phase of tuberculosis than in the acute phase of the infection (during which CD4+ lymphocytes play the primary role) (45). Further work is necessary to dissect the alcohol-induced alterations in CD8+ lymphocytes.
Previously investigators have examined the effects of alcohol consumption in experimental models of nontuberculous mycobacterial infection (3-6, 30, 31). Most describe a worse infection burden in animals given alcohol, but presently no unifying concept explains the deleterious effects of alcohol on mycobacterial growth. Our work points to defective effector CD4+ lymphocytes in the alcohol-consuming mice, but other host defense components may also be adversely affected by alcohol and may contribute to the failure to control mycobacterial growth. We have not excluded effects on components of the innate immune system (i.e., antigen-presenting cells, neutrophils, or natural killer cells), but we do demonstrate aberrancies in the effector CD4+-lymphocyte subset.
Our alcohol-consuming mice gained less weight than the control mice over the course of the experiments, and it is difficult to exclude with certainty a nutritional effect on the loss of control of mycobacterial growth in this model. Original reports of the use of the LED in rodents also describe lesser weight gain in the alcohol-consuming animals than in the controls (26). Alcohol-consuming rodents ingest less food while on the liquid diet but do not have nutritional deficiencies despite altered consumption (27). The caloric intake of our mice on the LED and LCD diets was 8 to 9 kcal/day, on the lower end of the normal range for adult BALB/c mice (43). Our data, in which there were no differences in the LALN and lung CD4+-lymphocyte numbers between the LED and LCD mice not infected with M. tuberculosis, provide evidence that dietary nutritional effects alone are unlikely to account for differences in the cell numbers. Additionally, in our infected mice, which had fewer CD4+ lung lymphocytes in the LED group, there were no differences between the two diet groups in the numbers of CD4+ LALN lymphocytes, again suggesting that nutritional differences are unlikely to account for the alterations in lymphocyte numbers.
Our model utilizes the pulmonary route for low-dose infection with virulent M. tuberculosis in mice consuming alcohol chronically. Our finding of fewer CD4+ lymphocytes in the lungs of alcohol-consuming, M. tuberculosis-infected mice is likely causally linked to the inability of these mice to contain the mycobacterial challenge. The alcohol-induced inability to increase the CD4+-lymphocyte population in response to pulmonary infections, as demonstrated here for tuberculosis, is at least partially due to diminished IL-2 production and lymphocyte proliferation in the regional lymph nodes. Additionally, granuloma formation is abnormal in the alcohol-consuming mice. In the work presented here we have not excluded alterations in the recruitment of CD4+ lymphocytes to the lungs or their persistence in the lungs (i.e., by accelerated apoptosis or necrosis), but ongoing experiments with this model will examine those processes. In addition to depressed CD4+-lymphocyte expansion in the lymph nodes and fewer CD4+ lymphocytes in the lungs of the alcohol-consuming mice, there is a diminished capability of the CD4+ lung lymphocytes to secrete IFN-γ (on a per-cell basis) when stimulated with M. tuberculosis-infected macrophages. This work suggests that chronic alcohol consumption adversely affects multiple facets of the effector CD4+-lymphocyte population, thereby compromising the containment of a challenge by M. tuberculosis. These findings provide initial insight into how alcohol may adversely affect the host's ability to control tuberculous infection and provide a preliminary understanding of the increased prevalence of tuberculosis among alcohol-consuming subjects.
Acknowledgments
This work was supported by AA011760, La BOR HEF{2000-05}-06, and the LSUHSC Research Enhancement Fund (2002-2003).
We thank Connie Poretta for expert assistance with the flow cytometry analyses and Luis Marrero for help with the histopathology studies.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Anderson, G., E. Jenkinson, N. Moore, and J. Owen. 1993. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature 362:70-73. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P., A. Bloch, P. Davidson, and D. Snider. 1991. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 324:1644-1650. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. 1994. Effect of ethanol on the interaction between the macrophage and Mycobacterium avium. Alcohol 11:69-73. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez, L., M. Petrofsky, P. Kolonoski, and L. Young. 1992. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J. Infect. Dis. 165:75-79. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez, L., and L. Young. 1991. Ethanol augments intracellular survival of Mycobacterium avium complex and impairs macrophage responses to cytokines. J. Infect. Dis. 163:1286-1292. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez, L., L. Young, J. Martinelli, and M. Petrofsky. 1993. Exposure to ethanol up-regulates the expression of Mycobacterium avium complex proteins associated with bacterial virulence. J. Infect. Dis. 168:961-968. [DOI] [PubMed] [Google Scholar]
- 7.Borgdorff, M., J. Veen, N. Kalisvaart, and N. Nagelkerke. 1998. Mortality among tuberculosis patients in The Netherlands in the period 1993-1995. Eur. Respir. J. 11:816-820. [DOI] [PubMed] [Google Scholar]
- 8.Bourke, G., L. Daly, and J. McGilvray. 1985. Interpretation and uses of medical statistics, 3rd ed. Blackwell Scientific Publications, Oxford, England.
- 9.Braun, K., P. Pearce, and C. Peterson. 1995. Acetaldehyde-serum protein adducts inhibit interleukin-2 secretion in concanavalin A-stimulated murine splenocytes: a potential common pathway for ethanol-induced immunomodulation. Alcohol. Clin. Exp. Res. 19:345-349. [DOI] [PubMed] [Google Scholar]
- 10.Buskin, S., J. Gale, N. Weiss, and C. Nolan. 1994. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. Am. J. Public Health 84:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso, A., N. Serbina, E. Klein, K. Triebold, B. Bloom, and J. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 12.Chan, J., Y. Xing, R. Magliozzo, and B. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudry, M., K. Messingham, S. Namak, A. Colantoni, C. Fontanilla, L. Duffner, M. Sayeed, and E. Kovacs. 2000. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol 21:239-243. [DOI] [PubMed] [Google Scholar]
- 14.Collins, C., S. Norris, G. McEntee, O. Traynor, L. Bruno, H. V. Boehmer, J. Hegarty, and C. O'Farrelly. 1996. RAG1, RAG2 and pre-T cell receptor alpha chain expression by adult human hepatic T cells: evidence for extrathymic T cell maturation. Eur. J. Immunol. 26:3114-3118. [DOI] [PubMed] [Google Scholar]
- 15.Combadiere, B., C. Blanc, T. Li, G. Carcelain, C. Delaugerre, V. Calvez, R. Tubiana, P. Debre, C. Katlama, and B. Autran. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur. J. Immunol. 30:3598-3603. [DOI] [PubMed] [Google Scholar]
- 16.Diel, R., S. Schneider, K. Meywald-Walter, C.-M. Ruf, S. Rüsch-Gerdes, and S. Niemann. 2002. Epidemiology of tuberculosis in Hamburg, Germany: long-term population-based analysis applying classical and molecular epidemiological techniques. J. Clin. Microbiol. 40:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn, J., J. Chan, K. Triebold, D. Dalton, T. Stewart, and B. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas, A., B. Rocha, and A. Coutinho. 1986. Lymphocyte population kinetics in the mouse. Immunol. Rev. 91:5-37. [DOI] [PubMed] [Google Scholar]
- 19.Friedman, L., G. Sullivan, R. Bevilaqua, and R. Loscos. 1987. Tuberculosis screening in alcohol and drug addicts. Am. Rev. Respir. Dis. 136:1188-1192. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding, C. 1997. Choosing and preparing antigen-presenting cells, p. 16.1.1-16.1.14. In R. Coico (ed.), Current protocols in immunology, vol. suppl. 23. John Wiley & Sons, New York, N.Y. [Google Scholar]
- 22.Hoag, K., N. Street, G. Huffnagle, and M. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13:487-495. [DOI] [PubMed] [Google Scholar]
- 23.Hudolin, V. 1975. Tuberculosis and alcoholism. Ann. N. Y. Acad. Sci. 252:353-364. [DOI] [PubMed] [Google Scholar]
- 24.Kline, S., L. Hedemark, and S. Davies. 1995. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N. Engl. J. Med. 333:222-227. [DOI] [PubMed] [Google Scholar]
- 25.Li, X., C. Grossman, C. Mendenhall, P. Hurtubise, S. Rouster, G. Roselle, and P. Gartside. 1998. Host response to mycobacterial infection in the alcoholic rat: male and female dimorphism. Alcohol 16:207-212. [DOI] [PubMed] [Google Scholar]
- 26.Lieber, C., and L. DeCarli. 1982. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol. Clin. Exp. Res. 6:523-531. [DOI] [PubMed] [Google Scholar]
- 27.Lieber, C., and L. DeCarli. 1989. Liquid diet technique of ethanol administration: 1989 update. Alcohol. Clin. Exp. Res. 24:197-211. [PubMed] [Google Scholar]
- 28.Maguire, H., J. Dale, T. McHugh, P. Butcher, S. Gillespie, A. Costetsos, H. Al-Ghusein, R. Holland, A. Dickens, L. Marston, P. Wilson, R. Pitman, D. Strachan, F. Drobniewski, and D. Banerjee. 2002. Molecular epidemiology of tuberculosis in London 1995-7 showing low rate of active transmission. Thorax 57:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason, C., E. Dobard, J. Shellito, and S. Nelson. 2001. CD4+ lymphocyte responses to pulmonary infection with Mycobacterium tuberculosis in naïve and vaccinated BALB/c mice. Tuberculosis 81:327-334. [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall, C., F. Finkelman, R. Means, K. Sherman, V. Nguyen, C. Grossman, S. Morris, S. Rouster, and G. Roselle. 1999. Cytokine response to BCG infection in alcohol-fed mice. Alcohol 19:57-63. [DOI] [PubMed] [Google Scholar]
- 31.Mendenhall, C., C. Grossman, G. Roselle, S. Ghosn, P. Gartside, C. Rouster, P. Chalasani, G. Schmitt, K. Martin, and K. Lamping. 1990. Host response to mycobacterial infection in the alcoholic rat. Gastroenterology 99:1723-1726. [DOI] [PubMed] [Google Scholar]
- 32.Milne, D., J. Moy, P. Corris, H. Robertson, A. DeSoyza, J. Kirby, and A. Cunningham. 2000. Intragraft proliferating T lymphocytes are associated with moderate acute pulmonary rejection. Transplantation 69:1981-1984. [DOI] [PubMed] [Google Scholar]
- 33.Mogues, T., M. Goodrich, L. Ryan, R. LaCourse, and R. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss, A., J. Hahn, J. Tulsky, C. Daley, P. Small, and P. Hopewell. 2000. Tuberculosis in the homeless: a prospective study. Am. J. Respir. Crit. Care Med. 162:460-464. [DOI] [PubMed] [Google Scholar]
- 35.Müller, I., S. P. Cobbold, H. Waldmann, and S. H. E. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richmond, J., and R. McKinney. 1993. Biosafety in microbiological and biomedical laboratories, 3rd ed. U.S. Government Printing Office, Washington, D. C.
- 37.Saunders, B., A. Frank, I. Orme, and A. Cooper. 2002. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell. Immunol. 216:65-72. [DOI] [PubMed] [Google Scholar]
- 38.Scanga, C., V. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon-gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seelig, L., W. Steven, and G. Stewart. 1996. Effects of maternal ethanol consumption on the subsequent development of immunity to Trichinella spiralis in rat neonates. Alcohol. Clin. Exp. Res. 20:514-522. [DOI] [PubMed] [Google Scholar]
- 40.Seitzman, G., J. Sonstein, S. Kim, W. Choy, and J. Curtis. 1998. Lung lymphocytes proliferate minimally in the murine pulmonary immune response to intratracheal sheep erythrocytes. Am. J. Respir. Cell Mol. Biol. 18:800-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small, P., R. Shafer, P. Hopewell, S. Singh, M. Murphy, E. Desmond, M. Sierra, and G. Schoolnik. 1993. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N. Engl. J. Med. 328:1137-1144. [DOI] [PubMed] [Google Scholar]
- 42.Solsona, J., J. Cayla, J. Nadal, M. Bedia, C. Mata, J. Brau, J. Maldonado, C. Mila, J. Alcaide, N. Altet, and H. Galdos-Tanguis. 2001. Screening for tuberculosis upon admission to shelters and free-meal services. Eur. J. Epidemiol. 17:123-128. [DOI] [PubMed] [Google Scholar]
- 43.Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition. 1995, posting date. Nutrient requirements of laboratory animals, 4th revised ed. National Academy Press. [Online.] http://books.nap.edu/books/0309051266/html/80.html. Accessed 2 October 2003.
- 44.Toungoussova, O. S., P. Sandven, A. O. Mariandyshev, N. I. Nizovtseva, G. Bjune, and D. A. Caugant. 2002. Spread of drug-resistant Mycobacterium tuberculosis strains of the Beijing genotype in the Archangel Oblast, Russia. J. Clin. Microbiol. 40:1930-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Pinxteren, L. A. H., J. P. Cassidy, B. H. C. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]