Abstract
The majority of women who quit smoking during pregnancy relapse postpartum and many experience increased depressive symptoms and concerns about body shape and weight. Given the relationship between weight concerns and negative mood with smoking relapse, interventions designed to address the postpartum experience are indicated. However, there are several challenges to research with postpartum women. We describe the rationale of a randomized controlled trial of postpartum smoking relapse prevention intervention and discuss methods to address the specific challenges to recruiting, retaining and conducting health behavior interventions among postpartum former smokers. Pregnant women who had quit smoking for at least one month prior to the 34 week of pregnancy and who were motivated to stay quit postpartum were recruited. Women were randomized either to a postpartum specific intervention designed to address concerns about mood, stress and weight using cognitive-behavioral techniques or to a support-only condition designed to control for time and attention. Intervention continues through six months postpartum and women complete follow-up assessments at 12-, 24- and 52-weeks after delivery. Women (n = 300) who had quit smoking as a result of pregnancy were recruited and are being followed. The intervention described in this report is designed to address stress, negative mood and concerns about weight that mediate smoking relapse postpartum to sustain abstinence and improve maternal and infant health.
Keywords: Postpartum, Relapse prevention, Smoking cessation, Mood, Weight concerns
1. Introduction
Although women are more likely to quit smoking during pregnancy than at any other time of life [1,2], the majority of women who quit smoking during pregnancy will resume smoking within the first six months of the postpartum period [3–5]. Smoking has many negative health consequences for women, including increased risk for lung cancer [6,7], bladder cancer [8], cervical cancer [9], reproductive complications, menstrual dysfunction [10], respiratory symptoms [11] and myocardial infarction [12]. In addition, given the links between environmental tobacco smoke exposure and infant health problems like sudden infant death syndrome, ear infections, respiratory illness and asthma [13,14] as well as later deficits in cognitive and behavioral performance [15–17], interventions designed to prevent postpartum smoking can have important public health benefits for mothers and their children.
There also are unique aspects of the postpartum period that may increase the likelihood that efforts to encourage women to maintain smoking abstinence at this time will be effective. Women who quit during pregnancy spend up to eight months smoke-free [18], and consequently have overcome the acute nicotine withdrawal symptoms, and broken many habitual associations to smoking. Thus, interventions designed to prevent women from resuming smoking after childbirth can decrease the overall smoking rate among women and reduce the exposure of infants and young children to environmental tobacco smoke.
To develop successful postpartum smoking relapse prevention interventions, it is important to understand factors that might increase urges to smoke postpartum. To date, the use of alcohol [19], minority status [20], higher levels of prepregnancy nicotine dependence [20–22], younger age [23] and lower educational attainment [24] have been associated with a resumption of smoking after pregnancy. Having a partner who smokes has also received consistent support as a factor related to postpartum relapse [4,19,21,22,25–27]. However, the relationship between breast feeding and smoking has not been supported consistently [4,22,25,26,28,29]. In addition to these demographic and situational factors, there are two psychosocial factors, mood changes and concerns about body shape and weight, that also increase postpartum smoking relapse risk and represent modifiable intervention targets.
1.1. Mood, weight concerns and postpartum smoking
The experience of depressive symptoms is common postpartum [30–32] and depressive symptoms are strongly linked to smoking relapse [33–36] independently of nicotine dependence [37]. Depressive symptoms during pregnancy [38,39] and postpartum [40] also increase the risk of postpartum smoking. Similarly, maladaptive eating attitudes and behaviors and concerns about shape and weight increase postpartum [41,42]. Specifically, concerns about weight gain or the use of smoking as a weight control strategy may increase a woman’s vulnerability to smoking during the postpartum period. Concerns about weight [4,21], the use of snacking as a strategy to cope with smoking urges during pregnancy [21] and having gained more than an average amount of weight during pregnancy [20] are associated with postpartum smoking relapse.
Recent findings from a sample of women smokers who quit as a result of pregnancy provide additional support for addressing mood and weight concerns to prevent postpartum smoking relapse. First, women’s motivation to maintain cessation postpartum indicates that even after controlling for intention to breast feed, partner’s smoking status, years of smoking and prepregnancy nicotine dependence, factors that have previously been associated with postpartum smoking relapse, weight concerns are related to motivation to stay quit postpartum [43]. Second, results from a prospective study of women who quit smoking during pregnancy and were followed through 6 months postpartum also suggest a role for mood and weight concerns in predicting smoking relapse. Specifically, feeling confident to manage weight without smoking and the experience of positive emotional states decreased the risk of postpartum relapse during the first six months postpartum, while feeling concerned about weight gain related to not smoking and the use of smoking as a tool to manage weight increased the likelihood of resuming smoking [44]. Thus, ample evidence suggests that weight concerns increase the likelihood of smoking relapse during the postpartum period. Importantly, smoking-related weight concerns are modifiable [45,46]. A cognitive behavioral intervention designed to ameliorate women’s concerns about smoking-related weight gain has been shown to be an efficacious smoking cessation treatment [45].
The current trial is based on the assumption that the ability of women to sustain smoking abstinence postpartum can be enhanced by an intervention designed to address postpartum weight and other concerns. Given that therapeutic support and monitoring may increase rates of sustained abstinence, an experimental design that controls for the effects of therapeutic time and attention is the best way to parse the effects of a cognitive-behavioral relapse prevention program developed specifically to address the empirically and conceptually supported factors related to postpartum relapse from those of nondirective, supportive therapeutic time and attention. Thus, this randomized controlled clinical trial compares a cognitive behavioral intervention focused on mood and weight issues to a supportive behavioral intervention to prevent postpartum smoking relapse.
2. Study design and methods
2.1. Overview of study design
The goal of this ongoing investigation is to determine whether a cognitive behavioral relapse prevention intervention designed to address mood and weight concerns during the postpartum period will decrease the rate of postpartum relapse to smoking. Women who quit smoking as a result of pregnancy, have quit for at least one month prior to delivery and are motivated to remain abstinent postpartum complete baseline assessments and are randomly assigned during the third trimester of pregnancy to either a cognitive behavioral relapse prevention intervention specifically designed for women who quit smoking during pregnancy, Strategies to Avoid Returning to Smoking (STARTS), or a nonspecific, supportive condition (SUPPORT). Intervention occurs during the first six months postpartum because the risk of relapse is greatest during the six months immediately following delivery [4,5]. Women complete assessments at the prenatal baseline and at 3, 6 and 12 months postpartum. The study was reviewed and approved annually by the University of Pittsburgh Institutional Review Board and participants provide informed consent.
2.2. Recruitment
Pregnant women were recruited from local programs that supported smoking cessation during pregnancy, obstetric and pediatric offices and other agencies that support women. Based on our past experience recruiting pregnant women who had quit smoking [44,47], we expected a substantial proportion of women to report lower levels of income and less education than in general samples of women smokers. Indeed, one of the primary recruitment sources was a program designed specifically to promote smoking cessation among women receiving prenatal care in clinics serving lower income women [48]. In addition, we expected our racial and ethnic breakdown to mirror that of past samples in which approximately 40% of women were African American and 60% were Caucasian American.
Because recruitment of pregnant and postpartum women, particularly lower-income women who have successfully quit smoking, as required for this trial is challenging [49,50], two strategies were proposed to facilitate recruitment. First, when the trial was designed the local women’s hospital provided a prenatal tobacco cessation program free of charge. All women enrolled in that program were provided with details about the postpartum relapse program. Second, clinicians working on this trial were also frequent visitors to the satellite clinics affiliated with the local women’s hospital to assist with proactive recruitment efforts. Specifically, having the study clinician visits the satellite clinics helped the staff and providers involved in the obstetrical care become familiar with the study, and served as a reminder to the medical staff to offer women the brochure and contact information about the trial in a proactive recruitment strategy.
2.3. Eligibility and screening
Women are initially screened for eligibility by phone. Those meeting initial eligibility are scheduled for an additional in person screening during which smoking abstinence is biochemically verified and additional psychiatric criteria evaluated. To be eligible, women initially had to self-report smoking daily for at least one month during the three months prior to becoming pregnant, report smoking at least 5 cigarettes/day before quitting, and report motivation to remain smoke-free postpartum. Motivation is assessed on a 4-point scale ranging from “not at all motivated” (0) to “extremely motivated” (3), and women with a score of at least 2 are considered motivated to quit.
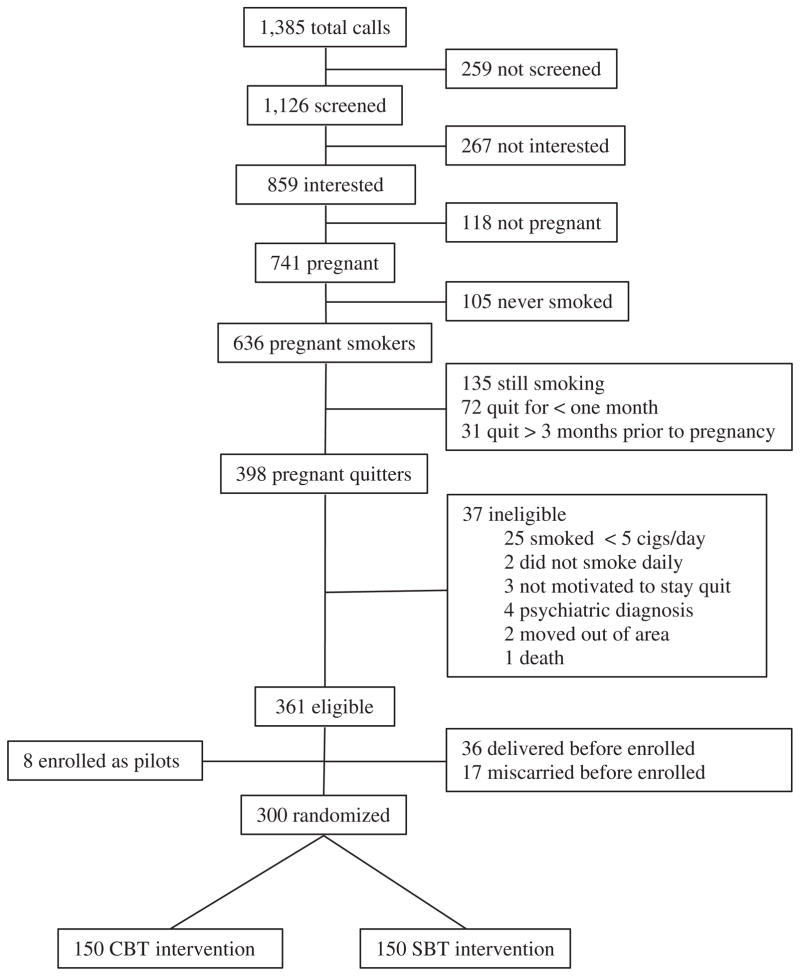
Women meeting initial screening criteria are scheduled for an appointment to further document current nonsmoking. This appointment was scheduled after 34 weeks of gestation to balance the length of time women had quit prior to delivery with the potential for women who have smoked during pregnancy to deliver earlier than nonsmokers. Additional eligibility documented at this appointment included smoking and psychiatric status. Specifically, women had to self-report no smoking during the past 2 weeks which was documented using the time line follow-back methodology [51] as well as an expired-air carbon monoxide level ≤8. In addition, psychiatric status was documented using either the Prime-MD [51] or SCID [51]. Women who reported acute psychiatric disorders, including other substance use problems that warranted immediate treatment in this interview, were excluded from this trial (n = 4; see Fig. 1).
Fig. 1.
Flow of participants in trial.
2.4. Sample size estimation
Based upon our previous work with a similar population [44], it is estimated that 15 and 20% of women will not complete assessments at 6 and 12 months postpartum. Women who are lost to follow-up will be assumed to be smoking. Thus, sample size was based on the estimate that 65% of women will resume smoking at six months without intervention and findings from previous postpartum relapse prevention interventions. Previous postpartum relapse prevention studies have demonstrated between 11% and 27% improvements in abstinence rates at six months postpartum [50–53]. Because our intervention is intensive and continues through six months postpartum, we estimated a decrease of 18% in relapse rates for STARTS relative to no treatment at six-months postpartum. However, because we believe that the supportive, nonspecific treatment (i.e., SUPPORT) will also increase rates of sustained abstinence above those of no treatment, we expect 62% of women to relapse to smoking in SUPPORT and estimate a 16% improvement in relapse rates in STARTS relative to SUPPORT. These estimates correspond to abstinence rates of 53% vs. 37% at six months postpartum in STARTS vs. SUPPORT, respectively. Using an alpha of .05 and a two-tailed test for differences in proportions, 150 women per group (300 total) yields 80% power to detect a 16% improvement in the proportion of women remaining abstinent at six months postpartum.
2.5. Randomization
Women are randomly assigned to either the STARTS or SUPPORT group using separate randomization schedules for African American and Caucasian women. Randomization schedules were generated by a statistician at the start of the study, and women were informed of their group assignment immediately after completing the psychiatric interview at the first in-person appointment.
3. Study interventions
In both intervention conditions, women receive written information on the dangers of postpartum smoking as well as relapse prevention strategies. The conditions also offer equal numbers of sessions with an identical balance of in-person vs. telephone sessions. Specifically, the first session occurs prior to delivery and is designed to foster a relationship with the clinician and program before the stress of adapting to the newborn occurs. In addition, we attempt to meet women in the hospital in the first days after delivery or within the first week at home. The schedule and mode of intervention delivery was selected to maximize contact with the intervention and minimize burden on participants postpartum.
Postpartum sessions in both conditions alternate between face-to-face sessions and telephone contacts, as shown in Fig. 2. To minimize burden and maximize compliance, the face-to-face sessions were designed to occur in locations most convenient to the mothers. Most commonly, those locations include the homes of women or their relatives.
Fig. 2.
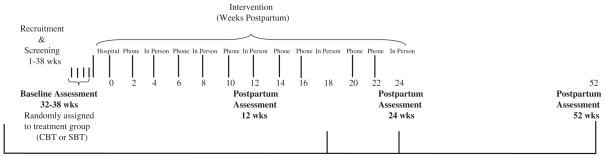
Study design.
As in other manual based interventions [54,55], the topics and skills of the STARTS intervention are presented in a standardized fashion. However, treatment is individualized by focusing each session on the woman’s unique situation. Differences between the STARTS condition, which is designed to address postpartum-linked psychosocial issues that relate to smoking urges, and the SUPPORT condition, designed to address only the behavioral urges to smoke are reviewed below.
3.1. STARTS condition: cognitive behavioral treatment
The cognitive-behavioral approach to behavior change is based upon the idea that dysfunctional or negative thoughts and beliefs affect behavior. There is a large evidence base for cognitive-behavioral treatment (CBT) of mood [56,57], eating [60], nicotine dependence [61] and other disorders [62]. For STARTS, evidence supported CBT techniques were adapted to postpartum women. Table 1 shows the order in which the treatment topics were delivered. STARTS balance the presentation of information on a specific topic with the teaching and reinforcement of cognitive behavioral skills and intervention is divided into three continuous phases.
Table 1.
Order of sessions and topics in STARTS.
| Educational topic | Cognitive-behavioral skill | ||
|---|---|---|---|
| Prepartum | Phase 1: orientation | ||
| Prenatal sessiona | Provide treatment rationale | Relationship building | |
| Model of relapse | Problem identification | ||
| Dangers of postpartum smoking | Self-monitoring I | ||
| Postpartum | 2 | Motivation | Goal setting |
| 4a | Review rationale/ambivalence | Listing pros & cons | |
| Phase 2: CBT for mood and weight concerns | |||
| 6 | Balancing baby and you | Self-monitoring II | |
| 8a | Stress management | Relaxation/breathing retraining | |
| 10 | Social support & pleasant events | Define dysfunctional thoughts | |
| 12a | Mood management | Types of dysfunctional thoughts I | |
| 14 | Myths about smoking and weight | Types of dysfunctional thoughts II | |
| 16 | Postpartum weight changes | Challenging cognitions | |
| 18a | Healthy eating and exercise for moms | Cognitive restructuring | |
| Phase 3: problem area focus/skills consolidation | |||
| 20 | Review mood and weight concerns | Beliefs and values | |
| 22 | Review mood and weight concerns | Beliefs and values | |
| 24a | Review and termination | Review |
Indicates face-to-face meeting.
3.1.1. Phase 1: orientation
Phase 1 begins during the prenatal period and includes psychoeducation about mood and weight concerns during the postpartum period and skills to enhance motivation for sustained abstinence. In this phase, the relationship between the interventionist and woman is forged and expectations about and from the program are reviewed. Specifically, the interventionist reminds women that the primary goal is to help them remain smoke-free postpartum and reminds women that we expect them to remain in touch, do assignments, and generally be active participants in the treatment.
In addition, during this first phase, the links between depressed mood, stress and weight concerns are explained and drawn for women. To begin, the interventionist explains: “We have heard from women that concerns about returning to a prepregnancy weight, the stresses of having a new baby, and other changes in mood and lifestyle after childbirth can interfere with staying quit. We therefore want to talk about your concerns and share ideas for relaxation, healthy eating and stress management, even if you have not had any desire to smoke.”
3.1.2. Phase 2: addressing mood and weight concerns
In the second phase, specific cognitive behavioral strategies designed to address cognitions and behaviors similar to those used in previous studies addressing mood [61,62] and weight concerns [46,63] among smokers are presented and practiced. Women use self-monitoring forms to track situations, thoughts and feelings related to smoking urges. Sessions begin with a review of urges or episodes of smoking and proceed with highlighting and reevaluating thoughts that lead to smoking urges. When women report that self-monitoring has not been completed, the participant and interventionist work together during the first part of the treatment session to complete a monitoring sheet. During this period, or while reviewing monitoring sheet that has been completed by participants, interventionists listen for and highlight cognitions that might relate to feelings of stress, overwhelm and sadness and target these thoughts as well. Maladaptive thoughts about the use of smoking to modulate mood or deal with stress are challenged.
Women also discuss strategies to increase social contacts and add pleasant events to their days and talk about the importance of balancing their needs with those of their new baby. Relaxation techniques and other mood regulation strategies are discussed and practiced in person and reinforced over the phone. For example, interventionists explain the complicated relationship between stress and smoking and discuss evidence that seems counterintuitive suggesting that smoking can maintain stress levels in smokers [66] and practice deep breathing exercises as an alternative to smoking.
Similarly, to address the relationship between smoking and weight during the postpartum period, sessions address mother’s thoughts about their current body shape and weight, and women will work to moderate maladaptive cognitions and beliefs about the importance of shape and weight. For example, women’s thoughts about being unattractive at anything other than her prepregnancy weight will be targeted for modification. Weight concerns specific to smoking also will be targeted by addressing common thoughts relating smoking to weight control, such as the desire to smoke rather than eat when feeding other children. Women receive information about healthy rates of weight loss after pregnancy [65,66], the dangers of strict calorie restriction and the benefits of physical activity. Physical activity is introduced as an alternative to smoking and a tool to improve mood and assist with weight concerns. Women are advised to participate in moderate activity in the form of walking and provided with a prescription for walking consistent with the current guidelines from the American College of Sports Medicine [69].
3.1.3. Phase 3: review and consolidation
In the final phase of STARTS, women are asked to review and select a mood or weight concern issue most relevant to them. To facilitate this review, interventionists provide women with a checklist of the various areas the woman’s interest in additional treatment to address depressed mood, weight or stress. Together, the interventionist and woman choose a topic for the final few sessions among the following broad areas: urges to smoke, feelings of stress, depressive symptoms or concerns about weight. Finally, participants will discuss termination and plans for continued management of mood, weight concerns and smoking urges posttreatment.
3.2. SUPPORT condition: standard behavioral relapse prevention
Given that data on the rates of postpartum smoking relapse without intervention have been established [42,52], the fact that usual care for smokers who have quit should include follow-up and the additional sample size needed to complete a three-group design, the efficacy of the cognitive behavioral relapse prevention program, STARTS, is evaluated in comparison to a nondirective, supportive condition. Thus, the SUPPORT condition is designed to control for the effects and amount of therapeutic time and attention. As in STARTS, a manual of basic relapse prevention materials is provided to women in SUPPORT and topics proceed in the general order shown in Table 1, without the cognitive-behavioral skill focus.
Although women in SUPPORT may also express some of the same adjustment to motherhood issues as women in STARTS, interventionists respond with behavioral questions rather than efforts to highlight and probe such topics. For example, a woman reporting that she feels most like smoking following an argument with her boyfriend about expenses is asked to think about what she might do next time they argue to decrease smoking urges. In this example the interventionist would take care not to ask about her thoughts or feelings about the argument and how those thoughts and feelings relate to smoking urges.
As in the STARTS group, women in SUPPORT received and reviewed information about the dangers of smoking and how it affects their babies/children. In addition to discussing behavioral responses, interventionists also helped women to create social support networks, formulated and wrote out “stay quit” plans to prevent relapse, discussed how to manage urges ahead of time, made lists of factors motivating maintained smoking cessation and identified ways to manage urges behaviorally. Thus, treatment addressed general topics related to relapse prevention and focused on challenges that the participant was experiencing from week to week and how to manage them without turning to smoking.
3.3. Interventionist training and fidelity assessment
Interventionists are master’s-level clinicians, trained prior to the start of the study. Clinicians receive general training in cognitive behavioral approaches to health behavior change, are provided with both a STARTS and SUPPORT treatment manual and attend a training that will include didactic materials, role-play and feedback. Following training, all clinicians were assigned a pilot case that was treated using the STARTS intervention. All sessions are audio-recorded and rated for fidelity to the intervention. Clinicians were required to achieve a 90% accurate score on a post-training test of knowledge and ratings of 80% or greater on a rating scale used to rate treatment fidelity in a pilot case, prior to being assigned a study participant.
To ensure fidelity and maintain distinction between the STARTS and SUPPORT conditions, interventionists participate in ongoing weekly group supervision. Tips on how to respond to mood or weight cognitions from women randomized to SUPPORT are provided as well as suggestions on how to probe mood and weight thoughts among women in STARTS who reported few such thoughts spontaneously. In addition, given that in-person sessions are designed to occur in locations convenient to postpartum women, many issues related to the presence of others during intervention, distraction from children or media (e.g., radio, televisions) in the home while meeting and concerns about clinician and participant relationships were discussed regularly during supervision meetings. Finally, to assess treatment fidelity, at the end of the trial, 10% of the tapes from both STARTS and SUPPORT conditions will be rated by an independent clinician. These ratings ask about the presence of specific components in session and include a global rating of the session as STARTS or SUPPORT.
4. Study assessments
Women complete assessments four times during the study period. As shown in Fig. 2, the prenatal assessment serves as a baseline, and women will complete assessments at 3, 6 and 12 months postpartum. All women are compensated with the amount increasing for later time points, to help incentivize completion at the 6 and 12 month points. In addition, assessment of both primary and secondary outcomes will be completed separately from the intervention and by a member of the study team not involved in providing intervention. Assessments include both self-report and biochemical measures, as described below.
4.1. Primary outcome
The primary outcome of the trial is continuous smoking abstinence. We will examine the proportion of women who maintain abstinence at 6 and 12 months postpartum. Abstinence is defined by self report and confirmed biochemically. Specifically, women are queried about any smoking since their last assessment using a time line follow-back format we have used in previous studies [45]. In addition, expired-air CO will be measured at each assessment and salivary cotinine will be collected, as recommended by the Society for Research on Nicotine and Tobacco’s subcommittee on biochemical validation (2001). Relapse is defined as seven consecutive days of smoking or a CO greater than 8 ppm [68,69]. Days to a first lapse and a full relapse postpartum are determined by counting the number of days between delivery and the first day of any smoking (i.e., a puff of more), and between delivery and seven consecutive days of smoking or a CO greater than 8 ppm, respectively. A cotinine level of less than 15 ng/ml [72] will be used to verify self-report of nonsmoking. In addition to continuous abstinence, we will examine differences between the two intervention groups in the number of days women remain abstinent from smoking.
4.2. Secondary outcomes
We also expect women in STARTS to evidence greater improvements in mood, stress and smoking related weight concerns relative to women in SUPPORT. Depressive symptoms will be assessed with two measures. Women will complete the Center for Epidemiological Studies-Depression Scale [73] to assess current depressive symptomatology. The CES-D was selected because it appears to be less sensitive than other depression scales to somatic symptoms that may be common during the postpartum period [74]. In addition, women will complete the Edinburgh Postnatal Depression Scale [75], a widely used 10-item assessment specific to postpartum depression. Responses on the CES-D and EPDS will be carefully reviewed, and women who endorse extreme scores or suicidality will be contacted for further evaluation and referral if necessary. The Perceived Stress Scale [76], designed to assess the degree to which an individual appraises situations as stressful, also will be administered. The PSS is a 14-item scale with adequate reliability that has been used in other smoking cessation studies [77]. In addition, the use of smoking as a weight control strategy [78], self-efficacy about weight management after quitting smoking [79] and cessation-specific weight concerns [45,77] will be assessed repeatedly.
4.3. Covariates
Demographic variables that have been related to post-partum smoking relapse will be collected. Specifically, mother’s age, race, ethnicity, income, parity and information about the pregnancy (e.g., whether pregnancy was intentional) will be collected at baseline. Measures of confidence in remaining abstinent, smoking status of others in the home and alcohol use will be repeated. In addition, women’s intention to breast feed will be measured at baseline and information on method of infant feeding will be collected at each assessment.
5. Planned statistical analyses
The primary analysis involves comparing the proportion of women remaining continuously abstinent, between the two intervention conditions at the end of treatment (6 months postpartum) and one year postpartum. First, chi-square tests will be used to compare the proportion of women continuously abstinent in both STARTS and SUPPORT. Second, models will be used to compare the main binary outcome, abstinent or relapsed, between the groups controlling for other variables that have been related to postpartum relapse (parity, breast feeding, age, alcohol use, race).
In addition, analyses will examine the role of psychosocial variables, particularly mood, stress and weight concerns as potential mediators of outcome, regardless of the efficacy of the interventions. We will use the following steps to determine if these variables mediate the relationship between treatment (STARTS vs. SUPPORT) and outcome (abstinence vs. relapse): (1) the effects of treatment on outcome will be estimated using general mixed models with a logit link, with the coefficient (c) representing the total effect of the intervention on outcome without taking any mediators into account; (2) the simultaneous effects of both intervention and mediator will be estimated with the coefficient (c′) representing the effect of the intervention after controlling for the effects of the mediator; and (3) the difference between (c) and (c′) will be evaluated, as the estimate of the mediated effect. The difference between (c) and (c′) measures the extent to which the mediator accounts for the relationship between treatment and outcome.
6. Discussion
Preventing relapse to smoking postpartum can substantially improve the health of mothers and their children. Efforts to improve cessation treatments during pregnancy have decreased the proportion of women that smoking during pregnancy, and pregnancy is a common time for smoking cessation [1,2]. However, postpartum smoking relapse is common and considerably less is known about how to sustain tobacco smoking abstinence postpartum [80]. There are unique features of the postpartum period that suggest intervention targets for smoking relapse prevention [81] and the STARTS intervention, described in this report, is designed to address the stress, negative mood and concerns about weight that mediate smoking relapse postpartum.
The current study has also several innovative features. First, it is the first randomized controlled trial of an intervention designed to address putative mediators of smoking postpartum: mood, stress and weight concerns. Evidence based techniques to address mood [61,62], stress and smoking-related weight concerns [46] have been incorporated into the intervention to target potentially modifiable behaviors and cognitions that increase vulnerability to relapse.
Second, the interventions being compared in this trial have been adapted to meet the challenge of conducting an intervention with postpartum women. The intervention was designed to be delivered through both in person and telephone sessions. Although in early planning women reported an interest in easy access to treatment, the use of online forums was not rated highly [47] and thus only telephone and face-to face visits are used. However, the in-person sessions occur in locations that are easily and readily accessible by postpartum women (e.g., homes, family health clinics, restaurants, coffee shops, pediatrician offices), minimizing the amount of travel and disruption for study participants. Finally, data collection and participant recruitment methods have been designed to maximize adherence. Study clinicians have invested considerable time and energy integrating into local clinics that serve lower-income pregnant and postpartum women. This integration creates a seamless line of referrals and promotes rapport among the study participants and interventionists.
6.1. Conclusions
In conclusion, postpartum smoking relapse is problematic. Results of this trial will inform the development of efficacious treatments to sustain rates of smoking abstinence postpartum, and help to elucidate the effects of therapeutic time and attention on postpartum smoking relapse. If either the STARTS or SUPPORT conditions prove to be efficacious, future work will need to examine the ways in which components of each intervention can be disseminated more broadly. For example, the benefits of having a concerned individual calling to inquire about smoking postpartum may prove to be as effective as cognitive strategies in elevating mood. If mood changes are found to mediate postpartum smoking relapse it might be easier and more cost-effective to disseminate a program involving brief supportive ‘check-in calls’ than a more intensive cognitive behavioral postpartum program. Thus, results of the current trial will provide direction for future evaluations designed to determine the effective components and costs of efforts to prevent relapse to smoking postpartum.
Acknowledgments
Support for this ongoing trial was provided by grant R01DA021608 (PI: Levine).
Footnotes
References
- 1.Ebrahim SH, Floyd RL, Merritt RK, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283:361–6. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- 2.Williamson DF, Serdula MK, Kendrick JS, Binkin NJ. Comparing the prevalence of smoking in pregnant and nonpregnant women, 1985–1986. JAMA. 1989;261:70–4. [PubMed] [Google Scholar]
- 3.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80:541–4. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride CM, Pirie PL. Postpartum smoking relapse. Addict Behav. 1990;15:165–8. doi: 10.1016/0306-4603(90)90020-x. [DOI] [PubMed] [Google Scholar]
- 5.Mullen PD, Quinn VP, Ershoff DH. Maintenance of nonsmoking postpartum by women who stopped smoking during pregnancy. Am J Public Health. 1990;80:992–4. doi: 10.2105/ajph.80.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kure EH, Ryberg D, Hewer A. Mutations in lung tumors: relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17:2201–5. doi: 10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- 7.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–92. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 8.Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominquez M, Crowder JS, et al. Gender and smoking related bladder cancer risk. J Natl Cancer Inst. 2001;93:538–45. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- 9.Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002;94:1406–14. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, LaVecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162:504–14. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 11.Langhammer A, Johnsen R, Holmen J, Bjermer GL. Cigarette smoking gives more respiratory symptoms among women than among men. J Epidemiol Community Health. 2000;54:917–22. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott E, Hippe M, Schrohr P, de Heis H, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–7. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dybing E, Sanner T. Passive smoking, sudden infant death syndrome (SIDS) and childhood infections. Hum Exp Toxicol. 1999;18:202–5. doi: 10.1191/096032799678839914. [DOI] [PubMed] [Google Scholar]
- 14.Ey JL, Holberg CJ, Aldous MB, Wright AL, Martinez FD, Taussig LM. Passive smoking exposure and otitis media in the first year of life: group health medical associates. Pediatrics. 1995;95:670–7. [PubMed] [Google Scholar]
- 15.Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Alcohol Res Health. 2000;24:242–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn RS, Zuckerman B, Bauchner H, Homer CJ, Wise PH. Women’s health after pregnancy and child outcomes at age 3 years: a prospective cohort study. Am J Public Health. 2002;92:1312–8. doi: 10.2105/ajph.92.8.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maughan B, Taylor C, Taylor A, Butler N, Bynner J. Pregnancy smoking and childhood conduct problems: a casual association? J Child Psychol Psychiatry. 2001;42:1021–8. doi: 10.1111/1469-7610.00800. [DOI] [PubMed] [Google Scholar]
- 18.Dolan-Mullen P, Ramirez G, Groff JY. A meta-analysis of randomized trials of prenatal smoking cessation interventions. Am J Obstet Gynecol. 1994;171:1328–34. doi: 10.1016/0002-9378(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 19.Severson HH, Andrews JA, Lichtenstein E, Wall M, Zoref L. Predictors of smoking during and after pregnancy: a survey of mothers of newborns. Prev Med. 1995;24:23–8. doi: 10.1006/pmed.1995.1004. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael SL, Ahluwalia IB, Group PW. Correlates of postpartum smoking relapse: results from the pregnancy risk assessment monitoring system (PRAMS) Am J Prev Med. 2000;19:193–6. doi: 10.1016/s0749-3797(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 21.McBride CM, Pirie PL, Curry SJ. Postpartum relapse to smoking: a prospective study. Health Educ Res. 1992;7:381–90. doi: 10.1093/her/7.3.381. [DOI] [PubMed] [Google Scholar]
- 22.Ratner PA, Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve-month follow-up of a smoking relapse prevention intervention for postpartum women. Addict Behav. 2000;25:81–92. doi: 10.1016/s0306-4603(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 23.Valanis B, Lichtenstein E, Mullooly JP, Labuhn K, Brody K, Severson HH, et al. Maternal smoking cessation and relapse prevention during health care visits. Am J Prev Med. 2001;20:1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- 24.Letourneau AR, Sonja B, Mazure CM, O’Malley SS, James D, Colson ER. Timing and predictors of postpartum return to smoking in a group of inner-city women: an exploratory pilot study. Birth. 2007;34:245–52. doi: 10.1111/j.1523-536X.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- 25.Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: long-term evaluation of a pediatric intervention. Prev Med. 1997;26:120–30. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- 26.Stotts AL, DiClemente CC, Carbonari JP, Mullen PD. Postpartum return to smoking: staging a “suspended” behavior. Health Psychol. 2000;19:324–32. doi: 10.1037//0278-6133.19.4.324. [DOI] [PubMed] [Google Scholar]
- 27.Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: impact on maternal smoking and relapse. Pediatrics. 1995;96:622–8. [PubMed] [Google Scholar]
- 28.Ratner PA, Johnson JL, Bottorff JL. Smoking relapse and early weaning among postpartum women: is there an association? Birth. 1999;26:76–82. doi: 10.1046/j.1523-536x.1999.00076.x. [DOI] [PubMed] [Google Scholar]
- 29.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92:1801–8. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess. 2005 Summ;:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopkins J, Marcus M, Campbell SB. Postpartum depression: a critical review. Psychol Bull. 1984;95:498–515. [PubMed] [Google Scholar]
- 32.Whiffen VE. Is postpartum depression a distinct diagnosis? Clin Psychol Rev. 1992;12:485–508. [Google Scholar]
- 33.Ginsberg D, Hall SM, Reus VI, Munoz RF. Mood and depression diagnosis in smoking cessation. Exp Clin Psychopharmacol. 1995;3:389–95. [Google Scholar]
- 34.Killen JD, Fortmann SP, Kraemer HC, Varady AN, Davis L, Barbara N. Interactive effects of depression symptoms, nicotine dependence, and weight change on late smoking relapse. J Consult Clin Psychol. 1996;64:1060–7. doi: 10.1037//0022-006x.64.5.1060. [DOI] [PubMed] [Google Scholar]
- 35.Pomerleau CS, Brouwer RJN, Pomerleau OF. Emergence of depression during early abstinence in depressed and non-depressed women smokers. J Addict Dis. 2001;20:73–80. doi: 10.1300/J069v20n01_07. [DOI] [PubMed] [Google Scholar]
- 36.Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence and lapse characteristics. J Consult Clin Psychol. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- 37.Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–7. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Blalock JA, Robinson JD, Wetter DW, Cinciripini PM. Relationship of DSM-IV-based depressive disorders to smoking cessation and smoking reduction in pregnant smokers. Am J Addict. 2006;15:268–77. doi: 10.1080/10550490600754309. [DOI] [PubMed] [Google Scholar]
- 39.Whitaker RC, Orzol SM, Kahn RS. The co-occurrence of smoking and a major depressive episode among mothers 15 months after delivery. Prev Med. 2007;45:476–80. doi: 10.1016/j.ypmed.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. Am J Prev Med. 2009;36:9–12. doi: 10.1016/j.amepre.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Baker CW, Carter AS, Cohen LR, Brownell KD. Eating attitudes and behaviors in pregnancy and postpartum: global stability versus specific transitions. Ann Behav Med. 1999;21:143–8. doi: 10.1007/BF02908295. [DOI] [PubMed] [Google Scholar]
- 42.Stein A, Fairburn CG. Eating habits and attitudes in the postpartum period. Psychosom Med. 1996;58:321–5. doi: 10.1097/00006842-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Levine MD, Marcus MD, Kalarchian MA, Weissfeld L, Qin L. Weight concerns affect motivation to remain abstinent from smoking postpartum. Ann Behav Med. 2006;32:147–53. doi: 10.1207/s15324796abm3202_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine MD, Marcus MD, Kalarchian MA, Houck PR, Cheng Y. Weight concerns, mood, and postpartum smoking relapse. Am J Prev Med. 2010;39:345–51. doi: 10.1016/j.amepre.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–13. [PubMed] [Google Scholar]
- 46.Levine MD, Marcus MD, Perkins KA. Women, weight and smoking: a cognitive behavioral approach to women’s concerns about weight gain following smoking cessation. Cogn Behav Pract. 2003;10:105–11. [Google Scholar]
- 47.Levine MD, Marcus MD, Leon-Verdin M. Similarities in affect, perceived stress, and weight concerns between black and white women who quit smoking during pregnancy. Nicotine Tob Res. 2008;10:1543–8. doi: 10.1080/14622200802323290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cluss PA, Levine MD, Landsittel D, Bender E. The Pittsburgh STOP program: an evidence-informed translational intervention for low income pregnant smokers. Am J Health Promot. 2011;25(5):S75–81. doi: 10.4278/ajhp.100616-QUAN-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez EN, Simmons VN, Quinn GP, Meade CD, Chirikos TN. Clinical trials and tribulations: lessons learned from recruiting pregnant ex-smokers for relapse prevention. Nicotine Tob Res. 2008;10:87–96. doi: 10.1080/14622200701704962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman-Phox K, Laraia BA, Adler N, Vieten C, Thomas M, Epel E. Recruitment and retention of pregnant women for a behavioral intervention: lessons from the maternal adiposity, metabolism and stress (MAMAS) study. Prev Chronic Dis. 2013:10. doi: 10.5888/pcd10.120096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown RS, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998:101–12. [Google Scholar]
- 52.Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–20. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JL, Ratner PA, Bottorff JL, Hall W, Dahinten S. Preventing smoking relapse in postpartum women. Nurs Res. 2000;49:44–52. doi: 10.1097/00006199-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 54.McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89:706–11. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McBride CM, Baucom DH, Peterson BL, Pollak KI, Palmer C, Westman E, et al. Prenatal and postpartum smoking abstinence a partner-assisted approach. Am J Prev Med. 2004;27:232–8. doi: 10.1016/j.amepre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Malik ML, Beutler LE, Alimohamed S, Gallagher-Thompson D, Thompson L. Are all cognitive therapies alike?. A comparison of cognitive and noncognitive therapy process and implications for the application of empirically supported treatments. J Consult Clin Psychol. 2003;71:150–8. doi: 10.1037//0022-006x.71.1.150. [DOI] [PubMed] [Google Scholar]
- 57.Wilson GT. Manual-based treatment and clinical practice. Clin Psychol Sci Pract. 1998;5:363–75. [Google Scholar]
- 58.Beck JS. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995. [Google Scholar]
- 59.Burns DD. The feeling good handbook. New York: Penguin Group; 1999. [Google Scholar]
- 60.Fairburn CG, Marcus MD, Wilson GT. Cognitive-behavioral therapy for binge eating and bulimia nervosa: a comprehensive treatment manual. New York: The Guilford Press; 1993. [Google Scholar]
- 61.Perkins KA, Conkin CA, Levine MD. Cognitive behavior therapy for smoking cessation: a practical guidebook to the most effective treatments. New York: Taylor & Francis; 2007. [Google Scholar]
- 62.Kendall PC. Cognitive-behavioral therapies with youth: guiding theory, current status, and emerging developments. J Consult Clin Psychol. 1993;61:235–47. doi: 10.1037//0022-006x.61.2.235. [DOI] [PubMed] [Google Scholar]
- 63.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 64.Hall SM, Munoz RF, Reus VI, Carol D, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo controlled study. J Consult Clin Psychol. 1996;64:1003–9. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 65.Perkins KA, Levine MD, Marcus MD, Shiffman S. Addressing women’s concerns about weight gain due to smoking cessation. J Subst Abuse Treat. 1997;14:173–82. doi: 10.1016/s0740-5472(96)00158-4. [DOI] [PubMed] [Google Scholar]
- 66.Parrott AC. Does cigarette smoking cause stress? Am Psychol. 1999;54:817–20. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- 67.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. Am J Public Health. 1993;83:1100–3. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker LO. Weight gain after childbirth: a women’s health concern. Ann Behav Med. 1995;17:132–41. doi: 10.1007/BF02895062. [DOI] [PubMed] [Google Scholar]
- 69.Pate RR, Pratt M, Blair SN, Haskell L, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the centers for disease control and prevention and the American college of sports medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 70.Ossip-Klein DJ, Bigelow G, Parker S, Curry S, Hall SM, Kirkland S. Classification and assessment of smoking behavior. Health Psychol. 1986;5:3–11. [PubMed] [Google Scholar]
- 71.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 72.Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;2:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 73.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 74.Coyle CP, Roberge JJ. The psychometric properties of the Center for Epidemiological Studies-Depression Scale (CES-D) when used with adults with physical disabilities. Psychol Health. 1992;7:69–81. [Google Scholar]
- 75.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 76.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 77.Cohen S. Contrasting the Hassles Scale and the Perceived Stress Scale: who’s really measuring appraised stress? Am Psychol. 1986;41:716–8. [Google Scholar]
- 78.Pomerleau CP, Ehrlich E, Tate JC, Marks JL, Flessland KA, Pomerleau OF. The female weight-control smoker: a profile. J Subst Abuse. 1993;5:391–400. doi: 10.1016/0899-3289(93)90007-x. [DOI] [PubMed] [Google Scholar]
- 79.Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addict Behav. 1998;23:609–22. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 80.Mullen PD. How can more smoking suspension during pregnancy become lifelong abstinence? Lessons learned about predictors, interventions and gaps in our accumulated knowledge. Nicotine Tob Res. 2004;6:S217–38. doi: 10.1080/14622200410001669150. [DOI] [PubMed] [Google Scholar]
- 81.Levine MD, Marcus MD. Do changes in mood and concerns about weight relate to smoking relapse in the postpartum period? Arch Womens Ment Health. 2004;7:155–66. doi: 10.1007/s00737-004-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]