Abstract
Inducible nitric oxide synthase (iNOS) is a cytoplasmic protein responsible for the generation of nitric oxide (NO · ) in macrophages. In this work, we hypothesized that the intracellular localization of iNOS is significant for effective delivery of NO · to phagosomes containing ingested microorganisms. Using immunofluorescence microscopy and Western blot analysis, iNOS was shown to localize in the vicinity of phagosomes containing latex beads in stimulated macrophages. iNOS also localized to phagosomes containing Escherichia coli. The colocalization of iNOS with ingested latex beads was an actin-dependent process, since treatment with the actin microfilament disrupter cytochalasin D prevented iNOS recruitment to latex bead phagosomes. In contrast to E. coli and inert particle phagosomes, mycobacterial phagosomes did not colocalize with iNOS. This study demonstrates that (i) iNOS can be recruited to phagosomes; (ii) this recruitment is dependent on a functional actin cytoskeleton; (iii) certain microorganisms have the ability to prevent or reduce colocalization with iNOS; and (iv) spatial exclusion of iNOS may play a role in Mycobacterium tuberculosis pathogenesis.
It is estimated that Mycobacterium tuberculosis, the causative agent of tuberculosis, infects approximately one-third of the world's population (WHO Fact Sheet [http://www.who.int/]). Tuberculosis causes more adult deaths worldwide than any other disease caused by a single infectious agent, and approximately two million people die of tuberculosis each year (WHO Fact Sheet [http://www.who.int/]). M. tuberculosis is an intracellular pathogen that survives and replicates within cells of the host immune system, primarily macrophages. Following phagocytosis into the macrophage, M. tuberculosis prevents phagosome-lysosome fusion by halting the maturation of the phagosome (1, 33). Recently, molecular insights into these phenomena have been generated (41). In comparison to model phagosomal compartments, phagosomes containing M. tuberculosis lack or have reduced levels of several essential late endosomal-lysosomal constituents, including mannose 6-phosphate receptors (46), and their cargo, e.g., mature cathepsin D, as well as the H+-ATPase pump (21, 39, 46). Mycobacterial phagosomes also lack the regulatory factors controlling organellar membrane fusion (13, 19, 20, 42), phagosome biogenesis (19), and delivery of lysosomal cargo from trans Golgi network or endosomal compartments (21).
By preventing phagosome maturation, M. tuberculosis interferes with bactericidal and immunological functions of the phagosome and antigen-presenting cells. In infected macrophages, M. tuberculosis may become exposed to reactive oxygen intermediates (24) and to another class of bactericidal agents, the reactive nitrogen intermediates (RNI). RNI include nitric oxide (NO · ) and its physiological metabolites. RNI are potent antimicrobial agents in murine macrophages (26) and are believed to play a similar role in human macrophages (27, 29). Numerous studies have been performed to examine the survival of mycobacteria in activated macrophages (6, 11, 18) as well as to correlate the levels of RNI in these macrophages with their antibacterial action (6, 11, 37, 38). While mycobacterial sensitivity to RNI has been investigated by several laboratories (30, 32, 47), it has not been explored to what extent M. tuberculosis actually encounters RNI in the macrophage in vivo and how NO · or its metabolites are delivered to the mycobacterial phagosome.
In this work, we focused our studies on the intracellular localization of inducible nitric oxide synthase (iNOS), the major enzyme generating NO · in immune cells. In macrophages, iNOS expression can be induced by gamma interferon (IFN-γ) and tumor necrosis factor alpha (or lipopolysaccharide [LPS]) (37, 38, 45). This isoform of NOS, also called NOS2, differs from neuronal NOS (NOS1) and endothelial NOS (NOS3) in that its activity is independent of intracellular Ca2+ levels, although, like the other NOSs, iNOS does interact with calmodulin (26, 36, 45). iNOS catalyzes the conversion of l-arginine to l-citrulline and NO · (26). NO · , as both a water- and lipid-soluble radical gas, is a potent RNI that can react with oxygen in solution to yield a variety of products, including 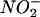 and
and 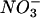 , and with superoxide to produce ONOO− (26). The rapid consumption of NO · due to its exquisite reactivity also implies that the proximity of NO · formation to its target may be important for its effective antibacterial activity. Thus, we hypothesized that the localization of iNOS relative to the phagosome containing ingested organisms may be of significance for the antibacterial action of NO · .
, and with superoxide to produce ONOO− (26). The rapid consumption of NO · due to its exquisite reactivity also implies that the proximity of NO · formation to its target may be important for its effective antibacterial activity. Thus, we hypothesized that the localization of iNOS relative to the phagosome containing ingested organisms may be of significance for the antibacterial action of NO · .
Here we tested whether iNOS colocalizes with newly formed phagosomes in infected, activated murine macrophages. To examine the intracellular localization of iNOS, we studied the distribution of iNOS relative to phagosomes containing latex beads (LB) and the M. tuberculosis var. bovis BCG, the vaccine strain variant of the M. tuberculosis complex. Using immunofluorescence microscopy and Western blot analysis, we show that iNOS is recruited to model phagosomes containing LB but that it does not colocalize with mycobacterial phagosomes. We propose that the ability of M. bovis BCG to prevent iNOS colocalization with mycobacterial phagosomes represents a means by which M. tuberculosis survives in macrophages and contributes to the establishment of a persistent, long-term infection.
MATERIALS AND METHODS
Bacterial strains and growth.
M. bovis BCG (ATCC 27291) was obtained from the American Type Culture Collection (ATCC; Rockville, Md.). In immunofluorescence studies, M. bovis BCG harbored the pMlahpC-gfp plasmid, which expresses green fluorescent protein (GFP) under the control of the M. tuberculosis ahpC promoter (15). Mycobacteria were cultured in Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% glycerol, 10% ADC (albumin-dextrose complex) enrichment without catalase, 0.05% Tween 80, and 10 μg of kanamycin/ml, when necessary. Cultures were incubated at 37°C in 5% CO2. Single-cell suspensions were produced as previously described (42). When necessary, mycobacteria were also grown on 7H11 plates (Difco) supplemented with 0.5% glycerol, 10% ADC enrichment without catalase, 0.05% Tween 80, and 10 μg of kanamycin/ml. Escherichia coli DH5α was obtained from Gibco BRL (Life Technologies, Rockville, Md.) as chemically competent cells. In immunofluorescence studies, E. coli harbored the pMRP9-1 plasmid, which expresses GFP as described previously (9). E. coli was grown in Luria-Bertani broth containing 25 μg of ampicillin/ml.
Cell culture and media.
The murine macrophage-like J774 cell line (ATCC TIB-67) was obtained from ATCC. J774 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; BioWhittaker, Walkersville, Md.) supplemented with 4 mM l-glutamine (BioWhittaker) and 5% fetal bovine serum (FBS; HyClone, Logan, Utah). Bone marrow-derived macrophages (BMM) were harvested from femora of 6- to 8-week-old C57BL/6J and C57BL/6J iNOS−/− mice (Jackson Laboratory, Bar Harbor, Maine) as described previously (43). For immunofluorescence experiments, macrophages were suspended in DMEM with 10% FBS.
Cytokine treatment of macrophages.
J774 and BMM were stimulated with IFN-γ and LPS. For immunofluorescence experiments, macrophages were seeded onto no. 1 thickness, 12-mm-diameter glass coverslips in 24-well tissue culture plates (Costar, Cambridge, Mass.) at a density of 4.5 × 105 cells per coverslip and exposed to 500 U of IFN-γ/ml, 500 ng of LPS/ml, or both IFN-γ and LPS for 16 h prior to infection.
Fluorescence microscopy.
Macrophages were prepared as described above for infections with GFP-labeled M. bovis BCG and E. coli. Mycobacterial infections were done as coinfections with LB that had been carboxylate modified to fluoresce blue. For opsonization studies, bacteria and LB were diluted in DMEM plus 10% FBS and incubated at room temperature for 30 min before infection. Immunoglobulin G (IgG)-coated LB were prepared by coating beads with bovine serum albumin (BSA) followed by anti-BSA IgG antibody, as described previously (28). The IgG-coated LB were suspended in DMEM for infections. Infections were synchronized by spinning bacteria and LB onto the macrophages using a Sorvall RT 6000D centrifuge (Sorvall, Newtown, Conn.) for 5 min at 107 × g, followed by incubation at 37°C. At various times, coverslips were removed from the well and washed three times with phosphate-buffered saline (PBS). Macrophages were fixed using 3.7% paraformaldehyde in PBS at room temperature for 10 min. To permeabilize the cells, 0.2% saponin in PBS was added to the coverslips at room temperature for 5 min. The coverslips were washed three times with PBS prior to incubating with blocking solution (10% skim milk, 6% BSA fraction V, and 2% goat serum in PBS). Macrophages were incubated with a polyclonal rabbit anti-iNOS antibody (developed against murine iNOS as antigen for M. Marletta by Cocalico Biologicals, Inc., Reamstown, Pa.), followed by a secondary antibody. This antibody against iNOS recognizes iNOS by immunoblotting and by immunofluorescence, and its specificity has been established using iNOS peptides and purified protein. Alexa Fluor 568 was conjugated to goat anti-rabbit IgG (H+L) (Molecular Probes, Eugene, Oreg.) in blocking solution. IgG-coated LB were checked for internalization by using tetramethylrhodamine-conjugated goat anti-mouse IgG (Molecular Probes) against permeabilized and nonpermeabilized cells. Coverslips were mounted on glass slides with PermaFluor (Immunon, Pittsburgh, Pa.) and analyzed using an Olympus BX60 microscope (Olympus, Melville, N.Y.) with a dichroic mirror-emitter cube set at 8,300 (Chroma Technology Corporation, Battleboro, Vt.). GFP-labeled bacteria and LB were visualized using an excitation wavelength of 490 ± 10 nm. Alexa Fluor 568 fluorescence was visualized using an excitation wavelength 570 ± 20 nm. Images were captured and analyzed using LSR Esprit 1.20 (Life Science Resources, Olympus). All images were taken at a magnification of ×100. Percent iNOS colocalization was calculated by determining the mean iNOS colocalization ± the standard error (SE) for either individual experiments or for fields counted (containing at least 50 phagosomes per field), as indicated in the text.
Purification of LB phagosomes from macrophages.
LB phagosomal compartments (LBC) were isolated from LB-infected J774 macrophages as described previously (14), and the purity of phagosomal preparations was ascertained as previously described (19, 43).
Preparation of whole-cell lysates.
For Western blot analysis, whole-cell lysates were prepared from LB-infected J774 macrophages. Macrophages were either not treated or received IFN-γ and LPS as described above at 16 h preinfection. LB infection was done as described above for opsonization studies. After 1 h at 37°C, macrophages were washed three times with PBS and suspended in a final volume of 100 μl of lysis buffer (35).
Western blot analysis.
Protein concentrations were determined using bicinchoninic acid protein assay reagents (Pierce, Rockford, Ill.). Protein samples (5 μg each) were loaded onto a 10% or 10-to-20% Tris-glycine Novex prepoured gel (Invitrogen Corporation, Carlsbad, Calif.). Following electrophoresis, proteins were transferred to Immobilon P membranes (Millipore, Bedford, Mass.) by electroblotting (12). Membranes were incubated with rabbit antibodies to iNOS, g-actin, rab7 (from L. Huber, Research Institute of Molecular Pathology, Vienna, Austria), or syntaxin 8 (from W. Hong, Institute of Molecular and Cell Biology, Singapore). This was followed by incubation with peroxidase-conjugated AffiniPure goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) to allow visualization of the bound antibodies with the NEN Renaissance Western blot chemiluminescence reagent (NEN Life Science Products, Inc., Boston, Mass.).
Cytochalasin D and jasplakinolide treatment.
LB-infected, IFN-γ- and LPS-activated macrophages were treated with the actin microfilament disrupter cytochalasin D at 10 min postinfection using a final concentration of 30 μM. The actin microfilament stabilizer jasplakinolide (Molecular Probes) was used at a final concentration of 1.4 μΜ at 20 min postinfection. Infections were done as described above with the following modifications. At 10 min postinfection, macrophages were washed three times with DMEM plus 10% FBS and then incubated in DMEM plus 10% FBS for the remainder of the 1-h infection. Cytochalasin D or jasplakinolide was added as described above. Some macrophages were treated with cytochalasin D at 10 min postinfection followed by jasplakinolide at 20 min postinfection. Results are reported as the percent iNOS colocalization ± SE for at least 10 representative fields.
Statistical analysis.
Statistical analysis was done using SuperANOVA version 1.11 software (Abacus Concepts, Inc). P values were calculated using Fisher's protected least squares difference (SuperANOVA).
RESULTS
iNOS localizes to phagosomes in stimulated macrophages.
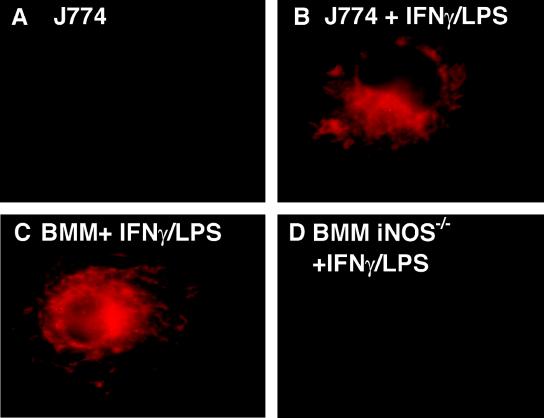
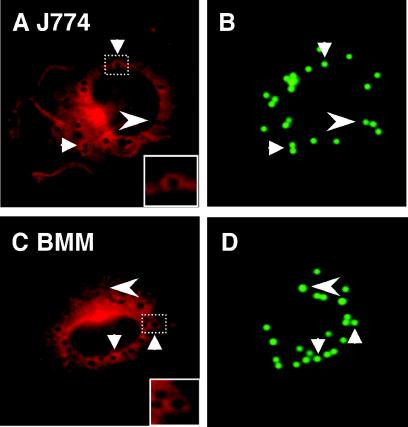
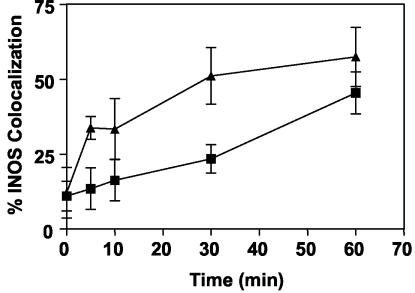
We first established that the antibody used to detect iNOS by immunofluorescence detected iNOS in activated macrophages and was specific for iNOS, as it did not show staining in BMM from iNOS−/− transgenic mice (Fig. 1). Next, we examined the intracellular localization of iNOS relative to phagosomes. J774 macrophages were infected with complement-opsonized fluorescent LB, and iNOS localization was examined by immunofluorescence. In macrophages stimulated with IFN-γ and LPS, a significant portion of LB phagosomes showed iNOS colocalization, seen as distinct rings of immunofluorescent iNOS around LB phagosomes (Fig. 2A and B). In unstimulated macrophages, no rings of iNOS immunofluorescence were seen, even when the images were examined at the highest sensitivity levels. The recruitment of iNOS to the phagosomes was time dependent, as colocalization of iNOS with LB phagosomes increased over time (Fig. 3). Colocalization of iNOS with LB in individual cells ranged from a minimum of 8% at the earliest time point tested to greater than 90%. After 1 h, the average percent iNOS colocalization with LB was 45.4% ± 7.0% (11 independent experiments). To ensure that iNOS recruitment to phagosomes is not unique to J774 cells, localization studies were also carried out in C57BL/6 BMM. BMM also demonstrated colocalization between iNOS and LB phagosomes (Fig. 2C and D), with 57.4% ± 9.9% (four independent experiments) iNOS colocalization at 1 h postinfection (Fig. 3). To determine if iNOS colocalization was dependent on the route of entry taken by LB, IgG-coated LB were used to infect J774 macrophages. IgG-coated LB also demonstrated colocalization with iNOS, at levels similar to that seen with complement-opsonized LB. At 1 h postinfection, iNOS colocalization with IgG-coated LB was 31.0% ± 4% (n = 4 fields). These studies indicate that iNOS is recruited to phagosomes in activated macrophages.
FIG. 1.
iNOS detection in macrophages by immunofluorescence microscopy. Images show the level of fluorescence observed with resting J774 cells (A), IFN-γ- and LPS-stimulated J774 macrophages (B), IFN-γ- and LPS-stimulated BMM from C57BL/6 mice (C), and IFN-γ- and LPS-stimulated BMM from C57BL/6 iNOS−/− mice (D). Magnification, ×100.
FIG. 2.
Recruitment of iNOS to model, LB phagosomes: J774 macrophages (A and B) and BMM from C57BL/6 mice (C and D) stimulated with IFN-γ and LPS. After phagocytosis of LB, cells were stained with anti-iNOS antibodies followed by a secondary antibody conjugated to Alexa 568. Arrows indicate examples of iNOS colocalization with LB phagosomes. Arrowheads indicate LB phagosomes that did not colocalize with iNOS. Insets (A and D) are enlarged images corresponding to the dashed boxes, as indicated.
FIG. 3.
Quantitative analysis of iNOS colocalization with phagosomes. Colocalization of LB phagosomes and iNOS over time in IFN-γ- and LPS-stimulated J774 macrophages and in IFN-γ- and LPS-stimulated BMM from C57BL/6 mice. Each symbol represents the mean percent iNOS colocalization for at least four microscopic fields containing a total of at least 50 LB phagosomes. Squares, J774; triangles, BMM.
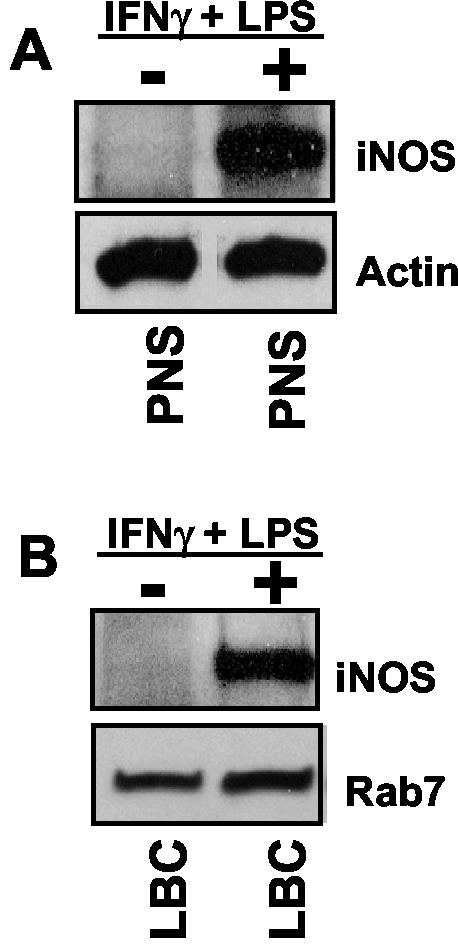
To further examine the association of iNOS and LB phagosomes, LBC were isolated and characterized for purity as previously described (19, 42). As seen in Fig. 4A, postnuclear supernatants (PNS) from unstimulated cells did not contain appreciable levels of iNOS. In stimulated cells, iNOS was present in PNS (Fig. 4A) and also on purified LBC (Fig. 4B). In these analyses, actin (Fig. 4A) and Rab7 (Fig. 4B) served as protein loading controls for PNS and LBC, respectively. The presence of iNOS on purified LBC is consistent with the interpretation that iNOS is recruited to phagosomes. To confirm this further, and to rule out nonspecific association of iNOS with phagosomes during purification, PNS from LB-infected but unstimulated J774 cells was mixed with PNS from uninfected but IFN-γ- plus LPS-stimulated macrophages. LBC were isolated by the same protocol as for the above experiments (19, 42), and preparations were subjected to Western blot analysis. No association of iNOS with LBC isolated from mixed PNS specimens was observed. Thus, iNOS detected on purified LBC is specifically recruited to and physically associated with LB phagosomes.
FIG. 4.
Biochemical analysis of iNOS recruitment to purified phagosomes. (A) Western blot of PNS from unstimulated (−) or IFN-γ- and LPS-stimulated (+) J774 macrophages used to purify LBC. (B) Western blot of LBC purified from unstimulated (−) or IFN-γ- and LPS-stimulated (+) J774 macrophages. Blots were probed with antibodies to iNOS and actin (A) or iNOS and Rab7 (B). Actin and Rab7 served as loading controls for PNS and LBC, respectively.
Mycobacteria block iNOS recruitment to phagosomes.
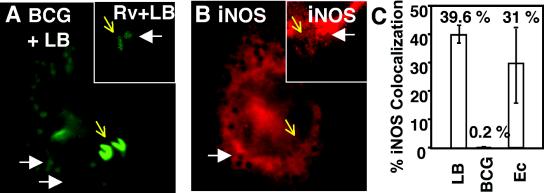
In contrast to LB phagosomes, iNOS did not colocalize with M. bovis BCG phagosomes (Fig. 5A and B). Out of 484 phagosomes (eight independent experiments) in IFN-γ- and LPS-stimulated J774 macrophages containing M. bovis BCG, only a single phagosome showed colocalization with iNOS after a 1-h infection (Fig. 5C). Identical results were observed with virulent M. tuberculosis H37Rv (Fig. 5A and B, insets; images are representative of three independent experiments). Similar results were obtained at 3 h after infection: M. tuberculosis H37Rv did not localize with iNOS at any of the time points tested (1 and 3 h postinfection). To ensure that iNOS was capable of being recruited to phagosomes in macrophages infected by mycobacteria, LB coinfection was used as a control. In macrophages infected with mycobacteria, LB phagosomes colocalized with iNOS (Fig. 5A and B), indicating that the ability of mycobacteria to affect iNOS colocalization is limited to the mycobacterial phagosome. Infection of C57BL/6 BMM showed identical results.
FIG. 5.
Exclusion of iNOS from mycobacterial phagosomes in IFN-γ- and LPS-stimulated macrophages infected with LB, M. bovis BCG, or M. tuberculosis H37Rv. (A) Fluorescence (green) indicates LB, M. bovis BCG, or M. tuberculosis H37Rv; (B) fluorescence (red) indicates iNOS. The large panels show BCG-LB coinfection. The insets show macrophages coinfected with M. tuberculosis H37Rv (Rv) and LB (images are representative of three independent experiments). White arrows, examples of iNOS recruitment to LB phagosomes; yellow arrows, M. bovis BCG or M. tuberculosis H37Rv (expressing GFP). Note that iNOS did not colocalize with M. bovis BCG or M. tuberculosis H37Rv. (C) LB, M. bovis BCG, and E. coli DH5α (Ec) in IFN-γ- and LPS-stimulated J774 macrophages were scored for percent iNOS colocalization at 1 h postinfection.
In order to examine a bacterial species other than mycobacteria, GFP-labeled E. coli DH5α was used to infect stimulated J774 macrophages. Unlike mycobacteria, E. coli phagosomes did colocalize with iNOS at 1 h postinfection. Percent colocalization between iNOS and E. coli in individual cells ranged from 4 to 50% at 1 h postinfection (three independent experiments) (Fig. 5C). Thus, the ability of mycobacteria to prevent iNOS colocalization may be limited to the species of bacteria adapted to macrophage parasitism.
A functional actin cytoskeleton is essential for recruitment of iNOS to phagosomes.
It has only recently been recognized that iNOS localizes to the apical membrane in epithelial cells (23) and is not diffusely distributed in the cytosol as previously thought. This subcortical plasma membrane anchoring of iNOS is achieved via interactions between the C-terminal three amino acids (SAL) of iNOS and the tandem, PDZ protein binding domains of the adaptor protein EBP50 (23). EBP50 in turn binds ezrin, and ezrin is a well-known linker between membranes (via its binding to PIP2 [2]) and the actin cytoskeleton (4). Importantly, actin has been recently shown to participate in phagosome maturation and function (10).
To examine whether the actin cytoskeleton plays a role in iNOS recruitment to LB phagosomes, LB-infected macrophages were treated with the actin microfilament disrupter cytochalasin D (Fig. 6). Cytochalasin D-treated macrophages showed a decrease in iNOS colocalization with LB phagosomes compared to untreated macrophages. Cytochalasin D-treated macrophages showed 6.9% ± 1.4% colocalization (n = 8 fields) between iNOS and LB phagosomes at 1 h postinfection, compared to 31.1% ± 2.9% colocalization (n = 7 fields) in untreated macrophages (P = 0.001) (Fig. 6). The effect of the actin microfilament stabilizer jasplakinolide was also examined. Macrophages treated with jasplakinolide also showed a decrease in iNOS colocalization with LB phagosomes relative to that of untreated macrophages. These macrophages showed 5.3% ± 0.6% iNOS colocalization (n = 8 fields) with LB phagosomes at 1 h postinfection (P = 0.001) (Fig. 6). Macrophages treated with cytochalasin D followed by jasplakinolide did not differ from the untreated control. Cytochalasin D and jasplakinolide-treated macrophages showed 37.6% ± 4.4% iNOS colocalization (n = 7 fields) with LB (Fig. 6), indicating that addition of jasplakinolide after cytochalasin D treatment reversed the effect. The ability of jasplakinolide to reverse the effect of cytochalasin D suggests that a cycle of actin depolymerization-polymerization (actin rearrangement) is required for proper iNOS localization relative to the phagosome. All of the results described above were generated using complement-opsonized LB. IgG-coated LB showed similar results, with cytochalasin D-treated macrophages showing 10.7% ± 2.4% colocalization (n = 7 fields) compared to the 42.0% ± 4.5% iNOS colocalization (n = 7 fields) seen with macrophages that were treated with both cytochalasin D and jasplakinolide (P = 0.001). Jasplakinolide-treated macrophages also showed an inhibition in iNOS colocalization, with only 4.4% ± 1.5% IgG-coated LB associating with iNOS (n = 10 fields) (P = 0.001, relative to cytochalasin D- and jasplakinolide-treated macrophages). Thus, a functional actin cytoskeleton is necessary for iNOS recruitment to the phagosome.
FIG. 6.
A functional actin cytoskeleton is necessary for iNOS recruitment to phagosomes. Quantitation of iNOS colocalization with LB phagosomes in IFN-γ- and LPS-stimulated J774 macrophages under conditions of no treatment, cytochalasin D, jasplakinolide, or cytochalasin D followed by jasplakinolide treatment is shown. Each column represents mean percent iNOS colocalization ± SE for at least seven microscopic fields containing a total of at least 50 LB phagosomes. **, significance of P = 0.001 relative to untreated phagosomes.
DISCUSSION
The purpose of our study was to investigate the intracellular colocalization of iNOS relative to newly formed phagosomes in macrophages during or immediately after phagocytosis and to examine whether intracellular mycobacteria show any differences relative to model, LB phagosomes. Our data show that iNOS is not randomly distributed in the cell and that it is recruited to newly formed phagosomes in macrophages. This recruitment is not dependent on the route of receptor-mediated phagocytosis, at least in the case of complement-opsonized and IgG-opsonized LB. In contrast to model phagosomes, iNOS does not show colocalization with M. tuberculosis var. bovis BCG and M. tuberculosis H37Rv. In contrast to mycobacteria, E. coli DH5α-containing phagosomes recruited iNOS during infection, indicating specificity of iNOS exclusion by the intracellular mycobacterial pathogens.
Our study extends the previous observations examining the localization of iNOS in activated macrophages (34, 44). Vodovotz et al. (44) performed immunoelectron microscopy and biochemical techniques, while Schmidt et al. (34) used Western blotting to examine iNOS in soluble and particulate protein fractions. The recruitment of the largely cytosolic protein iNOS to the vicinity of phagosomal membranes observed in our experiments is consistent with the findings of Schmidt et al. (34) and Vodovotz et al. (44). Both of these groups saw iNOS in the particulate fractions of stimulated RAW 264.7 macrophages (34, 44). While our work was in progress another group reported colocalization of iNOS with mutant Salmonella phagosomes (5), consistent with our observations and the observations by Schmidt et al. (34) and Vodovotz et al. (44).
To understand the nature of iNOS recruitment to LB phagosomes, we decided to examine the role of the actin cytoskeleton in this process. It has been proposed that actin filaments are involved in processes associated with phagosome biogenesis (10). Furthermore, recent studies have shown that iNOS can be anchored to the actin cytoskeleton via EBP50 and ezrin (23). In the present study, the colocalization of iNOS and LB has been shown to depend on actin cytoskeletal dynamics, as disruption of actin microfilaments by cytochalasin D prevented iNOS from colocalizing with LB. This effect was also seen with the actin microfilament stabilizer jasplakinolide. Jasplakinolide may stabilize actin microfilaments to the extent that the cytoskeleton is maintained locked in a certain state, thus preventing actin rearrangements that may be necessary for iNOS recruitment to the LB phagosome. Interestingly, the effect of cytochalasin D on actin microfilaments could be reversed by the subsequent addition of jasplakinolide, resulting in levels of iNOS colocalization with LB phagosomes similar to those seen with untreated, LB-infected macrophages. Thus, a cycle of actin rearrangement appears to be essential and perhaps sufficient in the relocalization of iNOS and its recruitment to phagosomes.
NO · has been implicated as one of the few bactericidal agents produced in vivo capable of affecting M. tuberculosis (18, 22, 27, 29). Nevertheless, the ability to induce macrophages to kill M. tuberculosis via iNOS and NO · has been contested (8), and variability in controlling different strains of M. tuberculosis via this mechanism has been reported (30, 32, 47). While the reason for these differences is not known, the apparent ability of M. tuberculosis to prevent recruitment of iNOS to the phagosome, and thus possibly reduce the efficacy of NO · action, may vary between the different strains or experimental conditions used and thus could potentially explain discrepancies with NO · susceptibilities of the tubercle bacillus.
The idea that enzymes involved in the generation of antibacterial agents are recruited to the plasma membrane in activated macrophages is not without precedent, as the components of NADPH oxidase are known to migrate to the plasma membrane in stimulated macrophages (7, 16, 17, 25). The recruitment of NADPH oxidase subunits and their assembly on the newly formed phagosome are necessary for oxidative burst (7, 16, 17, 31). Two of the cytosolic oxidase components, p67-phox and p47-phox, have been well characterized. Upon activation of the host cell, p47-phox becomes phosphorylated, causing conformational changes to occur that allow it to interact with p67-phox and with the phagosomal membrane protein cytochrome b558 (7, 17, 31). In this process, Rac1, Rac2, and p40-phox are also recruited to the plasma membrane upon activation (16, 17, 25, 40), where both Rac proteins play a critical role in the activation of the NADPH oxidase complex (3, 16, 40). Based on this model, we postulate that in infected macrophages, iNOS and possibly other regulatory proteins may be recruited to the plasma membrane or nascent phagosomes of activated macrophages. Future studies will identify factors controlling iNOS recruitment in activated macrophages and the mechanism(s) by which iNOS is excluded from mycobacterial phagosomes.
Acknowledgments
This work was supported by National Institutes of Health grants AI42999 (to V.D.) and CA26731 and by the Howard Hughes Medical Institute (M.A.M.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Armstrong, J. A., and P. D. A. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barret, C., C. Roy, P. Montcourrier, P. Mangeat, and V. Niggli. 2000. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 151:1067-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokoch, G. M., and C. J. Der. 1993. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 7:750-759. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher, A., K. Edwards, and R. G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3:586-599. [DOI] [PubMed] [Google Scholar]
- 5.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, R. A., B. D. Volpp, K. G. Leidal, and W. M. Nauseef. 1990. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J. Clin. Investig. 85:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., J. E. Pearl, J. V. Brooks, S. Ehlers, and I. M. Orme. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68:6879-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 10.Defacque, H., M. Egeberg, A. Habermann, M. Diakonova, C. Roy, P. Mangeat, W. Voelter, G. Marriott, J. Pfannstiel, H. Faulstich, and G. Griffiths. 2000. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 19:199-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 12.Deretic, D., L. A. Huber, N. Ransom, M. Mancini, K. Simons, and D. S. Papermaster. 1995. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J. Cell Sci. 108:215-224. [DOI] [PubMed] [Google Scholar]
- 13.Deretic, V., L. E. Via, R. A. Fratti, and D. Deretic. 1997. Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis 18:2542-2547. [DOI] [PubMed] [Google Scholar]
- 14.Desjardins, M., L. A. Huber, R. G. Parton, and G. Griffiths. 1994. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124:677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhandayuthapani, S., L. E. Via, C. A. Thomas, P. M. Horowitz, D. Deretic, and V. Deretic. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17:901-912. [DOI] [PubMed] [Google Scholar]
- 16.Dusi, S., M. Donini, and F. Rossi. 1996. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J. 314:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el Benna, J., J. M. Ruedi, and B. M. Babior. 1994. Cytosolic guanine nucleotide-binding protein Rac2 operates in vivo as a component of the neutrophil respiratory burst oxidase. Transfer of Rac2 and the cytosolic oxidase components p47phox and p67phox to the submembranous actin cytoskeleton during oxidase activation. J. Biol. Chem. 269:6729-6734. [PubMed] [Google Scholar]
- 18.Flesch, I. E., J. H. Hess, I. P. Oswald, and S. H. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 19.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fratti, R. A., J. Chua, and V. Deretic. 2002. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 277:17320-17326. [DOI] [PubMed] [Google Scholar]
- 21.Fratti, R. A., J. Chua, I. Vergne, and V. Deretic. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100:5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbe, T. R., N. S. Hibler, and V. Deretic. 1999. Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: induction of the 16-kilodalton alpha-crystallin homolog by exposure to nitric oxide donors. Infect. Immun. 67:460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynne, P. A., K. E. Darling, J. Picot, and T. J. Evans. 2002. Epithelial inducible nitric-oxide synthase is an apical EBP50-binding protein that directs vectorial nitric oxide output. J. Biol. Chem. 277:33132-33138. [DOI] [PubMed] [Google Scholar]
- 24.Hart, P. D., J. A. Armstrong, C. A. Brown, and P. Draper. 1972. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect. Immun. 5:803-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyworth, P. G., B. P. Bohl, G. M. Bokoch, and J. T. Curnutte. 1994. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 269:30749-30752. [PubMed] [Google Scholar]
- 26.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 27.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May, R. C., E. Caron, A. Hall, and L. M. Machesky. 2000. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2:246-248. [DOI] [PubMed] [Google Scholar]
- 29.Nozaki, Y., Y. Hasegawa, S. Ichiyama, I. Nakashima, and K. Shimokata. 1997. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 65:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien, L., J. Carmichael, D. B. Lowrie, and P. W. Andrew. 1994. Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect. Immun. 62:5187-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, J. W., M. Ma, J. M. Ruedi, R. M. Smith, and B. M. Babior. 1992. The cytosolic components of the respiratory burst oxidase exist as a Mr approximately 240,000 complex that acquires a membrane-binding site during activation of the oxidase in a cell-free system. J. Biol. Chem. 267:17327-17332. [PubMed] [Google Scholar]
- 32.Rhoades, E. R., and I. M. Orme. 1997. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 65:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell, D. G., H. C. Mwandumba, and E. E. Rhoades. 2002. Mycobacterium and the coat of many lipids. J. Cell Biol. 158:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt, H. H., T. D. Warner, M. Nakane, U. Forstermann, and F. Murad. 1992. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol. Pharmacol. 41:615-624. [PubMed] [Google Scholar]
- 35.Sisk, T. J., T. Gourley, S. Roys, and C. H. Chang. 2000. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J. Immunol. 165:2511-2517. [DOI] [PubMed] [Google Scholar]
- 36.Stevens-Truss, R., and M. A. Marletta. 1995. Interaction of calmodulin with the inducible murine macrophage nitric oxide synthase. Biochemistry 34:15638-15645. [DOI] [PubMed] [Google Scholar]
- 37.Stuehr, D. J., and M. A. Marletta. 1987. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J. Immunol. 139:518-525. [PubMed] [Google Scholar]
- 38.Stuehr, D. J., and M. A. Marletta. 1987. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 47:5590-5594. [PubMed] [Google Scholar]
- 39.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 40.Tsunawaki, S., H. Mizunari, M. Nagata, O. Tatsuzawa, and T. Kuratsuji. 1994. A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox. Biochem. Biophys. Res. Commun. 199:1378-1387. [DOI] [PubMed] [Google Scholar]
- 41.Vergne, I., J. Chua, and V. Deretic. 2003. Mycobacterium tuberculosis phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking. Traffic 4:1-7. [DOI] [PubMed] [Google Scholar]
- 42.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326-13331. [DOI] [PubMed] [Google Scholar]
- 43.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111:897-905. [DOI] [PubMed] [Google Scholar]
- 44.Vodovotz, Y., D. Russell, Q. W. Xie, C. Bogdan, and C. Nathan. 1995. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J. Immunol. 154:2914-2925. [PubMed] [Google Scholar]
- 45.Xie, Q. W., H. J. Cho, J. Calaycay, R. A. Mumford, K. M. Swiderek, T. D. Lee, A. Ding, T. Troso, and C. Nathan. 1992. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256:225-228. [DOI] [PubMed] [Google Scholar]
- 46.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]
- 47.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]