Abstract
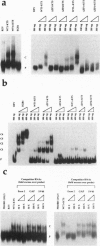
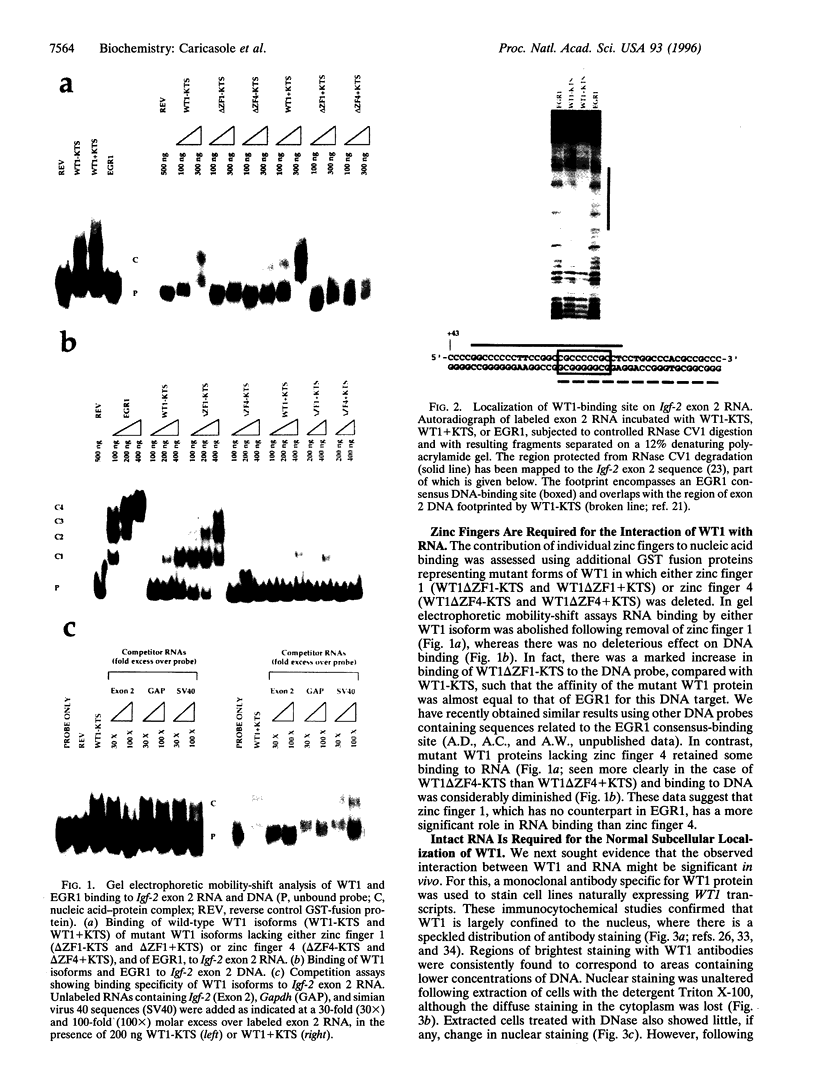
The Wilms tumor suppressor gene WT1 is implicated in the ontogeny of genito-urinary abnormalities, including Denys-Drash syndrome and Wilms tumor of the kidney. WT1 encodes Kruppel-type zinc finger proteins that can regulate the expression of several growth-related genes, apparently by binding to specific DNA sites located within 5' untranslated leader regions as well as 5' promoter sequences. Both WT1 and a closely related early growth response factor, EGR1, can bind the same DNA sequences from the mouse gene encoding insulin-like growth factor 2 (Igf-2). We report that WT1, but not EGR1, can bind specific Igf-2 exonic RNA sequences, and that the zinc fingers are required for this interaction. WT1 zinc finger 1, which is not represented in EGR1, plays a more significant role in RNA binding than zinc finger 4, which does have a counterpart in EGR1. Furthermore, the normal subnuclear localization of WT1 proteins is shown to be RNase, but not DNase, sensitive. Therefore, WT1 might, like the Kruppel-type zinc finger protein TFIIIA, regulate gene expression by both transcriptional and posttranscriptional mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Caricasole A., Ward A. A luciferase-reporter vector with blue-white selection for rapid subcloning and mutational analysis of eukaryotic promoters. Gene. 1993 Feb 14;124(1):139–140. doi: 10.1016/0378-1119(93)90777-z. [DOI] [PubMed] [Google Scholar]
- Caricasole A., Ward A. Transactivation of mouse insulin-like growth factor II (IGF-II) gene promoters by the AP-1 complex. Nucleic Acids Res. 1993 Apr 25;21(8):1873–1879. doi: 10.1093/nar/21.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B. R., Sukhatme V. P., Roberts A. B., Sporn M. B., Rauscher F. J., 3rd, Kim S. J. Repression of the transforming growth factor-beta 1 gene by the Wilms' tumor suppressor WT1 gene product. Mol Endocrinol. 1994 May;8(5):595–602. doi: 10.1210/mend.8.5.8058069. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Rupprecht H. D., Rohwer-Nutter P., Lopez-Guisa J. M., Madden S. L., Rauscher F. J., 3rd, Sukhatme V. P. DNA recognition by splicing variants of the Wilms' tumor suppressor, WT1. Mol Cell Biol. 1994 Jun;14(6):3800–3809. doi: 10.1128/mcb.14.6.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C., Hou X., Maheswaran S., Bennett P., Ngwu C., Re G. G., Garvin A. J., Rosner M. R., Haber D. A. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995 Oct 2;14(19):4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Goodyer P., Dehbi M., Torban E., Bruening W., Pelletier J. Repression of the retinoic acid receptor-alpha gene by the Wilms' tumor suppressor gene product, wt1. Oncogene. 1995 Mar 16;10(6):1125–1129. [PubMed] [Google Scholar]
- Haber D. A., Sohn R. L., Buckler A. J., Pelletier J., Call K. M., Housman D. E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Larsson S. H., Charlieu J. P., Miyagawa K., Engelkamp D., Rassoulzadegan M., Ross A., Cuzin F., van Heyningen V., Hastie N. D. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995 May 5;81(3):391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Little M. H., Williamson K. A., Mannens M., Kelsey A., Gosden C., Hastie N. D., van Heyningen V. Evidence that WT1 mutations in Denys-Drash syndrome patients may act in a dominant-negative fashion. Hum Mol Genet. 1993 Mar;2(3):259–264. doi: 10.1093/hmg/2.3.259. [DOI] [PubMed] [Google Scholar]
- Little M., Holmes G., Bickmore W., van Heyningen V., Hastie N., Wainwright B. DNA binding capacity of the WT1 protein is abolished by Denys-Drash syndrome WT1 point mutations. Hum Mol Genet. 1995 Mar;4(3):351–358. doi: 10.1093/hmg/4.3.351. [DOI] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., Park S., Bernard A., Morris J. F., Rauscher F. J., 3rd, Hill D. E., Haber D. A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin J. F., Friedman J. R., Meyer W. K., Vissing H., Thiesen H. J., Rauscher F. J., 3rd Krüppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4509–4513. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. F., Madden S. L., Tournay O. E., Cook D. M., Sukhatme V. P., Rauscher F. J., 3rd Characterization of the zinc finger protein encoded by the WT1 Wilms' tumor locus. Oncogene. 1991 Dec;6(12):2339–2348. [PubMed] [Google Scholar]
- Mundlos S., Pelletier J., Darveau A., Bachmann M., Winterpacht A., Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development. 1993 Dec;119(4):1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Nakagama H., Heinrich G., Pelletier J., Housman D. E. Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol Cell Biol. 1995 Mar;15(3):1489–1498. doi: 10.1128/mcb.15.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S., Ward A., Graham C. Discriminating translation of insulin-like growth factor-II (IGF-II) during mouse embryogenesis. Mol Reprod Dev. 1994 Nov;39(3):249–258. doi: 10.1002/mrd.1080390302. [DOI] [PubMed] [Google Scholar]
- Nielsen F. C., Ostergaard L., Nielsen J., Christiansen J. Growth-dependent translation of IGF-II mRNA by a rapamycin-sensitive pathway. Nature. 1995 Sep 28;377(6547):358–362. doi: 10.1038/377358a0. [DOI] [PubMed] [Google Scholar]
- Pieler T., Theunissen O. TFIIIA: nine fingers--three hands? Trends Biochem Sci. 1993 Jun;18(6):226–230. doi: 10.1016/0968-0004(93)90194-r. [DOI] [PubMed] [Google Scholar]
- Rathjen P. D., Nichols J., Toth S., Edwards D. R., Heath J. K., Smith A. G. Developmentally programmed induction of differentiation inhibiting activity and the control of stem cell populations. Genes Dev. 1990 Dec;4(12B):2308–2318. doi: 10.1101/gad.4.12b.2308. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Reddy J. C., Morris J. C., Wang J., English M. A., Haber D. A., Shi Y., Licht J. D. WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J Biol Chem. 1995 May 5;270(18):10878–10884. doi: 10.1074/jbc.270.18.10878. [DOI] [PubMed] [Google Scholar]
- Rotwein P., Hall L. J. Evolution of insulin-like growth factor II: characterization of the mouse IGF-II gene and identification of two pseudo-exons. DNA Cell Biol. 1990 Dec;9(10):725–735. doi: 10.1089/dna.1990.9.725. [DOI] [PubMed] [Google Scholar]
- Rupprecht H. D., Drummond I. A., Madden S. L., Rauscher F. J., 3rd, Sukhatme V. P. The Wilms' tumor suppressor gene WT1 is negatively autoregulated. J Biol Chem. 1994 Feb 25;269(8):6198–6206. [PubMed] [Google Scholar]
- Ryan G., Steele-Perkins V., Morris J. F., Rauscher F. J., 3rd, Dressler G. R. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995 Mar;121(3):867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- Sharma P. M., Bowman M., Yu B. F., Sukumar S. A rodent model for Wilms tumors: embryonal kidney neoplasms induced by N-nitroso-N'-methylurea. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9931–9935. doi: 10.1073/pnas.91.21.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov I. T., Attaran A., Kearsey S. E. BM28, a human member of the MCM2-3-5 family, is displaced from chromatin during DNA replication. J Cell Biol. 1995 Jun;129(6):1433–1445. doi: 10.1083/jcb.129.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov I. T., Pepperkok R., Philipova R. N., Kearsey S. E., Ansorge W., Werner D. A human nuclear protein with sequence homology to a family of early S phase proteins is required for entry into S phase and for cell division. J Cell Sci. 1994 Jan;107(Pt 1):253–265. doi: 10.1242/jcs.107.1.253. [DOI] [PubMed] [Google Scholar]
- Varanasi R., Bardeesy N., Ghahremani M., Petruzzi M. J., Nowak N., Adam M. A., Grundy P., Shows T. B., Pelletier J. Fine structure analysis of the WT1 gene in sporadic Wilms tumors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3554–3558. doi: 10.1073/pnas.91.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Madden S. L., Deuel T. F., Rauscher F. J., 3rd The Wilms' tumor gene product, WT1, represses transcription of the platelet-derived growth factor A-chain gene. J Biol Chem. 1992 Nov 5;267(31):21999–22002. [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Deuel T. F. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. J Biol Chem. 1993 May 5;268(13):9172–9175. [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Enger K. T., Deuel T. F. A second transcriptionally active DNA-binding site for the Wilms tumor gene product, WT1. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8896–8900. doi: 10.1073/pnas.90.19.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Gurrieri M., Huang J., Deuel T. F. WT1, the Wilms' tumor suppressor gene product, represses transcription through an interactive nuclear protein. Oncogene. 1995 Mar 16;10(6):1243–1247. [PubMed] [Google Scholar]
- Ward A., Pooler J. A., Miyagawa K., Duarte A., Hastie N. D., Caricasole A. Repression of promoters for the mouse insulin-like growth factor II-encoding gene (Igf-2) by products of the Wilms' tumour suppressor gene wt1. Gene. 1995 Dec 29;167(1-2):239–243. doi: 10.1016/0378-1119(95)00645-1. [DOI] [PubMed] [Google Scholar]
- Werner H., Rauscher F. J., 3rd, Sukhatme V. P., Drummond I. A., Roberts C. T., Jr, LeRoith D. Transcriptional repression of the insulin-like growth factor I receptor (IGF-I-R) gene by the tumor suppressor WT1 involves binding to sequences both upstream and downstream of the IGF-I-R gene transcription start site. J Biol Chem. 1994 Apr 29;269(17):12577–12582. [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]