Abstract
Previously, we had shown that T cells accumulated in peribronchiolar and perivascular areas of lungs soon after intranasal infection with Streptococcus pneumoniae. We have now presented new evidence, using major histocompatibility class II-deficient mice, that CD4 cells are important for early protective immunity. In addition, we have also shown that a population of human CD4 cells migrates towards pneumococci and that in vivo-passaged pneumococci are substantially more potent at inducing migration than in vitro-grown bacteria. This migratory process is unique to a specific population of CD4 cells, is highly reproducible, and is independent of prior CD4 cell activation, and yet the migratory process results in a significant proportion of CD4 cells becoming activated. The production of pneumolysin is a key facet in the induction of migration of CD4 cells by in vivo bacteria, as pneumolysin-deficient bacteria do not induce migration, but the data also show that pneumolysin alone is not sufficient to explain the enhanced migration. Increased CD25 expression occurs during migration, and a higher percentage of cells in the migrated population express gamma interferon or interleukin 4 (IL-4) than in the population that did not migrate. There is evidence that the activation of IL-4 expression occurs during migration.
The pneumococcus is an important human pathogen that colonizes the upper respiratory tract, eventually leading to diseases of high morbidity and mortality such as pneumonia, septicemia, and meningitis. A key virulence factor in these events is the pneumococcal toxin pneumolysin, which is a 53-kDa protein produced by virtually all clinical isolates of the pneumococcus (20). Pneumolysin is a cytosolic protein that is released upon cell lysis. It is known to cause a variety of proinflammatory and cytotoxic activities which significantly contribute to the pathogenesis of invasive pneumococcal disease. The toxin exhibits cytolytic activity usually assayed as hemolysis against cells with cholesterol-containing membranes (5).
Another important activity of pneumolysin is its interaction with components of the cellular and humoral immune response. Pneumolysin is known to directly affect leukocytes at sublytic concentrations. The neutrophil respiratory burst is inhibited by pneumolysin in vitro (28), as is mitogen-induced proliferation and antibody production by human lymphocytes (11). Additionally, pneumolysin has a direct role in cytokine-mediated inflammation, shown by its capacity to stimulate tumor necrosis factor alpha and interleukin 1β (IL-1β) production from human monocytes, and nitric oxide, IL-6, and cyclo-oxygenase 2 production from macrophages, following in vitro sublytic exposure to pneumolysin (6, 16).
In vivo, antibodies to pneumolysin have been shown to inhibit the virulence of wild-type strains, and pneumolysin-deficient strains are complemented by coinfection with wild-type strains, suggesting that pneumolysin is required for successful bacterial virulence (2, 3, 23). More recently, it has been shown with a mouse model of bronchopneumonia that the virulence of a pneumolysin-deficient pneumococcus was significantly reduced, with low growth of bacteria in the lungs and reduced development of the cellular inflammatory response compared to pneumolysin-sufficient pneumococci (18). Indeed, the absence of pneumolysin was associated with a significant delay and lower severity of pulmonary inflammation and a significantly less intense accumulation of neutrophils and T cells at inflamed sites in the lungs (18). It has also been shown that in a mouse model of nasopharyngeal colonization, the absence of pneumolysin is associated with significantly lower numbers of pneumococci in the nasopharynx (19). While it has been stated that the inflammation induced by the pneumococcus is a result of the release of inflammatory cell wall components (33), our studies have suggested that the majority of inflammation is induced by pneumolysin.
The immunopathology of pneumococcal disease is characterized by an intense inflammatory reaction. Resident alveolar macrophages are thought to play a phagocytic role in the early stages of infection only to be outnumbered by heavy infiltration of neutrophils into infected lungs (13, 18). Indeed, the overwhelming majority of infiltrating host inflammatory cells associated with infection are neutrophils. However, the significant early involvement of T cells in the lungs during pneumococcal infection, and the fact that T cells are an important component of the inflammatory host immune response to the pneumococcal toxin pneumolysin, has also been shown (18). A rapid increase in T-cell infiltration to areas of inflamed bronchioles in the mouse lung following intranasal infection and the fact that these infiltrating T cells migrated specifically to areas of heavy pneumococcal invasion, particularly to peribronchiolar and perivascular areas, were demonstrated (18). More recently, it was verified that both the cytolytic and complement-activating activities of pneumolysin contribute to the influx of inflammatory cells but that the toxin's complement-activating activity was more important for the recruitment of T cells to inflammatory lesions than for its pore-forming activity (17).
There is also accumulating evidence of the importance of T cells in resistance to pneumococcal disease. It is well established that patients with AIDS and patients who have been splenectomized are at high risk for developing severe pneumococcal infections (10, 32). A more recent study has described lymphopenia in the blood of human patients with pneumococcal disease. Activated CD4+ cells producing gamma interferon or IL-2 disappeared from the circulation only to reappear again following successful antibiotic treatment and clinical improvement (21). The numbers of naïve T cells and IL-4-producing CD4+ T cells in the blood did not alter. It was suggested that T cells were sequestered in infected peripheral tissues during infection and subsequently released to the circulation after the infection was cleared. Other studies have pointed to a role for T cells in protection from pneumococcal disease. One study reported that IL-12-deficient patients develop severe pneumococcal infections (14), presumably due to a deficient T-cell response; while in another study, gamma interferon was implicated in the host defense against pneumococcal infection (29).
We have now further investigated the involvement of T cells in pneumococcal disease by using major histocompatibility complex class II (MHC-II) knockout mice that exhibit a lack of CD4+ T cells (9, 25, 31). MHC-II molecules play a central role in the selection of the T-cell repertoire and in the establishment and regulation of the adaptive immune response. We have studied in more detail the interactions between the pneumococcus, pneumolysin, and human CD4 T cells in transmigration assays. For these studies, we used wild-type pneumococci recovered from in vivo and in vitro growth, pneumococci with a specific pneumolysin point mutation, and pneumolysin-deficient pneumococci. Pneumococci from different growth conditions were analyzed because we had unpublished evidence to suggest that pneumococcal isolates recovered from in vivo conditions were more virulent than those grown under in vitro conditions. We were interested to see if this had any bearing on their interactions with T cells.
MATERIALS AND METHODS
Bacteria.
Streptococcus pneumoniae serotype 2 strain D39 was obtained from the National Collection of Type Cultures, London, United Kingdom (NCTC 7466). The pneumolysin-negative mutant (PLN-A) and the autolysin-negative mutant (ALN) are insertion duplication mutants of D39 (4). Purified pneumolysin with 100% hemolytic activity (300 hemolytic units [HU] per μg of purified protein) and its W433>F mutant with no detectable hemolytic activity were purified as previously described (12). Bacteria were identified as pneumococci prior to experiments by Gram staining, catalase testing, alpha-hemolysis on blood agar plates, and determination of optochin sensitivity. The capsular polysaccharide serotypes were confirmed by the Quellung reaction.
To obtain pneumococci grown in vivo, bacteria were cultured and passaged through mice as described previously (18) and subsequently recovered and stored at −70°C. When required, suspensions were thawed at room temperature and bacteria were harvested by centrifugation before resuspension in sterile phosphate-buffered saline (PBS).
Infection of mice.
Female MHC-II knockout mice (Aβ0/0) (National Institute for Medical Research, London, United Kingdom) and their isogenic parent strain C57BL/6 mice were used. All mice were 8 to 10 weeks old when used and weighed 30 to 35 g (Harlan, Bichester, United Kingdom). These mice did not have detectable levels of anti-type 2 or antipneumolysin antibodies. The MHC-II knockout mice displayed no anatomical or behavioral defects, and histological analysis of a number of tissues showed no alterations in organ structure or composition (data not shown). As described before (18), the mice were lightly anesthetized with 2.5% (vol/vol) fluothane (Astra-Zeneca, Macclesfield, United Kingdom) over oxygen (1.5 to 2 liters/min), and 50 μl of PBS containing 106 CFU of S. pneumoniae was then administered into the nostrils of mice. The inoculum dose was confirmed by viable counting via plating on blood agar plates following infections.
At prechosen time intervals following infection, groups of mice were deeply anesthetized with 5% (vol/vol) fluothane (Astra-Zeneca), and blood was collected by cardiac puncture. Subsequently, the mice were sacrificed by cervical dislocation, and the lungs were removed into 10 ml of sterile distilled water, weighed, and then homogenized in a Stomacher-Lab blender (Seward Medical, London, United Kingdom). Viable counts in homogenates and blood were determined by serial dilution in sterile nanopure water and plating onto blood agar plates (Oxoid, Basingstoke, United Kingdom) supplemented with 5% (vol/vol) horse blood.
T-cell purification.
CD4 lymphocytes were purified from the peripheral blood of healthy human donors. Briefly, heparinized blood samples were layered onto Lymphoprep (density gradient of 1.077; Axis-Shield PoCAS, Oslo, Norway) and centrifuged at 800 × g for 30 min. The mononuclear cells were removed and washed and resuspended in RPMI medium containing 10% (vol/vol) fetal calf serum. The cells were then exposed to anti-CD4-conjugated Dynabeads (Miltenyi Biotec, Bergisch Gladbach, Germany) to allow for positive selective of CD4 lymphocytes. The viability of the lymphocyte population obtained was 99.4% ± 0.1% (n = 10) as assessed by trypan blue exclusion. CD4-T-cell purity was assessed by flow cytometry, which denoted a purity of >95% ± 2.2% (n = 10).
Transmigration assay.
Purified CD4-T-lymphocyte migration was examined by using 6.5-mm-diameter tissue culture-treated polycarbonate membrane Transwell filter plates (pore size, 5 μm) according to the manufacturer's instructions (Costar, Corning Inc., New York, N.Y.). Briefly, Transwell filters were prewetted with 30 μl of RPMI medium. Subsequently, 600 μl of PBS containing purified pneumolysin or W433>F (at various concentrations) or pneumococci (at 107 CFU) was placed in the lower macrowell and covered with the prewetted Transwell filter. One hundred microliters of RPMI medium containing 5 ×105 CD4 cells was then added to the upper chamber of the Transwell and incubated at 37°C at 5% CO2 for 1.5 h. The underside of each Transwell was then washed with medium into the lower macrowell. Cells that had migrated across the filter were counted with a hemocytometer. Each experiment was repeated three times, with each sample within one experiment done in triplicate.
T-cell activation.
To determine the extent of expression of CD25, resting cells and cells taken from the upper and lower chambers of the transwell after the transmigration assay were washed in PBS and incubated with fluorescein isothiocyanate-conjugated CD25 monoclonal antibody for 1 h (BD Biosciences, San Diego, Calif.). Following incubation, cells were washed in PBS and analyzed by using flow cytometry.
Before the determination of IL-4 and gamma interferon secretion following the transmigration assay, T cells were incubated for 16 h with CD3 (1 μg/ml) and CD28 (10 μg/ml) monoclonal antibodies (R&D, Abingdon, United Kingdom). Cells were then washed in PBS and incubated with the respective cytokine catch reagent (Miltenyi Biotec) at 37°C for 45 min. Cells were then washed in PBS and incubated for 15 min with the respective detection antibody. Cells were resuspended in PBS and analyzed by flow cytometry.
Pneumolysin hemolytic activity assay, enzyme-linked immunosorbent assay, and sonication.
The hemolytic activity of pneumolysin was determined as previously described in detail (2). Following the assay, plates were scored for the complete lysis of sheep blood as indicated by the absence of a pellet of red blood cells. The hemolytic titer of a sample was calculated as the reciprocal of the greatest dilution that caused complete lysis of sheep blood. Purified pneumolysin had a specific activity of at least 300 HU per μg of protein, where 1 HU per ml of pneumolysin lysed 50% of sheep erythrocytes in 30 min at 37°C. The W433>F mutant and the pneumolysin-deficient strain PLN-A caused no lysis of blood cells. Negative controls included PBS alone and bacterial growth medium (brain heart infusion) alone. None of the negative controls caused lysis of blood cells either. In order to release cytoplasmic pneumolysin, approximately 107 CFU of pneumococci per ml were sonicated for 15-s periods on ice with 30-s rest periods for a total of 10 min at an amplitude of 8 MHz per s (Sanyo Ultrasonicator). Extracellular pneumolysin levels for whole and sonicated pneumococci were measured by standard sandwich enzyme-linked immunosorbent assay (7) using a rabbit polyclonal antipneumolysin antiserum (1:1,000). This same antibody was used in one experiment to block the hemolytic activity of pneumococcal samples during transmigration assays (used at a 1:1,000 ratio and preincubated with relevant pneumococci 30 min before the transmigration assay). The antipneumolysin antiserum was generously supplied by Tim Mitchell, University of Glasgow, Glasgow, United Kingdom.
Statistics.
Data were analyzed for statistical significance by analysis of variance followed by the Bonferroni test. Statistical significance was considered to be P values of <0.05.
RESULTS
Growth of wild-type pneumococci in lung tissue and blood of MHC-II knockout and control mice.
In order to investigate whether or not CD4+ T cells had any effect upon pneumococcal infection, MHC-II knockout (Aβ0/0) mice were used in a study of pneumonia and septicemia. These MHC-II knockout mice have been studied extensively and are known to exhibit major decreases in CD4+ T cells (20-22). Indeed, very low numbers of peripheral CD4+ T cells are present (1% CD4-positive T cells in total lymphocyte population compared to 40 to 50% CD4-positive T cells in MHC-II-proficient mice). This 1% population of CD4+ T cells are thought to represent maturation intermediates dependent on MHC-I molecules (22). The CD8+-T-cell population in these mice is normal.
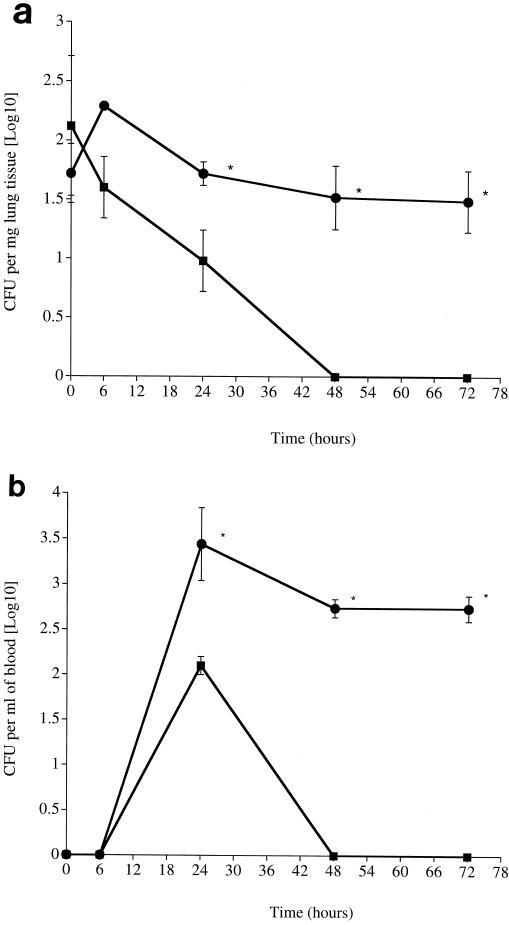
There were clear differences in the numbers of wild-type pneumococci in control and MHC-II knockout mice in both the lung (Fig. 1a) and blood (Fig. 1b). The number of wild-type pneumococci in the lungs of MHC-II knockout mice was significantly higher than in control mice over the whole 72-h period postinfection (P < 0.01 for all time points). Notably, control mice had cleared the infection from their lungs by 48 h postinfection, while MHC-II knockout mice still had bacteria in their lungs at 72 h postinfection (Fig. 1a). Wild-type pneumococci were detected in the blood of both control and knockout mice by 24 h postinfection; however, knockout mice had significantly higher numbers of bacteria over the 24- to 72-h period postinfection than control mice (P < 0.01). Again, control mice had cleared their bacteremia by 48 h postinfection, while MHC-II knockout mice still had bacteria in their blood at 72 h postinfection with no indication of declining numbers (Fig. 1b).
FIG. 1.
(a) Time course of S. pneumoniae infection in lungs of control C57BL/6 (squares) and MHC-II knockout (circles) mice infected intranasally (n = 10 mice for each time point). The error bars indicate the standard error of the means. (b) Time course of S. pneumoniae infection in blood of control C57BL/6 (squares) and MHC-II knockout (circles) mice infected intranasally (n = 10 mice for each time point). The error bars indicate the standard error of the mean. Asterisk, statistical signifiance at P of <0.01.
All MHC-II knockout mice showed signs of illness, such as starry coat and lethargy, at 24 and 48 h postinfection. By 72 h, knockout mice had become moribund and had to be sacrificed. Control mice (C57BL/6) showed no signs or symptoms of illness throughout the period of the experiment.
Effects of in vivo-recovered and in vitro-grown pneumococci on CD4-T-cell migration.
After revealing the involvement of CD4 T cells in pneumococcal infection, we wanted to know whether pneumococci could directly influence CD4-T-cell behavior and if this depended upon the presence of the pneumococcal toxin pneumolysin and/or in vitro and in vivo growth conditions.
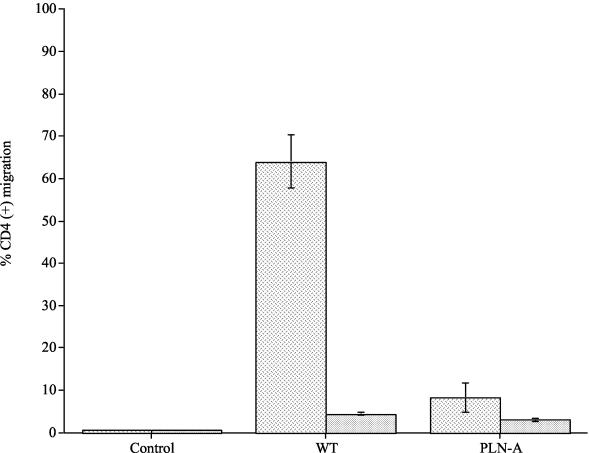
By using an in vitro assay of T-cell transmigration, wild-type pneumococci and PLN-A recovered in vivo were compared to the in vitro-grown wild type and PLN-A (Fig. 2). All pneumococci attracted significantly more CD4 cells than controls (P < 0.01); however, in vivo-grown wild-type pneumococci attracted significantly more CD4 cells than the in vitro-grown wild type or in vitro- or in vivo-grown PLN-A (P < 0.01 for all). There was no significant difference between in vivo- or in vitro-grown PLN-A compared to the in vitro-grown wild type (P > 0.05). Less than 1% CD4 migration was observed for PBS control samples. Pneumococci were never detected as having migrated from the lower to the upper well of the Transwell apparatus.
FIG. 2.
Percentage of CD4-T-cell migration to in vivo-recovered and in vitro-grown wild-type (WT) and pneumolysin-deficient (PLN-A) pneumococci. CD4 migration to in vivo-recovered pneumococci is represented as dashed columns, and migration to in vitro-grown pneumococci is represented as dotted columns (each experiment was repeated three times, with each sample within one experiment done in triplicate). Data represent the mean of all experiments, with error bars indicating the standard error of the mean.
Effects of purified pneumolysin and W433>F mutant on migration of CD4 T cells.
We next compared the abilities of purified wild-type pneumolysin and a pneumolysin with a single amino acid change that eliminated cytolytic activity (W433>F) to cause CD4 migration in vitro.
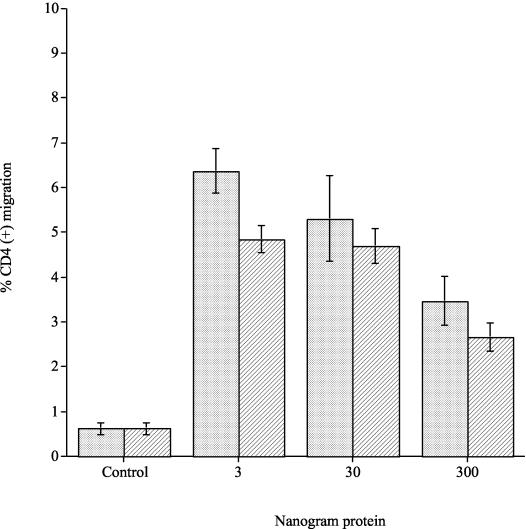
The migrations of CD4 cells to 3, 30, and 300 ng of purified wild-type pneumolysin and the W433>F mutant were compared. Concentrations of 3 to 300 ng of wild-type pneumolysin induced significant increases in the percentage of CD4 cell migration compared to control samples (P < 0.01) (Fig. 3). Similarly, 3 to 300 ng of W433>F caused a significant increase (P < 0.01) in CD4 cell migration compared to the control. There were no significant differences in CD4 cell migration between concentrations of either wild-type pneumolysin or W433>F at concentration ranges of 3 to 30 ng (P > 0.05). There was, however, a significant difference at concentrations of 300 ng when wild-type pneumolysin and the W433>F mutant were compared in that 300 ng of either toxin significantly reduced CD4 cell migration compared to 3 ng of either toxin (P < 0.01). Hence, at low concentration ranges, both wild-type pneumolysin and W433>F significantly increased CD4 cell migration compared to controls yet were significantly less able to do so at high concentrations (300 ng). Less than 1% CD4 cell migration was observed in PBS control samples. CD4 cell viability by the end time point of the experiments (at over 90% as determined by trypan blue exclusion) was not affected by the range of amounts of wild-type or W433>F pneumolysin used.
FIG. 3.
Percentage of CD4-T-cell migration to purified wild-type pneumolysin and the W433>F mutant. Migration to purified pneumolysin is represented as dotted columns, and migration to the W433>F mutant is represented as lined columns (each experiment was repeated three times, with each sample within one experiment done in triplicate). Data represent the mean of all experiments, with error bars indicating the standard error of mean.
In order to determine whether the population of CD4 cells that migrated to wild-type and W433>F pneumolysin was a defined subpopulation, CD4 cells that had migrated towards wild-type and/or W433>F pneumolysin were collected and reassayed (repeat migration) with amounts of wild-type and W433>F pneumolysin that had previously induced the maximum level of CD4 cell migration (3 ng). Both wild-type and W433>F pneumolysin caused a significant level of CD4 cell migration compared to control samples in the first migration assay (P < 0.01) (Table 1). When the assay was repeated with cells collected from the bottom well of the transmigration apparatus, nearly all of the CD4 cells from the first migration assay migrated for a second time, indicating that this was a specific subpopulation of CD4 cells highly responsive to pneumolysin that migrated towards the purified toxins (Table 1). There was no significant difference between CD4 cell migration to wild-type or W433>F pneumolysin during first or second migration assays (P > 0.05).
TABLE 1.
CD4 T cells assayed for migration to purified wild-type pneumolysin and the W433>F mutant and then reassayed for (repeat) migrationa
| Sample | First migration (% of total population) | Repeat migration (% of total first migration population) |
|---|---|---|
| Control | 0.4 ± 0.2 | 0.5 ± 0.2 |
| Wild-type pneumolysin | 7.9 ± 1 | 78 ± 3 |
| W433>F pneumolysin | 5.8 ± 0.2 | 94 ± 4 |
Each experiment was repeated three times, with each sample within one experiment done in triplicate. Data are the means of all experiments ± the standard errors of the means.
Amount of pneumolysin produced by pneumococci recovered in vivo or grown in vitro.
Total protein and pneumolysin levels from wild-type pneumococci recovered in vivo were compared to wild-type pneumococci grown in vitro. Total protein and pneumolysin levels were measured in pneumococcal samples collected and analyzed following T-cell transmigration assays.
There was no significant difference (P > 0.05) in total protein concentrations (1,044 mg per 107 CFU and 1,032 mg per 107 CFU, respectively) or in the amount of pneumolysin protein (59 ng per 107 CFU in vivo and 64 ng per 107 CFU in vitro) following sonication of in vitro- or in vivo-grown bacteria. There was no significant difference in the hemolytic activity of sonicated in vivo-recovered or in vitro-grown wild-type pneumococci (∼10,000 HU per μg protein per 107 CFU for both; P > 0.05). Similarly, there was no significant difference in the concentrations of extracellular pneumolysin protein released into the medium during CD4 cell migration assay from whole, intact in vivo-recovered and in vitro-grown wild-type pneumococci (12 ng per 107 CFU and 14 ng per 107 CFU, respectively). Interestingly, however, the same in vivo-recovered pneumococci released four times as much hemolytic activity as in vitro-grown pneumococci (160 and 40 HU per 107 CFU, respectively; P < 0.01). Neither pneumolysin protein nor any hemolytic activity was detected in any sonicated or nonsonicated PLN-A sample.
Further analysis of the influence of in vivo-recovered wild-type pneumococci on CD4-T-cell migration.
In order to determine whether the significantly enhanced migration of CD4 cells was exclusive to pneumococci recovered in vivo, CD4 cells that had originally migrated to in vivo-recovered pneumococci were collected and then reassayed (repeat migration) against in vivo-recovered and in vitro-grown pneumococci for a second time. As before, a significantly (P < 0.01) greater percentage of CD4 cells migrated towards the in vivo-recovered pneumococci than to the in vitro-grown pneumococci (Table 2, first migration).
TABLE 2.
CD4 T cells assayed for migration towards in vivo-passaged pneumococci and reassayed for (repeat) migration towards in vivo-passaged and in vitro-grown pneumococcia
| Sample | First migration (% of total population) | Repeat migration (% of total first migration population) |
|---|---|---|
| Control | 0.5 ± 0.1 | 0.6 ± 0.1 |
| In vivo pneumococci | 49 ± 5b | 100 ± 9 |
| In vitro pneumococci | 2.7 ± 0.3 | 0.4 ± 0.1 |
CD4 T cells that were assayed for migration towards in vivo-passaged pneumococci were collected (following migration) from the bottom wells and reassayed for (repeat) migration towards in vivo-passaged and in vitro-grown pneumococci. Each experiment was repeated three times, with each sample within one experiment done in triplicate. Data are the means of all experiments ± the standard errors of means.
The first migration population that was reassayed in the repeat migration.
All of the original CD4 cells remigrated to in vivo pneumococci in the second migration assay (Table 2, repeat migration), whereas CD4 cell migration to in vitro-grown pneumococci was effectively zero (P < 0.01).
To see whether this significant difference in migration to in vivo and in vitro bacteria was due to CD4 cell activation, CD3 CD28 preactivated CD4 cells were used. Preactivation of CD4 cells did not alter their ability to migrate to in vivo pneumococci (41.2% ± 1% compared to 49% ± 5% for nonactivated CD4) and it did not affect their ability to selectively migrate in the second (repeat) migration assay as well (94% ± 3% compared to 100% ± 9% for nonactivated CD4), suggesting that the migration process is not dependent upon prior CD4 cell activation.
Characterization of T cells.
Migration was associated with the activation of a high, but not the total, number of T cells. A total of 44.4% ± 2.6% (n = 3) of CD4 cells that migrated to in vivo-recovered pneumococci also expressed CD25, whereas only 7.0% ± 0.3% of nonmigrating cells were CD25 positive. Prior to the migration assay, only 10.4% ± 0.3% of CD4 cells expressed CD25.
The levels of expression of IL-4 and gamma interferon by cells migrating and not migrating towards in vivo-recovered pneumococci were compared (Table 3). The pattern of cytokine release from migrated and nonmigrated cells was not simply a reflection of the starting population. Cells with a capacity to release cytokine on stimulation selectively migrated; significantly higher percentages of migrating cells released IL-4 or gamma interferon than nonmigrating cells (P < 0.01 for both). The percentage of nonmigrating cells that released gamma interferon was significantly less (P < 0.01) than that of the control population, whereas a significantly greater (P < 0.05) percentage of migrated cells released IL-4 on stimulation compared with the controls cells.
TABLE 3.
Secretion of gamma interferon and IL-4 by T cells that migrated or did not migrate towards in vivo-passaged pneumococci after stimulation with CD3 and CD28a
| T cells | % of cells positive for secretion of:
|
|
|---|---|---|
| Gamma interferon | IL-4 | |
| Resting | 59.2 ± 12.1 | 7.1 ± 2.3 |
| Not migrated | 9.7 ± 3.3 | 7.7 ± 2.5 |
| Migrated | 49.3 ± 5.0 | 34.6 ± 8.6 |
Data are the means of six experiments ± the standard errors of means.
Effects of pneumolysin-blocking antibody and pneumococcal autolysin mutation on CD4-T-cell migration.
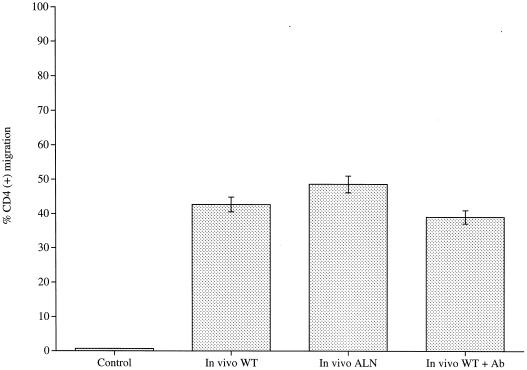
A rabbit polyclonal antipneumolysin antiserum that neutralized hemolytic activity was added to in vivo-recovered pneumococci, and CD4 cell migration to these bacteria was then assayed. The presence of this antiserum had no significant effect (P > 0.05) on CD4 cell migration compared to migration to in vivo-recovered pneumococci alone (Fig. 4). The concentration of antibody used in the migration assay was confirmed as completely inhibitory of the hemolytic activity of 107 CFU of wild-type pneumococci. To test whether autolysis of bacteria and hence lytic release of pneumolysin was required to induce CD4 cell migration, in vivo-recovered autolysin-negative pneumococci were assayed. No significant difference (P > 0.05) in CD4 cell migration levels was observed compared to the migration to autolysin-positive wild-type in vivo-recovered pneumococci (Fig. 4). This result shows that autolysin-induced pneumococcal lysis is not required for CD4 cell migration.
FIG. 4.
Percentage of CD4-T-cell migration to in vivo-recovered wild-type pneumococci (WT), in vivo-recovered autolysin-negative pneumococci (ALN), and in vivo-recovered wild-type pneumococci in the presence of blocking antibody (WT + Ab) (each experiment was repeated three times, with each sample within one experiment done in triplicate). Data represent the mean of all experiments, with error bars indicating the standard error of mean.
DISCUSSION
It has been demonstrated previously that T cells migrated over time to areas of heavy pneumococcal invasion, particularly to peribronchiolar and perivascular areas rich in pneumolysin production (7, 18). The work presented here, together with our previous work, has uncovered an early role for T cells during pneumococcal disease. We have shown that MHC-II knockout mice, which are effectively CD4-T-cell negative, are significantly more susceptible to pneumococcal bronchopneumonia and septicemia than their isogenic wild-type parents, indeed, so much so that these mice succumb to their pneumococcal infections.
This paper reports several new and significant observations relevant to the role of CD4 T lymphocytes in the host immune response to pneumococcal infection: CD4 cells appear to have an important role in early protective responses to pneumococcal infection; a subpopulation of CD4 cells migrates towards pneumococci; CD4 cells migrate towards the toxin pneumolysin; migration to in vivo-recovered pneumococci is drastically greater than to in vitro-grown pneumococci; the enhanced migration is dependent on the presence of pneumolysin; and neither the enhanced migration nor migration to pure pneumolysin is dependent on the hemolytic activity of the toxin. The observation of pneumolysin-induced migration of CD4 cells is consistent with a previous report on T-cell migration during pneumococcal infection. It was shown that the absence of pneumolysin resulted in a significantly less intense accumulation of T cells at inflamed sites in the lungs (18) and that the toxin's hemolytic (cytolytic) activity appeared not to be crucial for this recruitment. In support of this conclusion, here we found that the removal of hemolytic activity, either with antibody or by mutation, did not prevent migration.
We have also shown that CD4 T cells migrate towards in vivo-recovered pneumococci in significantly greater numbers than they do to in vitro-grown pneumococci. The population of CD4 cells that migrates does so persistently and exclusively to in vivo-recovered pneumococci. As levels of migration to in vitro-grown pneumococci and those for in vivo- or in vitro-grown PLN-A are not significantly different from each other, this suggests that the T-cell response to in vivo-recovered pneumococci we observed is unique to only these cells and growth conditions.
Pneumolysin is essential to the enhanced CD4 migration to in vivo-grown pneumococci. Thus, there was no enhancement of migration to in vivo-passaged, pneumolysin-deficient pneumococci. However, the evidence suggests that it is not the amount or activity of pneumolysin per se that explains the significantly greater levels of CD4 migration. There was no detectable difference in the amount or specific activity of the pneumolysin produced by in vivo- and in vitro-grown pneumococci, although more HU were released from in vivo-grown bacteria. Nevertheless, the amount of toxin released in both cases was within the range (3 to 300 ng) over which there was a maximal induction of migration. It is also noteworthy that, by itself, the amount of pneumolysin present in pneumococci grown in vivo was not sufficient to induce the enhanced migration. Therefore, we suggest that another as-yet-unknown factor present within in vivo pneumococci works in concert with pneumolysin to greatly enhance CD4-T-cell migration. We also propose that this is an effector molecule that works in concert only with pneumolysin from in vivo-recovered wild-type pneumococci, because mixing purified pneumolysin with in vivo-recovered PLN-A did not reproduce the enhanced T-cell recruitment (data not shown). Either the synthesis of the effector molecule is linked directly to pneumolysin synthesis in vivo or the effector becomes intimately associated with pneumolysin in the in vivo-recovered pneumococci. An effector protein that synergizes with the cholesterol-dependent cytolysin of Streptococcus pyogenes to enhance cytotoxicity has been recently described (24). It appears, however, that the stimulators of T-cell migration do not have to be presented on whole bacteria because whole bacteria never enter the upper well of the Transwell apparatus.
There are two outstanding questions. First, how does growth under in vivo conditions alter the characteristics of the pneumococcus and/or pneumolysin to enhance T-cell migration? Second, what are the characteristics of the subpopulation of CD4 cells that migrate specifically towards in vivo-recovered pneumococci?
It is not unreasonable to suggest that growth under in vivo conditions alters the characteristics of the pneumococcus. For example, there is evidence to suggest that under in vivo growth conditions, the mRNA levels of several well-characterized pneumococcal virulence factors are up-regulated compared to in vitro growth conditions (27). Indeed, it was reported that the expression of the pneumolysin gene in vivo, 24 h following pneumococcal infection, is up to 10-fold higher than its expression under in vitro conditions at an equivalent time point (27). The authors of that study did not, however, provide data on levels of pneumolysin protein in vivo compared to those in vitro, although we found no evidence of increases in amount or total cytolytic activity of pneumolysin protein or total cytolytic activity. It is tempting, however, to speculate that some other property of pneumolysin may be altered by in vivo growth. We found, for example, that the specific hemolytic (cytolytic) activity of released, but not intracellular, pneumolysin was increased after in vivo growth; this could indicate increased protein structural stability. Furthermore, the specific migration-inducing activity of the toxin may be increased independently of hemolytic activity; we know hemolytic activity is not a requirement for migration.
Why does a subpopulation of CD4 cells have a specific sensitivity to pneumolysin and pneumococci recovered in vivo? We cannot fully answer this question at present. We have shown, however, that the process of migration to in vivo-passaged pneumococci does lead to significantly increased levels of CD25-expressing CD4 cells specifically within the migrated cell population. However, activation as defined by CD25 expression is not a prerequisite for migration, since 56% of migrated cells were not expressing detectable CD25. It is also noteworthy that CD25 expression is minimal prior to migration and that the preactivation of cells before the migration assay does not increase their capability to migrate towards in vivo pneumococci. This finding suggests that the sequence of events that leads to CD4 cell migration is initiated only when these cells are in close proximity to in vivo pneumococci. To our knowledge, the scale and rapidity of CD25 activation seen in these experiments is unprecedented for gram-positive bacteria. Some information on pneumolysin-induced intracellular signals is beginning to be reported (6, 8), but there are no specific data on cellular activation in this regard. Therefore, to the best of our knowledge, this is the first report of such an occurrence. Clearly, the mechanisms underlying this intriguing phenomenon warrant further in-depth investigation. It was previously shown that different cells vary in their sensitivity to pneumolysin, and cell sensitivity can be modulated (15). Differences in sensitivity might result either from differences in plasma membrane properties or in signaling. A related toxin, perfringolysin, binds to cholesterol-rich lipid rafts (35), as do other pore-forming toxins (1). Differences in cholesterol-rich lipid rafts on plasma membranes have been suggested as the explanation for differential sensitivity of rabbit and human erythrocytes to staphylococcal alpha-toxin (32). Cholesterol-rich microdomains, known as lipid rafts, form distinct microenvironments for the preferential enrichment or exclusion of certain molecules important for T-cell signaling and adhesion. Hence, lipid rafts represent meeting places where specific protein-protein interactions take place on the T-cell surface. Intracellular signaling and hence activation are thus thought to be initiated at sites of such contact. Indeed, an increasing number of raft-associated proteins are being identified, many of them associated with regulating T-cell receptor signaling. It is conceivable that pneumolysin instigates CD4-T-cell activation and subsequent migration by binding to an as-yet-unknown receptor compartmentalized within specific plasma membrane microdomains. We are currently investigating these possibilities.
When we looked at the cytokines released by the T cells following the migration assay, there were two interesting observations. One was that cells with the capacity to secrete gamma interferon had a vastly greater tendency to migrate towards pneumococci than to not migrate. This also was true for T cells with the capacity to secrete IL-4; however, it also seems that pneumococcal products can prime the T cells to release IL-4 when subsequently stimulated. Compare the migrated cells with the control resting cells in Table 3. The need for priming by a bacterial product would be consistent for the production of an anti-inflammatory cytokine that limits pathogen-initiated pathology. Previous reports on the involvement of T cells in the host response to pneumococcal infection have concentrated on a strong involvement of so-called type 1 cells (21); however, our data suggest that chemotaxis in response to pneumococci will result in the release of immunosuppressive, as well as immunostimulatory, cytokines at the sites of infection. T cells that express CD25 or secrete high levels of immunosuppressive cytokines, including IL-4, have been termed regulatory T cells (22, 30), and such cells can be induced by bacterial pathogens (26). Taken together, the data suggest that more investigation of regulatory T cells in pneumococcal disease is worthwhile.
In conclusion, we have shown that CD4-T-cell-negative mice are significantly more susceptible to pneumococcal lung infection and bacteremia than mice with functional CD4 T cells. We have also established that in vivo pneumococcal populations can trigger CD4 cell migration in a fashion that is not reproducible by in vitro-grown pneumococci. We propose that this may be due to an effector molecule acting in concert with pneumolysin released only from in vivo pneumococcal populations. The migratory process is unique to a specific population of CD4 cells, is highly reproducible, and is independent of prior CD4 cell activation, yet the migratory process results in a significant proportion of CD4 cells becoming activated. The new evidence we provide here for T-cell interactions with pneumolysin and pneumococci emphasizes the important role of the T cell in the host response to pneumococcal infection.
Editor: J. N. Weiser
REFERENCES
- 1.Abrami, L., M. Fivaz, P. E. Glauser, R. G. Parton, and F. G. van der Goot. 1998. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J. Cell Biol. 140:525-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansmann, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton, K. A., M. P. Everson, and D. E. Briles. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Structure and function of pneumolysin, the multifunctional thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 5:2611-2616. [DOI] [PubMed] [Google Scholar]
- 6.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canvin, J. R., A. P. Marvin., M. Sivakumaran., J. C. Paton., G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119-123. [DOI] [PubMed] [Google Scholar]
- 8.Cockeran, R., C. Durandt, C. Feldman, T. J. Mitchell, and R. Anderson. 2002. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 186:562-565. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell 66:1051-1066. [DOI] [PubMed] [Google Scholar]
- 10.Ejstrud, P., B. Kristensen, J. B. Hansen, K. M. Madsen, H. C. Schonheyder, and H. T. Sorensen. 2000. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand. J. Infect. Dis. 32:521-525. [DOI] [PubMed] [Google Scholar]
- 11.Ferrante, A., B. Rowan-Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, R. J. C., J. Rossjohn, M. W. Parker, R. K. Tweten, P. J. Morgan, T. J. Mitchell, N. Errington, A. J. Rowe, P. W. Andrew, and O. Byron. 1998. Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. J. Mol. Biol. 284:1223-1237. [DOI] [PubMed] [Google Scholar]
- 13.Gingles, N. A., J. E. Alexander, A. Kadioglu, P. W. Andrew, A. Kerr, T. J. Mitchell, E. Hopes, P. Denny, S. Brown, H. B. Jones, S. Little, G. C. Booth, and W. L. McPheat. 2001. The role of genetic resistance in invasive pneumococcal infection: identification and study of susceptible and resistant inbred mouse strains. Infect. Immun. 69:426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraguchi, S., N. K. Day, R. P. Nelson, Jr., P. Emmanuel, J. E. Duplantier, C. S. Christodoulou, and R. A. Good. 1998. Interleukin 12 deficiency associated with recurrent infections. Proc. Natl. Acad. Sci. USA 95:13125-13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst, R. A., H. Yesilkaya, E. Clitheroe, A. Rutman, N. Dufty, T. J. Mitchell, C. O'Callaghan, and P. W. Andrew. 2002. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infect. Immun. 70:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jounblat, R., A. Kadioglu, T. J. Mitchell, and P. W. Andrew. 2003. Pneumococcal behavior and host response during bronchopneumonia are affected by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun. 71:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadioglu, A., N. Gingles, K. Grattan, T. J. Mitchell, and P. W. Andrew. 2000. The host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 2:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadioglu, A., S. Taylor, F. Iannelli, G. Pozzi, T. J. Mitchell, and P. W. Andrew. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 70:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanclerski, K., and R. Mollby. 1987. Production and purification of Streptococcus pneumoniae hemolysin (pneumolysin). J. Clin. Microbiol. 25:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp, K., H. Bruunsgaard, P. Skinhoj, and B. K. Pedersen. 2002. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect. Immun. 70:5019-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavelle, E. C., E. McNeela, M. E. Armstrong, O. Leavy, S. C. Higgins, and K. H. G. Mills. 2003. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J. Immunol. 171:2384-2392. [DOI] [PubMed] [Google Scholar]
- 23.Lock, R. A., D. Hansman, and J. C. Paton. 1992. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb. Pathog. 5:461-467. [DOI] [PubMed] [Google Scholar]
- 24.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in Gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 25.Madsen, L., N. Labrecque, J. Engberg, A. Dierich, A. Svejgaard, C. Benoist, D. Mathis, and L. Fugger. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA 96:10338-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuirk, P., and K. H. G. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 27.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 28.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shevach, E. M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389-400. [DOI] [PubMed] [Google Scholar]
- 31.Tascon, R. E., E. Stavropoulos, K. V. Lukacs, and M. J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8 T cells requires the production of gamma interferon. Infect. Immun. 66:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres, J. M., O. Cardenos, A. Vasquez, and D. Schlossberg. 1998. Streptococcus pneumoniae bacteremia in a community hospital. Chest 113:387-390. [DOI] [PubMed] [Google Scholar]
- 33.Tuomanen, E., R. Rich, and O. Zak. 1987. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am. Rev. Respir. Dis. 135:869-874. [DOI] [PubMed] [Google Scholar]
- 34.Valeva, A., I. Walev, and S. Bhakdi. 2000. The nature of the high affinity-binding site for staphylococcal alpha toxin: introducing the concept of a quasi receptor. Med. Microbiol. Immunol. 189:51-55. [Google Scholar]
- 35.Waheed, A. A., Y. Shimada, H. F. G. Heijnen, M. Nakamura, M. Inomata, M. Hayashi, S. Iwashita, J. W. Slot, and Y. Ohno-Iwashita. 2001. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc. Natl. Acad. Sci. USA 98:4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]