Abstract
The clinical outcomes and survival of tyrosine kinase inhibitor (TKI)-treated patients with chronic myeloid leukemia (CML) have been significantly improved. The aim of this editorial is to outline critical steps of TKI administration practices during the long-term clinical course of CML based on data obtained from randomized clinical trials and international recommendations. The efficacy of TKI treatment, TKI side effects, off-target complications, and long-term morbidities due to both the disease and the drug are common arguments in the management of CML. Complete hematological response, early complete cytogenetic response, faster major molecular response, and deeper, more durable molecular responses (MR4, MR4.5, MR5) are the ultimate goals for TKI-receiving patients with CML.
Conflict of interest:None declared.
Keywords: Chronic myeloid leukemia, Tyrosine kinase inhibitor, Imatinib, Nilotinib, Dasatinib, Bosutinib, Ponatinib
Abstract
Kronik myeloid lösemi (KML) tedavisinde tirozin kinaz inhibitörü (TKI) grubu ilaçların kullanımı klinik gidişi ve hastaların yaşam süresini son derece olumlu etkilemiştir. Bu yazının amacı, KML’nin uzun klinik seyri boyunca yapılan TKI uygulamalarının kritik basamaklarını randomize klinik çalışma verileri ve uluslararası rehberler ışığında irdelemektir. KML yönetiminde TKI tedavisinin etkinliği, TKI yan etkileri, ilaçların hedef-dışı komplikasyonları, ve hem hastalık seyrine hem de uygulanan ilaçlara bağlı uzun dönem morbiditeler önemli tartışma noktalarını teşkil etmektedir. Tam hematolojik yanıt, erken tam sitogenetik yanıt, daha çabuk gelişen, daha derin (MR4, MR4.5, MR5), ve dayanıklı major moleküler yanıtlar TKI tedavisi alan KML hastalarında klinik seyir boyunca ulaşılması gereken hedefler konumundadırlar.
INTRODUCTION
Current initial frontline therapy for chronic myeloid leukemia (CML) is chronic oral administration of tyrosine kinase inhibitor (TKI) [1,2]. During the last decade, the introduction of TKI to the treatment regimen of CML has significantly affected the survival of patients. Imatinib mesylate was the first TKI in the clinic. The survival benefit of imatinib in CML is excellent [3]. Next-generation TKIs, namely dasatinib [4,5], nilotinib [6], bosutinib [7], and ponatinib [8], were then developed for the management of CML patients. The clinical outcomes and survival of tyrosine kinase inhibitor (TKI)-treated patients with chronic myeloid leukemia (CML) have been significantly improved. The proper clinical and laboratory monitoring of CML patients is absolutely necessary to reach those successful outcomes [9,10,11]. Complete hematological response (CHR), early complete cytogenetic response (CCyR), faster major molecular response (MMR), and deeper, more durable molecular responses (MR4, MR4.5, MR5) are the ultimate goals for TKI-receiving patients with CML [12]. During the era of 3rd generation TKIs, excellent molecular responses are the most important targets in CML. The surrogate markers of CML outcome (rate, depth, and time to cytogenetic and molecular response) are vital in the clinical management of the disease [1,13,14,15,16,17].
Critical evaluations of CML patients to hit those targets should be made at the baseline and at the 3rd, 6th, 12th, 18th, and 24th months after TKI administration. The treatment milestones are checked during the time-line of evaluation [12]. Therapeutic expectations have increased in the field of CML. The functional cure of the disease is now possible with TKIs. Likewise, the molecular responses of MR4 or MR4.5 could lead to the discontinuation of the drug with proper molecular monitoring (TFR; treatment-free remission) within the context of clinical trials [18]. On the other hand, disease progression (accelerated phase (AP) CML or blastic crisis (BC)) under TKI is a great disaster [19,20,21]. Survival after progression into AP/BC is still significantly shorter, even in the TKI era. However, the risk of progression has been decreased with the introduction of more powerful TKIs [22,23,24]. Major attention should be given for the prevention of disease progression, particularly for the treatment-nave CML or TKI-refractory diseases. Clinical response, the depth of response, and the impact of TKI use on the disease outcome should always be the focuses during the long-term management of CML [12].
Investigational efforts tried to improve the results of CML first-line therapy of imatinib obtained from the International Randomized Study of Interferon and STI571 trials. Those attempts included imatinib dose increase, particularly in high-Sokal risk patients [25]; imatinib-based combinations [26]; and the setting of second-generation TKIs as first-line therapy [24,27]. Dose optimization studies of TKI, such as the German CML IV [28] and TIDEL [29], have been taken into account for increments in safety, efficacy, tolerability, adherence, and acceptably manageable drug toxicity. The aim of this review is to outline critical steps of TKI administration practices during the long-term clinical course of CML based on data obtained from randomized clinical trials (RCTs) and international recommendations. The efficacy of TKI treatment, TKI side effects, off-target complications, and long-term morbidities due to both the disease and the drug are common arguments in the management of CML. Standardized definitions of molecular response in CML under TKI have been given by the European LeukemiaNet (ELN). MR4 is achieved with a BCR-ABL expression of <0.01%, MR4.5 with <0.0032% BCR-ABLIS, and MR5 with <0.001% BCR-ABLIS [28].
Baseline Evaluation and Management of the Patient with CML
Standard baseline evaluation of the de novo CML patient includes exact medical diagnosis of CML, basic laboratory evaluation covering complete blood count (CBC) [30] and peripheral blood smear (PBS), bone marrow histopathology, conventional cytogenetics and/or FISH analyses for the Ph* chromosome, and quantitative molecular analyses for BCR-ABL1. Tumor load and disease phase should be defined [12]. Newly diagnosed chronic-phase CML patients should be stratified based on the Sokal [31], Euro/Hasford [32], and EUTOS [33] CML prognostic scoring systems. Novel recent investigations for de novo CML patients have examined the validity of gene expression profiling, genetic polymorphisms, next-generation genomics, multidrug resistance genes (MDR, OCT1), fusion transcripts, and preexisting BCR-ABL kinase domain mutations [34,35,36,37,38,39,40,41,42,43].
Current initial TKI treatment for chronic-phase CML is imatinib at 400 mg p.o. [12]. Second-generation TKIs, namely dasatinib at 100 mg p.o. [27] and nilotinib at 600 mg p.o. [24], have also been registered for the first-line therapy of CML. There is a tendency for the prescription of more powerful TKIs in high-Sokal risk CML patients and high-risk patients with complex karyotypic abnormalities at the beginning of the disease for the prevention of disease progression. Likewise, young and low prognostic risk CML patients are candidates for second-generation TKIs for the sake of drug discontinuation in the future. However, heterogeneous presentation and course of CML, individual characteristics, compliance and preferences of the patients, comorbidities, different toxicity profiles of the drugs, and the physician-clinical center experience must all be considered during the initial decision making for first-line TKI usage in newly diagnosed chronic-phase CML [12,24,27].
Evaluation and Management at the 3rd Month after the Initiation of TKI in the Patient with CML
Standard disease assessments at the 3rd month of oral TKI administration for the chronic-phase CML patient include critical clinical evaluation and CBC/PBS to reveal CHR, cytogenetic analyses to evaluate the cytogenetic response, and quantitative molecular BCR-ABL analyses to identify molecular response [12]. Optimal response at the 3rd month of imatinib administration is CHR and minor cytogenetic response. However, particularly after the introduction of the powerful second-generation TKIs, namely nilotinib and dasatinib, to the first-line therapy of CML, the expectations in response become higher. Recent RCT studies [24,27,44,45,46,47,48,49,50] indicated that the critical BCR-ABL transcript level (10% cut-off value) the 3rd month following the start of TKI treatment may have prognostic significance in patients with CML. This scientific observation has been made with imatinib in GIMEMA [44], German CML IV [26], Hammersmith [51], DASISION [52], and ENESTnd [22] trials, and with dasatinib in DASISION [49] and with nilotinib in ENESTnd [22] trials. Challenges for the widespread routine use of the 10% BCR-ABL transcript cut-off at the 3rd month of TKI are present. First, the estimated ratio of BCR-ABL/ABL is highly technique-dependant. Many laboratories in the world are still not qualified for the international harmonization of scale (IS). High ratio values on the IS scale, house-keeping control gene problems, variations in samples, delays in the exact molecular assessment time after TKI administration, and early unexpected variation kinetics of response in indivi dual CML patients complicate the universal application of the 10% BCR-ABL transcript cut-off at the 3rd month of TKI. Furthermore, the tumor burden at diagnosis, prognostic scoring, gene profile, cytoreduction with TKI dosage, treatment adherence, and numerous confounding effects may obscure the real-life picture at the 3rd month of TKI usage outside clinical trials. Nevertheless, any CML patient that does have a BCR-ABL over 10% after 3 months of TKI presents a strong warning, requiring more careful and more frequent monitoring based on the clear RCT data. If the CML patient exhibits no CHR and/or no minor cytogenetic response, the failure of the first-line TKI is evident. If the initial failed TKI treatment for CML was imatinib, then nilotinib or dasatinib should be given. If 1 of the 2 second-generation TKIs (nilotinib or dasatinib) was used as the first-line therapy and failed, the other (dasatinib or nilotinib) could be administered particularly based on the mutation data. During the treatment decision for 2nd line TKIs, a mutational analysis shall be performed. Increasing the dose of imatinib has been tried in the literature but seems to be a dying practice in the era of stronger TKIs. Drug tolerability and adherence to the treatment should always be sought. Effective management of the treatment-related adverse effects is a vital part of the CML care [12].
Evaluation and Management at the 6th Month after the Initiation of TKI in the Patient with CML
Standard disease assessments at the 6th month of oral TKI administration for the chronic-phase CML patient include critical clinical evaluation to establish CHR, cytogenetic analyses to evaluate the cytogenetic response, and quantitative molecular BCR-ABL analyses to identify molecular response. Optimal response at the 6th month of imatinib is at least partial cytogenetic response (Ph* chromosome lower than 35%) [12]. However, particularly after the introduction of the powerful second-generation TKIs, namely nilotinib and dasatinib, to the first-line therapy of CML, the expectations in response become higher. CCyR at 6 months and/or BCR-ABL below 1% following 6 months of second-generation TKIs are considered as optimal. Any CML patient that does have a BCR-ABL over 10% and/or Ph* chromosome over 35% after 6 months of TKI treatment (particularly nilotinib and dasatinib) may be accepted as a failed case and the treatment strategy may be changed. Those higher treatment milestones could be applied to the first-line imatinib-receiving CML patients and a switch to second-generation TKIs may be performed. Cumulative incidence of MMR is higher with both nilotinib and dasatinib. An early switch from imatinib to a second-generation TKI is rational since RCTs have indicated the higher probability of obtaining better responses as well as progression-free survival and overall survival [24,27]. Prevention of disease progression seems to be better achieved with more powerful second-generation TKIs. Specific long-term drug adverse effects (such as pleuropulmonary syndrome for dasatinib and metabolic syndrome for nilotinib), as well as increased treatment costs, should be considered. Drug tolerability and adherence to the treatment should always be sought [12].
Evaluation and Management at the 12th Month after the Initiation of TKI in the Patient with CML
Standard disease assessments at the 12th month of oral TKI administration for the chronic-phase CML patient include critical clinical evaluation to establish CHR, cytogenetic analyses to examine the cytogenetic response, and quantitative molecular BCR-ABL analyses to identify molecular response [12]. Additional karyotypic abnormalities should be searched in the BM cytogenetics. Optimal response at the 12th month of imatinib usage is at least CCyR. However, particularly after the introduction of the powerful second-generation TKIs, to the first-line therapy of CML, the expectations in response become higher [1,6,8,15,16,22,28,44,45,48,50,51,53,54,55,56,57,58,59,60,61,62,63]. CCyR at 12 months and BCR-ABL below 0.1% following 6 months of second-generation TKIs are considered as optimal based on international guidelines. Any CML patient that does have a BCR-ABL over 1% and/or Ph* chromosome over 1% after 12 months of TKI usage (particularly nilotinib and dasatinib) may be accepted as a failed case and the treatment strategy may be changed. Those higher treatment milestones could be applied to the first-line imatinib-receiving CML patients and a switch to second-generation TKIs may be performed. Drug tolerability and adherence to the treatment should always be sought [12].
Evaluation and Management at the 18th Month after the Initiation of TKI in the Patient with CML
Standard disease assessments at the 18th month of oral TKI administration for the chronic-phase CML patient include critical clinical evaluation to establish CHR and CCyR, and quantitative molecular BCR-ABL analyses to identify molecular response [12]. Optimal response at the 18th month of imatinib is at least MMR. However, particularly after the introduction of the powerful second-generation TKIs, namely nilotinib and dasatinib, to the first-line therapy of CML, the expectations in response become higher [1,4,6,10,11,17,23,38,39,42,44,45,47,48,49,50,51,52,53,54,55,56,57]. CCyR at 18 months and BCR-ABL below 0.1% following 18 months of second-generation TKIs are considered as optimal. Any CML patient that does have a BCR-ABL over 1% and/or Ph* chromosome over 1% (absence of CCyR) after 18 months of TKI usage (particularly nilotinib and dasatinib) may be accepted as a failed case and the treatment strategy may be changed [15]. Those higher treatment milestones could be applied to the first-line imatinib-receiving CML patients and a switch to second-generation TKIs may be performed. Drug tolerability and adherence to the treatment should always be sought [12].
Evaluation and Management at the 24th Month and Thereafter Following the Initiation of TKI in the Patient with CML
Standard disease assessments at the 24th month of oral TKI administration for the chronic-phase CML patient include critical clinical evaluation to establish CHR and CCyR, and quantitative molecular BCR-ABL analyses to identify molecular response [12]. Optimal response at the 24th month of imatinib is at least the continuation of MMR. However, particularly after the introduction of the powerful second-generation TKIs, namely nilotinib and dasatinib, to the first-line therapy of CML, the expectations in response become higher. CCyR at 24 months and BCR-ABL below 0.1% following 24 months of second-generation TKIs are considered as optimal. Any CML patient that does have a BCR-ABL over 1% and/or Ph* chromosome over 1% after 24 months of TKI usage (particularly nilotinib and dasatinib) may be accepted as a failed case and the treatment strategy may be changed [15]. Those higher treatment milestones could be applied to the first-line imatinib-receiving CML patients and a switch to second-generation TKIs may be performed. Drug tolerability and adherence to the treatment should always be sought. Quality of life is especially a matter of concern in CML patients receiving long-term, maybe lifetime, TKI drugs [12].
In the case of intolerance to any TKIs and/or multi-TKI-resistant CML cases with or without mutations, third-line treatment includes bosutinib, ponatinib, allogeneic stem cell transplantation, and experimental therapies [64,65,66]. ABL domain mutations leading to the increments in the BCR-ABL oncogenicity may be detected during the TKI therapy. The TKI regimen may be altered with another TKI based on the type of the mutation. Sometimes the entire treatment strategy of CML has to be changed because of the presence of the ABL domain mutation. For instance, T315I is a unique mutation making the CML patient irresponsive to most TKIs (excluding ponatinib) and leads allografting to become an option in the case [15]. Combination treatments such as TKI plus interferon [67] are still a matter of research and are rarely used outside of clinical trials.
Evaluation for the discontinuation of TKIs in the superior TKI-responder patient with CML should be performed in the long term, for instance after 2 years. Patients with deeper molecular responses (MR4, MR4.5, MR5) are candidates for TKI discontinuation [68]. MR4 is achieved with a BCR-ABL expression of <0.01%, MR4.5 with <0.0032% BCR-ABLIS, and MR5 with <0.001% BCR-ABLIS [28,46]. Treatment-free remissions and re-induction of the remission with the same TKI seem to be possible based on the data from the STIM trial [68]. Pregnancy represents a cause for TKI discontinuation because of the negative impact of any TKI on organogenesis. Patients with AP/BC CML should be treated with the most powerful TKI available and multi-agent chemotherapy before allografting [19,20,21,65,69,70,71]. Since those patients with advanced-phase CML still do have a worse prognosis, prevention of disease progression is the most significant aspect of CML disease management.
Future Perspectives of CML
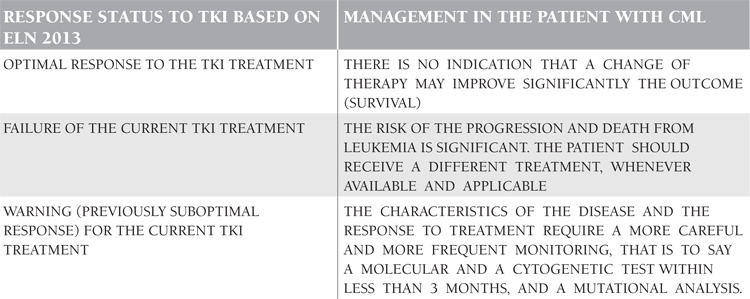
ELN 2013 recommendations have established how to proceed in clinical decision making in the CML patients receiving TKIs based on the response status (Table 1). 72 The future of CML and TKI treatment will reveal better understandings of the disease pathobiology, leukemic stem cells, signal transduction, and their translations to the patient’s care [34,35,36,37,38,39,40,41,42,43]. Cure of CML and the eradication of minimal residual disease via the multi-hit drugs with distinct biological actions would be possible. The cessation of therapy with the aim of cure, stem cell depletion, stem cell exhaustion, and immunological control of the disease may be the future strategies in the management of CML.
Table 1. European LeukemiaNet (ELN) 2013 recommendations for the clinical decision making in the chronic myeloid leukemia (CM)L patients receiving tyrosine kinase inhibitor (TKI) based on the response status during the follow-up and monitoring.
CONFLICT OF INTEREST STATEMENT
The authors of this paper have no conflicts of interest, including specific financial interests, relationships, and/ or affiliations relevant to the subject matter or materials included.
References
- 1.Ferdinand R, Mitchell SA, Batson S, Tumur I. Treatments for chronic myeloid leukemia: a qualitative systematic review. J Blood Med. 2012;3:51–76. doi: 10.2147/JBM.S33380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koca E, Haznedaroğlu İC. Imatinib mesylate and the management of chronic myeloid leukemia (CML) Turk J Haematol. 2005;22:161–172. [PubMed] [Google Scholar]
- 3.Anstrom KJ, Reed SD, Allen AS, Glendenning GA, Schulman KA. Long-term survival estimates for imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2004;101:2584–2592. doi: 10.1002/cncr.20674. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, Hellmann A, Zaritsky A, Golenkov A, Radich J, Hughes T, Countouriotis A, Shah N. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 5.Saydam G, Haznedaroglu IC, Temiz Y, Soysal T, Sucak G, Tombuloglu M, Ozdogu H, Yavuz S, Altintas A, Ozet G, Gulbas Z, Ferhanoglu B, Ilhan O. Retrospective evaluation of patients treated with dasatinib for Philadelphia positive leukemias: Turkish experience of 16 months. UHOD. 2009;19:195–204. [Google Scholar]
- 6.Luciano L, Seneca E, Annunziata M, Pezzullo L, Danise P, Palmieri F, Iovine M, Vallone R, Pane F. Efficacy of nilotinib in early chronic phase CML patients who have suboptimal cytogenetic or molecular response to imatinib A multicentric retrospective study. ASH Annual Meeting Abstracts. 2012;120:4454–4454. [Google Scholar]
- 7.Cortes JE, Khoury HJ, Lipton JH, Gambacorti-Passerini C, Brümmendorf TH, Kim DW, Leip E, Kelly V, Besson N, Turnbull K, Kantarjian HM. Baseline predictors of response to bosutinib in patients with chronic phase chronic myeloid leukemia following resistance or intolerance to imatinib plus dasatinib and/or nilotinib. ASH Annual Meeting Abstracts. 2012;120:2793–2793. [Google Scholar]
- 8.Hochhaus A, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley J, Khoury HJ, Talpaz M, DiPersio JF, DeAngelo D, Abruzzese E, Rea D, Baccarani M, Muller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hughes TP, Goldman JM, Shah N, Kantarjian HM, Cortes JE. Molecular responses with ponatinib in patients with Philadelphia chromosome positive (Ph+) leukemia: results from the PACE trial. ASH Annual Meeting Abstracts. 2012;120:3763–3763. [Google Scholar]
- 9.Simonsson B, Porkka K, Richter J. Second-generation BCR-ABL kinase inhibitors in CML. N Engl J Med. 2010;363:1673–1673. doi: 10.1056/NEJMc1007927. [DOI] [PubMed] [Google Scholar]
- 10.Haznedaroglu IC, Cetiner M, Ilhan O. The management of chronic myeloid leukemia in the era of second generation tyrosine kinase inhibitors. UHOD. 2011;21:1–3. [Google Scholar]
- 11.Haznedaroglu IC, Koca E, Aksu S, Goker H, Sayinalp N, Buyukasik Y, Ozcebe OI. The gray-zone concept, suboptimal response to imatinib, shall be removed from the ELN-CML recommendations. UHOD 2010;20(Suppl. 2010;20(Suppl 1):25–26. [Google Scholar]
- 12.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fausel C. Targeted chronic myeloid leukemia therapy: Seeking a cure. Am J Health Syst Pharm. 2007;64(Suppl 15):9–15. doi: 10.2146/ajhp070482. [DOI] [PubMed] [Google Scholar]
- 14.Fava C, Cortes J. Optimizing first-line therapy for patients with chronic myeloid leukemia. Semin Hematol 2009;46(Suppl. 2009;46(Suppl 3):5–10. doi: 10.1053/j.seminhematol.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Gora-Tybor J. Emerging therapies in chronic myeloid leukemia. Curr Cancer Drug Targets. 2012;12:458–470. doi: 10.2174/156800912800673202. [DOI] [PubMed] [Google Scholar]
- 16.Guilhot F, Roy L, Tomowiak C. Current treatment strategies in chronic myeloid leukemia. Curr Opin Hematol. 2012;19:102–109. doi: 10.1097/MOH.0b013e32834ff610. [DOI] [PubMed] [Google Scholar]
- 17.Jabbour E, Cortes JE, Ghanem H, O’Brien S, Kantarjian HM. Targeted therapy in chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:99–110. doi: 10.1586/14737140.8.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini O, Kantarjian HM, Rios MB, Jabbour E, O’Brien S, Jain P, Faderl S, Garcia-Manero G, Ravandi F, Borthakur G, Quintás-Cardama A, Cortes JE. Patient (Pt)-driven discontinuation of tyrosine kinase inhibitor therapy in chronic phase chronic myeloid leukemia (CML) - Single institution experience. ASH Annual Meeting Abstracts. 2012;120:3783–3783. [Google Scholar]
- 19.Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, Erben P, Cortes J, Paquette R, Bradley-Garelik MB, Zhu C, Dombret H. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116:3852–3861. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uz B, Bektas O, Eliacık E, Goker H, Erbilgin Y, Sayitoglu M, Sayinalp N, Aksu S, Buyukasik Y, Ozcebe O, Haznedaroglu IC. Allografting for Bosutinib, Imatinib, Nilotinib, Dasatinib, and Interferon Resistant Chronic Myeloid Leukemia without ABL Kinase Mutation. Case Rep Hematol. 2011;2011:263725–263725. doi: 10.1155/2011/263725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochhaus A, Hughes TP, Saglio G, Guilhot F, Al-Ali HK, Rosti G, Nakaseko C, De Souza CA, Kemp C, Fan X, Hoenekopp A, Larson RA, Kantarjian HM. Outcome of patients with chronic myeloid leukemia in chronic phase (CML-CP) based on early molecular response and factors associated with early response: 4-year follow-up data from ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials Newly Diagnosed Patients) ASH Annual Meeting Abstracts. 2012;120:167–167. [Google Scholar]
- 22.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW, Ogura M, Pavlovsky C, Junghanss C, Milone JH, Nicolini FE, Robak T, Van Droogenbroeck J, Vellenga E, Bradley-Garelik MB, Zhu C, Hochhaus A. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM. ENESTnd InvestigatorsNilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 24.Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, Alimena G, Ehrencrona H, Hjorth-Hansen H, Kairisto V, Levato L, Martinelli G, Nagler A, Lanng Nielsen J, Ozbek U, Palandri F, Palmieri F, Pane F, Rege-Cambrin G, Russo D, Specchia G, Testoni N, Weiss-Bjerrum O, Saglio G, Simonsson B. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113:4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 25.Berger U, Engelich G, Reiter A, Hochhaus A, Hehlmann R. Imatinib and beyond--the new CML study IV A randomized controlled comparison of imatinib vs imatinib/interferon-alpha vs imatinib/low-dose AraC vs imatinib after interferon-alpha failure in newly diagnosed chronic phase chronic myeloid leukemia. Ann Hematol. 2004;83:258–264. doi: 10.1007/s00277-003-0807-x. [DOI] [PubMed] [Google Scholar]
- 26.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, Nakamae H, Huguet F, Boqué C, Chuah C, Bleickardt E, Bradley-Garelik MB, Zhu C, Szatrowski T, Shapiro D, Baccarani M. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 27.Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, Schnittger S, Haferlach C, Göhring G, Proetel U, Kolb HJ, Krause SW, Hofmann WK, Schubert J, Einsele H, Dengler J, Hänel M, Falge C, Kanz L, Neubauer A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Branford S, Hughes TP, Spiekermann K, Baerlocher GM, Pfirrmann M, Hasford J, Saußele S, Hochhaus A. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26:2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 28.Yeung DT, Osborn MP, White DL, Branford S, Kornhauser M, Slader C, Issa S, Hiwase DK, Hertzberg MS, Schwarer AP, Filshie R, Arthur CK, Kwan YL, Forsyth CJ, Ross R, Mills AK, Grigg A. Early switch to nilotinib does not overcome the adverse outcome for CML patients failing to achieve early molecular response on imatinib, despite excellent overall outcomes in the TIDEL II Trial. ASH Annual Meeting Abstracts. 2012;120:3771–3771. [Google Scholar]
- 29.Hayran M, Koca E, Haznedaroglu IC, Unsal I, Durgun B, Guvenc F, Ozturk B, Ratip S, Ozcebe OI. Predicting chronic leukaemias from assessment of complete peripheral blood counts. J Intl Med Res. 2006;34:640–647. doi: 10.1177/147323000603400609. [DOI] [PubMed] [Google Scholar]
- 30.okal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 31.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, Alimena G, Steegmann JL, Ansari H. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 32.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, Guilhot F, Porkka K, Ossenkoppele G, Lindoerfer D, Simonsson B, Pfirrmann M, Hehlmann R. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 33.Bourgne C, Janel A, Chassagne J, Rapatel C, Tournilhac O, Guerci A, Lioret F, Briançon A, Berger J, Bamdad M, Boiret-Dupré N, Berger MG. Phosphorylation of spleen tyrosine kinase (Syk) appears to be related to the blast phase of chronic myeloid leukemia. ASH Annual Meeting Abstracts. 2012;120:4425–4425. [Google Scholar]
- 34.Gutknecht M, Joas S, Güttler L, Kanz L, Salih HR, Grünebach F, Rittig SM. Monocyte-derived dendritic cells induce functionally active regulatory T cells upon exposure to BCR-ABL tyrosine kinase inhibitors. ASH Annual Meeting Abstracts. 2012;120:2156–2156. [Google Scholar]
- 35.Gutknecht M, Joas S, Kanz L, Salih HR, Rittig SM, Gruenebach F. Inhibition of PI3K/Akt and Erk signaling pathways by BCR-ABL tyrosine kinase inhibitors up-regulates the immunosuppressive receptor osteoactivin in monocyte-derived dendritic cells. ASH Annual Meeting Abstracts. 2012;120:1050–1050. [Google Scholar]
- 36.Nievergall E, White DL, Yong ASM, Ramshaw HS, Busfield SJ, Vairo G, Lopez AF, Hughes TP, Hiwase DK. Effective elimination of CML progenitor and stem cells through combination of {alpha}-CD123 antibody-dependent cell-mediated cytotoxicity and tyrosine kinase inhibitor treatment. ASH Annual Meeting Abstracts. 2012;120:32–32. [Google Scholar]
- 37.Quintarelli C, De Angelis B, Errichiello S, Caruso S, Esposito N, Luciano L, Paratore S, Galimberti C, Soverini S, Terragna C, Cilloni D, Saglio G, Martinelli G, Giles F, Hochhaus A, Pane F. Analysis of bone marrow microenvironment factors as early markers of response in patients with newly diagnosed Bcr-Abl positive CML in chronic phase treated with nilotinib. ASH Annual Meeting Abstracts. 2012;120:2795–2795. [Google Scholar]
- 38.Rothe K, Lin KBL, Lin H, Leung A, Forrest DL, Gorski S, Jiang X. Identification of a unique autophagy gene expression signature in CD34+ CML stem/progenitor cells that correlates with clinical response to imatinib mesylate. ASH Annual Meeting Abstracts. 2012;120:1662–1662. [Google Scholar]
- 39.Schafranek L, Nievergall E, Powell JA, Hiwase DK, White DL, Hughes TP. Commitment of CML cells to apoptotic cell death depends on the length of exposure to Das and the level of STAT5 activity. ASH Annual Meeting Abstracts. 2012;120:3736–3736. [Google Scholar]
- 40.Schemionek M, Steidl U, Schwab A, Tenen DG, Mhairi C, Holyoake TL, Preisinger C, Mueller-Tidow C, Brümmendorf TH, Koschmieder S. Metastasis suppressor 1 is downregulated in CML stem cells and overexpression impairs early leukemic cell propagation. ASH Annual Meeting Abstracts. 2012;120:2776–2776. [Google Scholar]
- 41.Slupianek A, Falinski R, Znojek P, Stoklosa T, Flis S, Doneddu V, Synowiec E, Blasiak J, Bellacosa A, Skorski T. BCR-ABL1 kinase inhibits DNA glycosylases to enhance oxidative DNA damage and stimulate genomic instability. ASH Annual Meeting Abstracts. 2012;120:520–520. doi: 10.1038/leu.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabe Y, Jin L, Iwanami H, Kazuno S, Fujimura T, Matsushita H, Ueno T, Miida T, Andreeff M, Konopleva M, Kimura S. Analysis of dasatinib-induced molecular mechanisms of apoptosis in hypoxia-adopted CML cells utilizing quantitative proteomics technology. ASH Annual Meeting Abstracts. 2012;120:2773–2773. [Google Scholar]
- 43.Castagnetti F, Gugliotta G, Breccia M, Specchia G, Abruzzese E, Levato L, Cavazzini F, Iurlo A, Stagno F, Ferrero D, Porretto F, Martino B, Rupoli S, Intermesoli T, Fava C, Palandri F, Venturi C, Soverini S, Testoni N, Alimena G, Pane F, Saglio G, Martinelli G, Baccarani M, Rosti G. The BCR-ABL transcript levels at 3 and 6 months predict the long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib mesylate: a Gimema CML WP analysis. ASH Annual Meeting Abstracts. 2012;120:1678–1678. [Google Scholar]
- 44.Gugliotta G, Castagnetti F, Palandri F, Breccia M, Levato L, Stagno F, Rege-Cambrin G, Zaccaria A, Specchia G, Usala E, Gozzini A, Martino B, Capucci A, Musto P, Tiribelli M, Abruzzese E, Soverini S, Bochicchio MT, Testoni N, Saglio G, Martinelli G, Pane F, Alimena G, Baccarani M, Rosti G. Cytogenetic and molecular responses at 3 months are associated with a better outcome in early chronic phase (ECP) chronic myeloid leukemia (CML) patients treated with nilotinib. ASH Annual Meeting Abstracts. 2012;120:2797–2797. [Google Scholar]
- 45.Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O’Brien SG, Rudoltz MS, Mone M, Wehrle E, Modur V, Goldman JM. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116:3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain P, Kantarjian HM, Nazha A, Jabbour E, Quintás-Cardama A, Benjamini O, Pierce SA, Cardenas-Turanzas M, Verstovsek S, Borthakur G, Ravandi F, O’Brien S, Cortes JE. Early molecular and cytogenetic responses predicts for significantly longer event free survival (EFS) and overall survival (OS) in patients (pts) with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP) - an analysis of 4 tyrosine kinase inhibitor (TKI) modalities (standard dose imatinib, high dose imatinib, dasatinib and nilotinib) ASH Annual Meeting Abstracts. 2012;120:70–70. [Google Scholar]
- 47.Kim DDH, Lee HG, Kamel-Reid S, Lipton JH. BCR/ABL transcript at 3 months predicts long-term outcomes following second generation tyrosine kinase inhibitor therapy in the patients with CML in chronic phase who failed imatinib. ASH Annual Meeting Abstracts. 2012;120:2777–2777. [Google Scholar]
- 48.Saglio G, Kantarjian HM, Shah N, Jabbour EJ, Quintas-Cardama A, Steegmann JL, Boqué C, Chuah C, Pavlovsky C, Mayer J, Ukropec J, Wildgust M, Hochhaus A. Early response (molecular and cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): exploratory analysis of DASISION 3-year data. ASH Annual Meeting Abstracts. 2012;120:1675–1675. [Google Scholar]
- 49.White DL, Saunders VA, Yeung DT, Grigg A, Hughes TP. Early molecular response to imatinib in CP-CML patients: the significance of early dose intensity and OCT-1 activity in responders and efficacy of dose escalation and switch to nilotinib in non-responders. ASH Annual Meeting Abstracts. 2012;120:693–693. [Google Scholar]
- 50.Marin D, Hedgley C, Clark RE, Apperley J, Foroni L, Milojkovic D, Pocock C, Goldman JM, O’Brien S. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012;120:291–294. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 51.Kantarjian H, O’Brien S, Jabbour E, Shan J, Ravandi F, Kadia T, Faderl S, Garcia-Manero G, Borthakur G, Cortes J. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. J Clin Oncol. 2011;29:3173–3178. doi: 10.1200/JCO.2010.33.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi SY, Lee SE, Kim SH, Jang EJ, Lee J, Oh YJ, Bang JH, Park JE, Byun JY, Jeon HL, Woodman RC, Szczudlo T, Jootar S, Kim HJ, Sohn SK, Park JS, Kim SH, Zang DY, Oh SJ, Kim DW. Nilotinib versus high-dose imatinib in early chronic phase CML patients who have suboptimal molecular responses to standard-dose imatinib: updated data from RE-Nice Study. ASH Annual Meeting Abstracts. 2012;120:3775–3775. [Google Scholar]
- 53.Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390–1397. doi: 10.1182/blood-2012-03-378919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortes JE, Brümmendorf TH, Khoury HJ, Hochhaus A, Apperley J, O’Brien S, Leip E, Kelly V, Ruffner K, Kantarjian HM. Assessment of early cytogenetic response as a predictor of long-term clinical outcomes in a phase 1/2 study of bosutinib in chronic phase CML. ASH Annual Meeting Abstracts. 2012;120:2798–2798. [Google Scholar]
- 55.Cortes JE, Pasquini R, Kantarjian HM, Joske D, Meillon LA, Shen Z, Piccolo C, Zernovak O, Sivarathinasami R, Eng DF, Kim DW, Hughes TP. First-line treatment and management of chronic myeloid leukemia (CML) in clinical practice: update of > 1800 patients (Pts) in the WORLD CML Registry. ASH Annual Meeting Abstracts. 2012;120:3750–3750. [Google Scholar]
- 56.Falchi L, Kantarjian HM, Quintas-Cardama A, O’Brien S, Jabbour EJ, Ravandi F, Borthakur G, Garcia-Manero G, Verstovsek S, Burger JA, Luthra R, Cortes JE. Clinical significance of deeper molecular responses with four modalities of tyrosine kinase inhibitors as frontline therapy for chronic myeloid leukemia. ASH Annual Meeting Abstracts. 2012;120:164–164. [Google Scholar]
- 57.Hughes TP, Lipton JH, Spector N, Leber B, Pasquini R, Clementino N, Schwarer AP, Etienne G, Guerci-Bresler A, Branford S, Purkayastha D, Collins L, Szczudlo T, Cervantes F. Switching to nilotinib is associated with continued deeper molecular responses in CML-CP patients with minimal residual disease after ≥ 2 years on imatinib: ENESTcmr 2-year follow-up results. ASH Annual Meeting Abstracts. 2012;120:694–694. [Google Scholar]
- 58.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, Flinn IW, Kurokawa M, Moiraghi B, Yu R, Blakesley RE, Gallagher NJ, Saglio G, Kantarjian HM. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 59.Rosti G, Castagnetti F, Gugliotta G, Palandri F, Baccarani M. Second-generation BCR-ABL inhibitors for frontline treatment of chronic myeloid leukemia in chronic phase. Crit Rev Oncol Hematol. 2012;82:159–170. doi: 10.1016/j.critrevonc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Rosti G, Gugliotta G, Castagnetti F, Breccia M, Levato L, Rege-Cambrin G, Capucci A, Tiribelli M, Zaccaria A, Bocchia M, Stagno F, Cavazzini F, Specchia G, Martino B, Cedrone M, Intermesoli T, Palandri F, Soverini S, Bochicchio MT, Testoni N, Alimena G, Pane F, Saglio G, Martinelli G, Baccarani M. Five-year results of nilotinib 400 mg BID in early chronic phase chronic myeloid leukemia (CML): high rate of deep molecular response - update of the GIMEMA CML WP Trial CML0307. ASH Annual Meeting Abstracts. 2012;120:3784–3784. [Google Scholar]
- 61.Sail KR, Chen L, Jackson J, Ericson SG, Haislip S, Ibison T, Gilmore J, Saleh MN. Treatment patterns among patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) treated with imatinib in a community setting. ASH Annual Meeting Abstracts. 2012;120:3179–3179. [Google Scholar]
- 62.Wu B, Zhong H, Saglio G, Chen F. Different strategies for first-line treatment of chronic myeloid leukaemia: an economic analysis. ASH Annual Meeting Abstracts. 2012;120:4696–4696. [Google Scholar]
- 63.Quintás-Cardama A, Kantarjian H, Cortes J. Bosutinib for the treatment of chronic myeloid leukemia in chronic phase. Drugs Today (Barc) 2012;48:177–188. doi: 10.1358/dot.2012.48.3.1750274. [DOI] [PubMed] [Google Scholar]
- 64.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley J, Khoury HJ, Talpaz M, DiPersio JF, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Muller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah N. A pivotal phase 2 trial of ponatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant or intolerant to dasatinib or nilotinib, or with the T315I BCR-ABL mutation: 12-month follow-up of the PACE Trial. ASH Annual Meeting Abstracts. 2012;120:163–163. [Google Scholar]
- 65.Ahmed S, Saliba RM, De Lima M, Rondon G, Kebriaei P, Fisher TD, Jabbour E, Chen J, Kantarjian H, Anderlini P, Alousi AM, Hosing CM, Andersson B, Jones RB, Qazilbash MH, Shpall EJ, Giralt S, Champlin RE. Sequential treatment after allogeneic stem cell transplantation for chronic myelogenous leukemia. ASH Annual Meeting Abstracts. 2012;120:3129–3129. [Google Scholar]
- 66.Nicolini FE, Etienne G, Dubruille V, Roy L, Huguet F, Legros L, Giraudier S, Coiteux V, Guerci-Bresler A, Lenain P, Rea D, Ame S, Cony-Makhoul P, Gardembas M, Hermet E, Rousselot P, Gagnieu MC, Morisset S, Pivot C, Etienne M, Guilhot F, Dulucq S, Mahon FX. Pegylated interferon-{alpha} 2a in combination to nilotinib as first line therapy in newly diagnosed chronic phase chronic myelogenous leukemia provides high rates of MR4. 5. Preliminary results of a phase II study. ASH Annual Meeting Abstracts. 2012;120:166–166. [Google Scholar]
- 67.Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P. Intergroupe Français des Leucémies Myéloïdes ChroniquesDiscontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 68.Chae YK, Kantarjian HM, Romo CG, Whitaker C, Jabbour EJ, O’Brien S, Borthakur G, Ravandi F, Thomas DA, Pierce SA, Quintás-Cardama A, Cortes JE. Prognostic factors in the blastic and accelerated phase of patients with chronic myeloid leukemia (CML) in the tyrosine kinase inhibitor (TKI) era. ASH Annual Meeting Abstracts. 2012;120:3786–3786. [Google Scholar]
- 69.Ghanem H, Kantarjian HM, Jabbour EJ, Cortes JE, Quintas-Cardama A. Rare blast phase (BP) phenotypes of chronic myeloid leukemia (CML) ASH Annual Meeting Abstracts. 2012;120:4434–4434. [Google Scholar]
- 70.Strati P, Kantarjian HM, Thomas DA, O’Brien SM, Jabbour EJ, Quintas-Cardama A, Borthakur G, Faderl S, Ravandi F, Cortes JE. Hyper-CVAD plus imatinib or dasatinib for patients with lymphoid blastic phase of chronic myeloid leukemia. ASH Annual Meeting Abstracts. 2012;120:3766–3766. [Google Scholar]
- 71.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]


